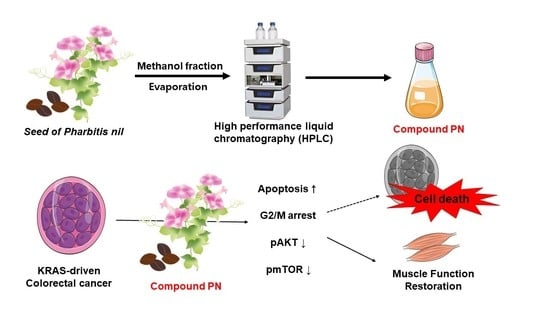
Active Compound of Pharbitis Semen (Pharbitis nil Seeds) Suppressed KRAS-Driven Colorectal Cancer and Restored Muscle Cell Function during Cancer Progression
Abstract
:1. Introduction
2. Results and Discussion
2.1. PN Suppressed Colorectal Cancer Cell Progress
2.2. PN Induced Apoptosis and Cell Cycle Arrest in the G2/M Phase
2.3. Inhibition of the AKT/mTOR Pathway Enhanced PN-Induced Cell Death
2.4. PN Restores Muscle Cell Function during Cancer Progression
3. Materials and Methods
3.1. Preparation of PN from the Seeds of Pharbitis nil
3.2. Cell Culture and Treatment
3.3. Cell Proliferation by MTS Assay
3.4. DAPI/PI Staining
3.5. Clonogenic Assay
3.6. Cell Apoptosis Analysis
3.7. Cell Cycle Analysis
3.8. Western Blotting
3.9. Myogenic Differentiation
3.10. Preparation of SW480-Conditioned Media
3.11. C2C12 Myogenic Differentiation in Cancer Conditioned Media
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- McCormick, F. KRAS as a Therapeutic Target. Clin. Cancer Res. 2015, 21, 1797–1801. [Google Scholar] [CrossRef] [Green Version]
- Misale, S.; Yaeger, R.; Hobor, S.; Scala, E.; Janakiraman, M.; Liska, D.; Valtorta, E.; Schiavo, R.; Buscarino, M.; Siravergna, G.; et al. P2.08 Emergence of Kras Mutations and Acquired Resistance to Anti Egfr Therapy in Colorectal Cancer. Ann. Oncol. 2012, 23, v24. [Google Scholar] [CrossRef]
- Lee, S.K.; Hwang, J.H.; Choi, K.Y. Interaction of the Wnt/beta-catenin and RAS-ERK pathways involving co-stabilization of both beta-catenin and RAS plays important roles in the colorectal tumorigenesis. Adv. Biol. Regul. 2018, 68, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Köhne, C.-H.; Hitre, E.; Zaluski, J.; Chien, C.-R.C.; Makhson, A.; D’Haens, G.; Pinter, T.; Lim, R.; Bodoky, G.; et al. Cetuximab and Chemotherapy as Initial Treatment for Metastatic Colorectal Cancer. N. Engl. J. Med. 2009, 360, 1408–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardelli, A.; Siena, S. Molecular Mechanisms of Resistance to Cetuximab and Panitumumab in Colorectal Cancer. J. Clin. Oncol. 2010, 28, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Lièvre, A.; Bachet, J.-B.; Le Corre, D.; Boige, V.; Landi, B.; Emile, J.-F.; Côté, J.-F.; Tomasic, G.; Penna, C.; Ducreux, M.; et al. KRAS Mutation Status Is Predictive of Response to Cetuximab Therapy in Colorectal Cancer. Cancer Res. 2006, 66, 3992–3995. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-Y.; Lee, I.-Y.; Huang, W.-S.; Lin, Y.-S.; Kuan, F.-C.; Shu, L.-H.; Cheng, Y.-C.; Yang, Y.; Wu, C.-Y. Danshen improves survival of patients with colon cancer and dihydroisotanshinone I inhibit the proliferation of colon cancer cells via apoptosis and skp2 signaling pathway. J. Ethnopharmacol. 2017, 209, 305–316. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.-Z.; Yu, Y.; Wang, J.-J. Inhibitory ASIC2-mediated calcineurin/NFAT against colorectal cancer by triterpenoids extracted from Rhus chinensis Mill. J. Ethnopharmacol. 2019, 235, 255–267. [Google Scholar] [CrossRef]
- Son, Y.; An, Y.; Jung, J.; Shin, S.; Park, I.; Gwak, J.; Ju, B.G.; Chung, Y.; Na, M.; Oh, S. Protopine isolated from Nandina domestica induces apoptosis and autophagy in colon cancer cells by stabilizing p53. Phytotherapy Res. 2019, 33, 1689–1696. [Google Scholar] [CrossRef]
- Zhou, H.; Sun, Y.; Zheng, H.; Fan, L.; Mei, Q.; Tang, Y.; Duan, X.; Li, Y. Paris saponin VII extracted from trillium tschonoskii suppresses proliferation and induces apoptosis of human colorectal cancer cells. J. Ethnopharmacol. 2019, 239, 111903. [Google Scholar] [CrossRef]
- Allaoui, A.; Gascón, S.; Benomar, S.; Quero, J.; De La Osada, J.; Nasri, M.; Yoldi, M.J.R.; Boualga, A. Protein Hydrolysates from Fenugreek (Trigonella foenum graecum) as Nutraceutical Molecules in Colon Cancer Treatment. Nutrients 2019, 11, 724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Ruiz-Torres, V.; Agulló-Chazarra, L.; Herranz-López, M.; Valdés, A.; Cifuentes, A.; Micol, V. Rosemary (Rosmarinus officinalis) extract causes ROS-induced necrotic cell death and inhibits tumor growth in vivo. Sci. Rep. 2019, 9, 808. [Google Scholar] [CrossRef]
- Seo, H.; Song, J.; Kim, M.; Han, D.-W.; Park, H.-J.; Song, M. Cordyceps militaris Grown on Germinated Soybean Suppresses KRAS-Driven Colorectal Cancer by Inhibiting the RAS/ERK Pathway. Nutrients 2018, 11, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.-J.; Park, J.-B.; Lee, S.-J.; Song, M. Phellinus linteus Grown on Germinated Brown Rice Increases Cetuximab Sensitivity of KRAS-Mutated Colon Cancer. Int. J. Mol. Sci. 2017, 18, 1746. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Sun, Y.; Yang, B.; Wang, Z.; Liu, Y.; Cao, Q.; Sun, X.; Kuang, H.-X. Optimization of polysaccharides extraction from seeds of Pharbitis nil and its anti-oxidant activity. Carbohydr. Polym. 2014, 102, 460–466. [Google Scholar] [CrossRef]
- Park, Y.J.; Choi, C.-I.; Chung, H.; Kim, K.H. Pharbilignan C induces apoptosis through a mitochondria-mediated intrinsic pathway in human breast cancer cells. Bioorganic Med. Chem. Lett. 2016, 26, 4645–4649. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Kang, J.-H.; Choi, S.; Son, Y.K.; Lee, K.R.; Seong, J.K.; Kim, S.Y.; Oh, S.H. Pharbitis Nil (PN) induces apoptosis and autophagy in lung cancer cells and autophagy inhibition enhances PN-induced apoptosis. J. Ethnopharmacol. 2017, 208, 253–263. [Google Scholar] [CrossRef]
- Ko, S.-G.; Koh, S.-H.; Jun, C.-Y.; Nam, C.-G.; Bae, H.; Shin, M.-K. Induction of Apoptosis by Saussurea lappa and Pharbitis nil on AGS Gastric Cancer Cells. Boil. Pharm. Bull. 2004, 27, 1604–1610. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.H.; Jung, I.C.; Kim, N.H.; Choi, S.; Lee, K.R.; Son, M.; Jin, M. Gastroprokinetic effects of DA-9701, a new prokinetic agent formulated with Pharbitis Semen and Corydalis Tuber. Phytomedicine 2008, 15, 836–843. [Google Scholar] [CrossRef]
- Bruggeman, A.R.; Kamal, A.H.; Leblanc, T.W.; Ma, J.D.; E Baracos, V.; Roeland, E. Cancer Cachexia: Beyond Weight Loss. J. Oncol. Pract. 2016, 12, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- De Castro, G.S.; Simoes, E.; Lima, J.D.; Ortiz-Silva, M.; Festuccia, W.T.; Tokeshi, F.; Alcântara, P.S.; Otoch, J.P.; Coletti, D.; Seelaender, M.C.L. Human Cachexia Induces Changes in Mitochondria, Autophagy and Apoptosis in the Skeletal Muscle. Cancers 2019, 11, 1264. [Google Scholar] [CrossRef] [Green Version]
- Villars, F.O.; Pietra, C.; Giuliano, C.; Lutz, T.A.; Riediger, T. Oral Treatment with the Ghrelin Receptor Agonist HM01 Attenuates Cachexia in Mice Bearing Colon-26 (C26) Tumors. Int. J. Mol. Sci. 2017, 18, 986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Z.-X.; Yang, Y.-T.; Yu, S.; Li, Y.-Z.; Wang, W.-W.; Huang, J.; Xie, X.-F.; Xiong, L.; Lei, S.; Peng, C. Pogostone induces autophagy and apoptosis involving PI3K/Akt/mTOR axis in human colorectal carcinoma HCT116 cells. J. Ethnopharmacol. 2017, 202, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Takigawa, A.; Mineno, T.; Yoshimitsu, H.; Nohara, T.; Ikeda, T.; Fukuda-Teramachi, E.; Noda, N.; Miyahara, K. Acylated Glycosides of Hydroxy Fatty Acid Methyl Esters Generated from the Crude Resin Glycoside (Pharbitin) of Seeds ofPharbitis nilby Treatment with Indium(III) Chloride in Methanol. J. Nat. Prod. 2010, 73, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Choi, S.U.; Lee, K.R. Diterpene Glycosides from the Seeds ofPharbitis nil. J. Nat. Prod. 2009, 72, 1121–1127. [Google Scholar] [CrossRef]
- Jung, D.Y.; Ha, H.; Lee, H.Y.; Kim, C.; Lee, J.-H.; Bae, K.; Kim, J.S.; Kang, S.S. Triterpenoid saponins from the seeds of Pharbitis nil. Chem. Pharm. Bull. 2008, 56, 203–206. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Moon, E.; Kim, K.H. Neolignan and monoterpene glycoside from the seeds of Pharbitis nil. Phytochem. Lett. 2017, 20, 98–101. [Google Scholar] [CrossRef]
- Kim, K.H.; Woo, K.W.; Moon, E.; Choi, S.U.; Kim, S.Y.; Choi, S.Z.; Son, M.W.; Lee, K.R. Identification of Antitumor Lignans from the Seeds of Morning Glory (Pharbitis nil). J. Agric. Food Chem. 2014, 62, 7746–7752. [Google Scholar] [CrossRef] [Green Version]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef]
- Mollah, M.L.; Park, D.K.; Park, H.J. Cordyceps militaris Grown on Germinated Soybean Induces G2/M Cell Cycle Arrest through Downregulation of Cyclin B1 and Cdc25c in Human Colon Cancer HT-29 Cells. Evid Based Complement Altern. Med. 2012, 2012, 249217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, C.Z.; Du, G.J.; Qi, L.W.; Calway, T.; He, T.C.; Du, W.; Yuan, C.S. Genistein induces G2/M cell cycle arrest and apoptosis via ATM/p53-dependent pathway in human colon cancer cells. Int. J. Oncol. 2013, 43, 289–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponnurangam, S.; Standing, D.; Rangarajan, P.; Subramaniam, D. Tandutinib inhibits the Akt/mTOR signaling pathway to inhibit colon cancer growth. Mol. Cancer Ther. 2013, 12, 598–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.D.; Yu, S.N.; Park, S.-G.; Kim, Y.W.; Nam, H.W.; An, H.H.; Kim, S.H.; Kim, K.-Y.; Ahn, S.C. Biological Activities of Pharbitis nil and Partial Purification of Anticancer Agent from Its Extract. J. Life Sci. 2017, 27, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Nie, Y.; Chen, H.; Guo, C.; Yuan, Z.; Zhou, X.; Zhang, Y.; Zhang, X.; Mo, D.; Chen, Y. Palmdelphin promotes myoblast differentiation and muscle regeneration. Sci. Rep. 2017, 7, 41608. [Google Scholar] [CrossRef] [PubMed]
- Pavlidou, T.; Rosina, M.; Fuoco, C.; Gerini, G.; Gargioli, C.; Castagnoli, L.; Cesareni, G. Regulation of myoblast differentiation by metabolic perturbations induced by metformin. PLoS ONE 2017, 12, e0182475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compound PN are not available from the authors. |






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Seo, H.; Kim, M.-R.; Lee, S.-J.; Ahn, S.; Song, M. Active Compound of Pharbitis Semen (Pharbitis nil Seeds) Suppressed KRAS-Driven Colorectal Cancer and Restored Muscle Cell Function during Cancer Progression. Molecules 2020, 25, 2864. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25122864
Song J, Seo H, Kim M-R, Lee S-J, Ahn S, Song M. Active Compound of Pharbitis Semen (Pharbitis nil Seeds) Suppressed KRAS-Driven Colorectal Cancer and Restored Muscle Cell Function during Cancer Progression. Molecules. 2020; 25(12):2864. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25122864
Chicago/Turabian StyleSong, Jisu, Heejung Seo, Mi-Ryung Kim, Sang-Jae Lee, Sooncheol Ahn, and Minjung Song. 2020. "Active Compound of Pharbitis Semen (Pharbitis nil Seeds) Suppressed KRAS-Driven Colorectal Cancer and Restored Muscle Cell Function during Cancer Progression" Molecules 25, no. 12: 2864. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25122864