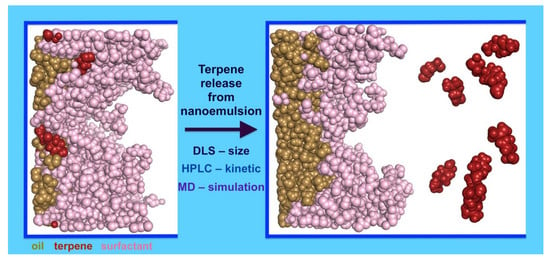
Influence of Terpene Type on the Release from an O/W Nanoemulsion: Experimental and Theoretical Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Droplet Size and Polydispersity Index of Terpene-Loaded Nanoemulsion
2.2. Release of Terpenes
2.3. Kinetic Analysis
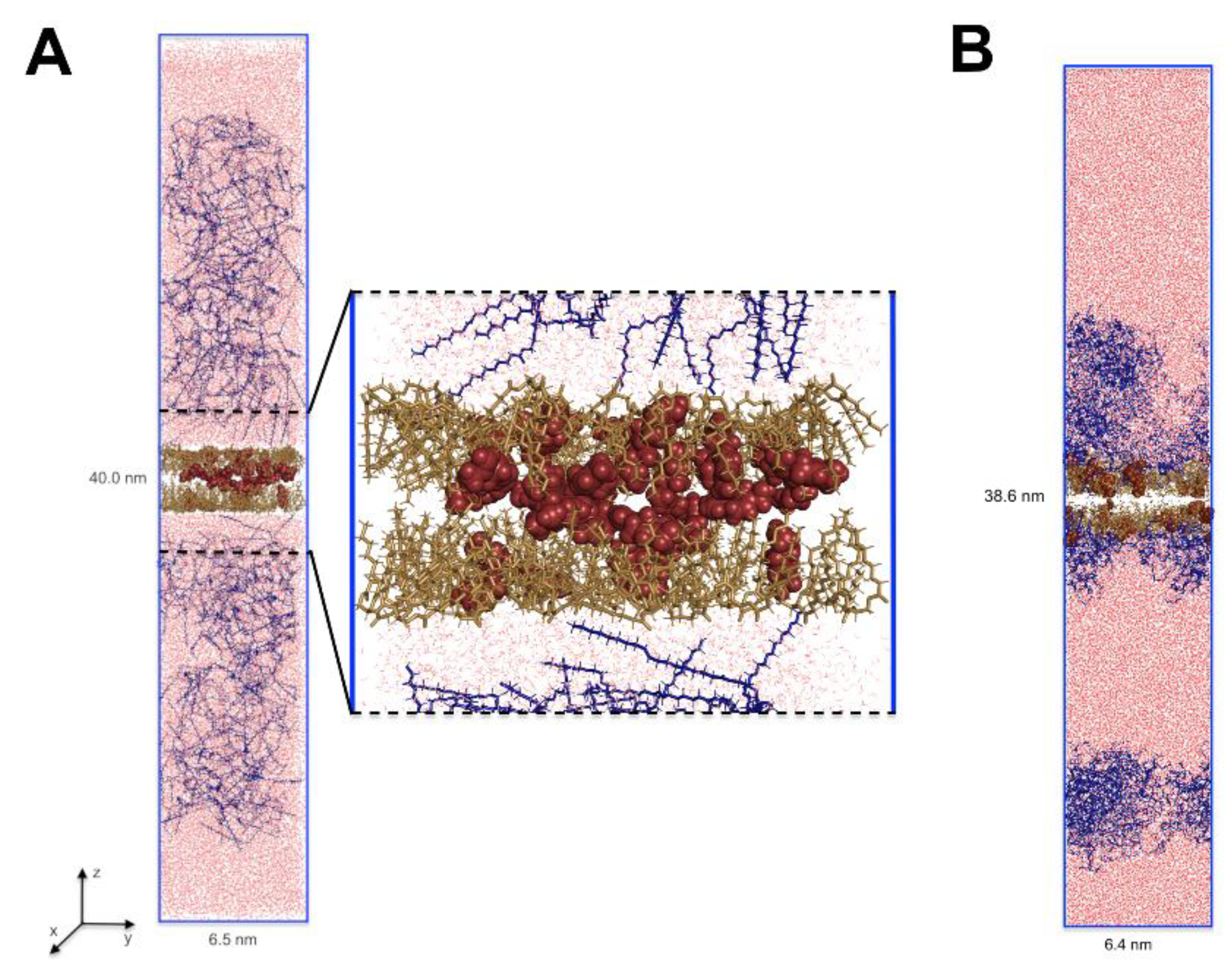
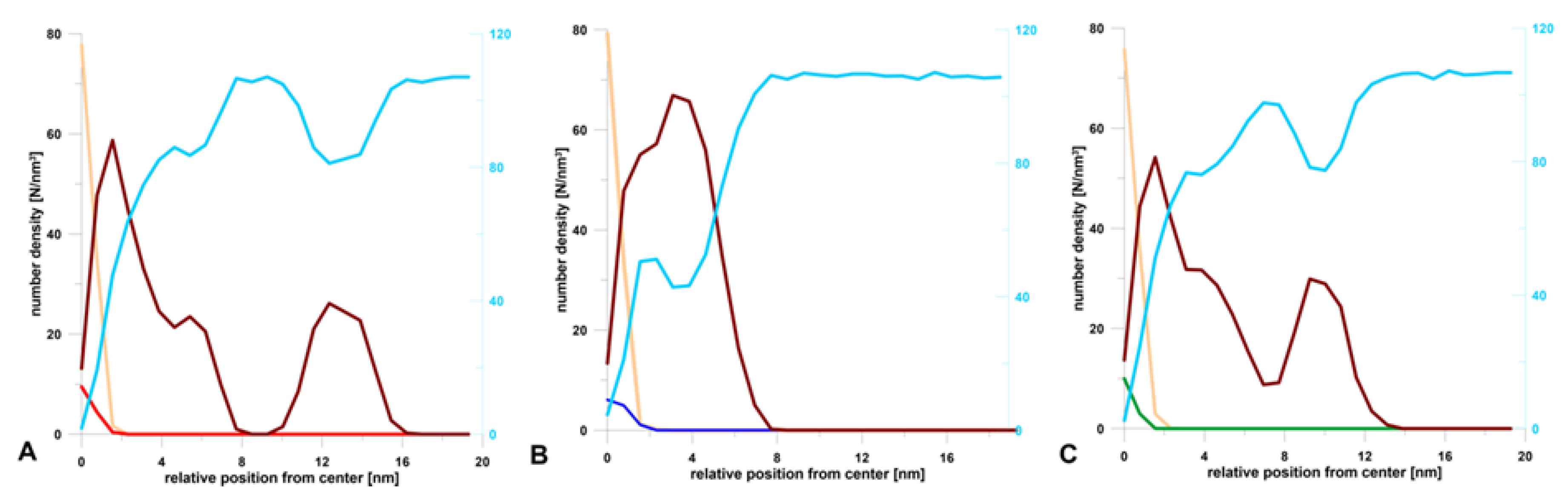
2.4. Molecular Dynamic Simulations
3. Materials and Methods
3.1. Materials
3.2. Nanoemulsion Preparation and Characterization
3.3. Release of Terpenoids
3.4. Evaluation of Release Kinetics
3.5. Computational Details
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eros, I.; Abu-Eida, E.Y.; Csóka, I.; Sánta, Z.; Cserne, A.; Kövér, T. Optimization of drug release from dermatological semisolid preparations. Drug Dev. Res. 2003, 59, 316–325. [Google Scholar] [CrossRef]
- Zeng, L.; An, L.; Wu, X. Modeling Drug-Carrier Interaction in the Drug Release from Nanocarriers. J. Drug Deliv. 2011. [Google Scholar] [CrossRef]
- Wang, G.; Tang, W.; Bidigare, R.R. Terpenoids As Therapeutic Drugs and Pharmaceutical Agents. In Natural Products; Drug Discovery and Therapeutic Medicine; Humana Press Inc.: Totowa, NJ, USA, 2005; pp. 197–227. [Google Scholar]
- Silva, M.; David, J.; Silva, L.; Santos, R.; David, J.; Lima, L.; Reis, P.; Fontana, R. Bioactive oleanane, lupane and ursane triterpene acid derivatives. Molecules 2012, 17, 12197–12205. [Google Scholar] [CrossRef]
- Perveen, S. Introductory Chapter: Terpenes and Terpenoids. In Terpenes and Terpenoids; IntechOpen: London, UK, 2018. [Google Scholar]
- Akhtar, N.; Verma, A.; Pathak, K. Exploring preclinical and clinical effectiveness of nanoformulations in the treatment of atopic dermatitis: Safety aspects and patent reviews. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 1–10. [Google Scholar] [CrossRef]
- Mishra, B.B.T.S.; Patel, B.B.; Tiwari, S. Colloidal nanocarriers: A review on formulation technology, types and applications toward targeted drug delivery. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 9–24. [Google Scholar] [CrossRef]
- Lovelyn, C.; Attama, A.A. Current State of Nanoemulsions in Drug Delivery. J. Biomater. Nanobiotechnol. 2011, 2, 626–639. [Google Scholar] [CrossRef] [Green Version]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter. 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Anton, N.; Vandamme, T.F. Nano-emulsions and micro-emulsions: Clarifications of the critical differences. Pharm. Res. 2011, 28, 978–985. [Google Scholar] [CrossRef]
- Miastkowska, M.; Lasoń, E.; Sikora, E. Wolińska-Kennard, K. Preparation and Characterization of Water-Based Nano-Perfumes. Nanomater 2018, 8, 981. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wong, H.L.; Shuhendler, A.J.; Rauth, A.M.; Wu, X.Y. Molecular interactions, internal structure and drug release kinetics of rationally developed polymer-lipid hybrid nanoparticles. J. Control. Release 2008, 128, 60–70. [Google Scholar] [CrossRef]
- Yukuyama, M.N.; Kato, E.T.; Lobenberg, R.; Bou-Chacra, N.A. Challenges and Future Prospects of Nanoemulsion as a Drug Delivery System. Curr. Pharm. Des. 2017, 23, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Mukhija, A.; Kishore, N. Drug partitioning in individual and mixed micelles and interaction with protein upon delivery form micellar media. J. Mol. Liq. 2018, 265, 1–15. [Google Scholar] [CrossRef]
- McClements, D.J. Edible nanoemulsions: Fabrication, properties, and functional performance. Soft Matter. 2011, 7, 2297–2316. [Google Scholar] [CrossRef] [Green Version]
- Enache, M.; Volanschi, E. Spectroscopic investigations of the molecular interaction of anticancer drug mitoxantrone with non-ionic surfactant micelles. J. Pharm. Pharm. 2012, 64, 688–696. [Google Scholar] [CrossRef]
- Albano, J.M.R.; de Paula, E.; Pickholz, M. Molecular Dynamics Simulations to Study Drug Delivery Systems. In Molecular Dynamics; InTechOpen: London, UK, 2018; pp. 74–90. [Google Scholar]
- Li, Y.; Hou, T. Computational Simulation of Drug Delivery at Molecular Level. Curr. Med. Chem. 2010, 17, 4482–4491. [Google Scholar] [CrossRef]
- Katiyar, R.S.; Jha, P.K. Molecular simulations in drug delivery: Opportunities and challenges. WIREs Comput. Mol. Sci. 2018, 8, e1358. [Google Scholar] [CrossRef]
- Saurabh, S.; Sivakumar, P.M.; Perumal, V.; Khosravi, A.; Sugumaran, A.; Prabhawathi, V. Molecular Dynamics Simulations in Drug Discovery and Drug Delivery. In A Integrative Nanomedicine for New Therapies; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- Miastkowska, M.; Konieczna, M.; Lasoń, E.; Tabaszewska, M.; Sikora, E.; Ogonowski, J. The release of perillyl alcohol from the different kind of vehicles. Curr. Pharm. Biotechnol. 2018, 19, 573–580. [Google Scholar] [CrossRef]
- Miastkowska, M.; Sikora, E.; Ogonowski, J.; Zielina, M.; Łudzi, A. The kinetic study of isotretinoin release from nanoemulsion. Colloids Surf. A Physicochem. Eng. Asp. 2016, 510, 63–68. [Google Scholar] [CrossRef]
- Miastkowska, M.; Wójtowicz, S. The kinetic study of dexamethasone release from multiple emulsions. Przem. Chem. 2020, 99, 87–91. [Google Scholar]
- Yener, G.; Dal, Ö.; Üner, M. Effect of vehicles on release of meloxicam from various topical formulations. Open Drug Deliv. J. 2009, 3, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Özsoy, Y.; Güngör, S.; Cevher, E. Vehicle effects on in vitro release of tiaprofenic acid from different topical formulations. IL Farmaco 2004, 59, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Sandeep, K.; Reddy, P.S. In vitro release of ibuprofen from different topical vehicles. Asian J. Pharm. Sci. Res. 2011, 1, 141–197. [Google Scholar]
- Nayak, A.S.; Jain, A.; Rathore, P.; Sumbhate, S.; Nayak, S. A comparative release study of lisinopril from different vehicles. Int. J. Pharm. Pharm. Sci. 2009, 1, 213–218. [Google Scholar]
- Khalil, R.M.; Basha, M.; Kamel, R. Nanoemulsions as parenteral drug delivery systems for a new anticancer benzimidazole derivative: Formulation and in-vitro evaluation. Egypt Pharm. J. 2015, 14, 166–173. [Google Scholar]
- Osterberg, T.; Norinder, U. Prediction of Polar Surface Area and Drug Transport Processes Using Simple Parameters and PLS Statistics. J. Chem. Inf. Comput. Sci. 2000, 40, 1408–1411. [Google Scholar] [CrossRef]
- Lasoń, E.; Sikora, E.; Ogonowski, J.; Tabaszewska, M.; Skoczylas, Ł. Release study of selected terpenes from nanostructured lipid carriers. Colloids Surf A Physicochem. Eng. Asp. 2016, 510, 87–92. [Google Scholar] [CrossRef]
- Chime, S.A.; Kenechukwu, F.C.; Attama, A.A. Nanoemulsions—Advances in Formulation, Characterization and Applications in Drug Delivery. In Application of Nanotechnology in Drug Delivery; IntechOpen: London, UK, 2014. [Google Scholar]
- Yew, H.-C.; Misran, M.B. Nonionic Mixed Surfactant Stabilized Water-in-Oil Microemulsions for Active Ingredient In Vitro Sustained Release. J. Surfact. Deterg. 2016, 19, 49–56. [Google Scholar] [CrossRef]
- Bruschi, M. Mathematical Models of Drug Release, Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Sawston/Cambridge, UK, 2015; pp. 63–86. [Google Scholar]
- Drais, H.K.; Hussein, A.A. Formulation and characterization of carvedilol nanoemulsion oral liquid dosage form. Int. J. Pharm. Pharm. Sci. 2015, 7, 209–216. [Google Scholar]
- Monteiro, L.M.; Lione, V.F.; do Carmo, F.A.; do Amaral, L.H.; da Silva, J.H.; Nasciutti, L.E.; Rodrigues, C.R.; Castro, H.C.; de Sousa, V.P.; Cabral, L.M. Development and characterization of a new oral dapsone nanoemulsion system: Permeability and in silico bioavailability studies. Int. J. Nanomed. 2012, 7, 5175–5182. [Google Scholar]
- Pradhan, M.; Singh, D.; Murthy, S.N.; Singh, M.R. Design, characterization and skin permeating potential of Fluocinolone acetonide loaded nanostructured lipid carriers for topical treatment of psoriasis. Steroids 2015, 101, 56–63. [Google Scholar] [CrossRef]
- Borges, V.R.A.; Simon, A.; Sena, A.R.C.; Cabral, L.M.; de Sousa, V.P. Nanoemulsion containing dapsone for topical administration: A study of in vitro release and epidermal permeation. Int J. Nanomed. 2013, 8, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An Overview of Micro- and Nanoemulsions as Vehicles for Essential Oils: Formulation, Preparation and Stability. Nanomater 2020, 10, 135. [Google Scholar] [CrossRef] [Green Version]
- Miastkowska, M.; Sikora, E.; Lasoń, E.; Garcia-Celma, M.J.; Escribano-Ferrer, E.; Solans, C.; Llinas, M. Nano-emulsions as vehicles for topical delivery of forskolin. Acta Biochim. Pol. 2017, 64, 713–718. [Google Scholar] [CrossRef]
- Shultz, A.; Jaksch, S.; Schubel, R.; Wegener, E.; Di, Z.; Han, Y.; Meister, A.; Kressler, J.; Kabanov, A.V.; Luxenhofer, R.; et al. Drug-Induced Morphology Switch in Drug Delivery Systems Based on Poly(2-oxazoline)s. ACS Nano 2014, 8, 2686–2696. [Google Scholar]
- Explore Chemistry. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/ (accessed on 22 April 2020).
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–233. [Google Scholar] [PubMed]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- ChemAxon. InstantJChem 15.2.16.0. 2015. Available online: http://www.chemaxon.com (accessed on 15 March 2020).
- Frisch, M.J.; Frisch, G.W.; Trucks, H.B.; Schlegel, G.E.; Scuseria, M.A.; Robb, J.R.; Cheeseman, G.; Scalmani, V.; Barone, G.A.; Petersson, H.; et al. Gaussian 16, Revision C. 01; Gaussian. Inc: Wallingford, CT, UK, 2016. [Google Scholar]
- Martinez, L.; Andrade, R.; Birgin, E.G.; Martinez, J.M. Packmol: A Package for Building Initial Configurations for Molecular Dynamics Simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Martínez, J.M.; Martínez, L. Packing optimization for automated generation of complex system’s initial configurations for molecular dynamics and docking. J. Comput. Chem. 2003, 24, 819–825. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; van der Spoel, D.; van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindah, E. Gromacs: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Śliwa, P.; Śliwa, K.; Sikora, E.; Ogonowski, J.; Oszmiański, J.; Nowicka, P. Incorporation of bioflavonoids from Bidens tripartite into micelles of non-ionic surfactants-experimental and theoretical studies. Colloids Surf. B Biointerfaces 2019, 184, 110553. [Google Scholar] [CrossRef] [PubMed]
- Śliwa, K.; Śliwa, P.; Sikora, E.; Ogonowski, J.; Oszmiański, J.; Nowicka, P. Application of Polyethylene/Polypropylene Glycol Ethers of Fatty Alcohols for Micelle-Mediated Extraction of Calendula anthodium. J. Surfactants Deterg. 2019, 22, 655–661. [Google Scholar] [CrossRef]
- Einstein, A. On the Motion of Small Particles Suspended in a Stationary Liquid. Ann. Phys. 1905, 322, 549–560. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |



| Sample Name | Terpene | Z-Ave (d. nm) ± S.D./PDI |
|---|---|---|
| NE | - | 15 ± 1/0.350 ± 0.015 |
| NE-PA | Perillyl alcohol | 17 ± 2/0.391± 0.57 |
| NE-F | Forskolin | 19 ± 2/0.380 ± 0.027 |
| NE-UA | Ursolic acid | 248 ± 15/0.478± 0.089 |
| Kinetic Model | Parameter | Formulation | |||
|---|---|---|---|---|---|
| NE-PA | NE-F | NE-UA | |||
| Zero-order | R2 | 0.9535 | 0.9785 | 0.9076 | |
| K0 (mg/h) | 0.432744 | 0.2304 | 0.1242 | ||
| First-order | R2 | 0.965 | 0.9812 | 0.8176 | |
| K1 (h−1) | 0.025333 | 0.1787 | 0.09189 | ||
| Higuchi | R2 | 0.996 | 0.9655 | 0.784 | |
| KH (mg/h1/2) | 1.619123 | 0.8379 | 0.4226 | ||
| Korsmeyer–Peppas | R2 | 0.9396 | 0.9564 | 0.7319 | |
| KHP (h−n) | 1.845 | 25.287 | 6.227 | ||
| n | 1.2444 | 0.51778 | 0.807 | ||
| Chemical Name | Structure | Molecular Weight (g/mol) | logP a | Water Solubility a (mg/mL)/logS b (mol/mL) at pH = 7.4 | Polar Surface Area b (Å2) | Molar Volume a (cm3) | S:O Solubility (mg/mL) |
|---|---|---|---|---|---|---|---|
| Perillyl alcohol |  | 152.2 | 1.94 | 1.9/−2.25 | 20.23 | 161.8 | 9 |
| Forskolin |  | 410.5 | 1.36 | 1.1/−3.42 | 113.29 | 331.3 | 6 |
| Ursolic acid |  | 456.7 | 6.58 | 0.00059/−4.73 | 57.53 | 414.7 | 2.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miastkowska, M.; Śliwa, P. Influence of Terpene Type on the Release from an O/W Nanoemulsion: Experimental and Theoretical Studies. Molecules 2020, 25, 2747. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25122747
Miastkowska M, Śliwa P. Influence of Terpene Type on the Release from an O/W Nanoemulsion: Experimental and Theoretical Studies. Molecules. 2020; 25(12):2747. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25122747
Chicago/Turabian StyleMiastkowska, Małgorzata, and Paweł Śliwa. 2020. "Influence of Terpene Type on the Release from an O/W Nanoemulsion: Experimental and Theoretical Studies" Molecules 25, no. 12: 2747. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25122747