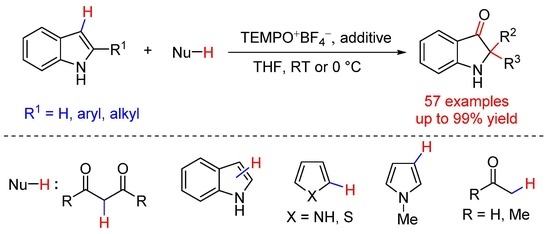
3.2. General Procedure for the Oxidative Dearomative Cross-Dehydrogenative Coupling Reactions
General procedure A: To a solution of 1 (0.1 mmol), 2 (0.2 mmol) and Cu(OTf)2 (0.005 eq.) in THF (1.0 mL) was added TEMPO+BF4− (0.1 mmol) at room temperature. The mixture was further stirred until the disappearance of starting indole by TLC analysis at room temperature. Then, the solvent was removed, and the residue was purified by flash chromatography using acetone-petroleum ether as eluent to afford the desired product.
General procedure B: To a solution of 1 (0.1 mmol) and 4 (0.2 mmol) in THF (1.0 mL) was added TEMPO+BF4− (0.1 mmol) at 0 °C. The mixture was further stirred until the disappearance of starting material 1 by TLC analysis at 0 °C. The solvent was removed and the residue was purified by flash chromatography using acetone-petroleum ether as eluent to afford the desired product.
General procedure C: To a solution of 1 (0.1 mmol) and MeOH (0.5 mmol) in THF (1.0 mL) was added TEMPO+BF4− (0.1 mmol) at 0 °C. The mixture was stirred at 0 °C until the disappearance of 1. Nucleophiles 4r–4t (0.2 mmol) were added to the mixture and the reaction was further stirred until the disappearance of intermediates by TLC analysis at 0 °C. Then, the solvent was removed and the residue was purified by flash chromatography using acetone-petroleum ether as eluent to afford the desired product.
General procedure D: To a solution of C2-substituted indole (0.2 mmol) or indole (0.3 mmol) in THF (1.0 mL) was added TEMPO+BF4− (0.1 mmol). The mixture was stirred at room temperature for 6 h. The solvent was removed and the residue was purified by flash chromatography using acetone-petroleum ether as eluent to afford the desired product.
Diethyl 2-(3-oxo-2-phenylindolin-2-yl)malonate (3a). According to procedure A, 3a was obtained as a yellow solid in 98% yield (36.0 mg; flash chromatographic condition: petroleum ether-acetone 90:10). 1H NMR (600 MHz, CDCl3) δ 7.56 (d, J = 7.7 Hz, 1H), 7.54–7.51 (m, 2H), 7.49–7.45 (m, 1H), 7.30 (t, J = 7.6 Hz, 2H), 7.25 (t, J = 7.3 Hz, 1H), 6.97 (d, J = 8.2 Hz, 1H), 6.81 (t, J = 7.4 Hz, 1H), 6.09 (s, 1H), 4.72 (s, 1H), 4.10–3.99 (m, 3H), 3.91 (dq, J = 10.8, 7.1 Hz, 1H), 1.02 (t, J = 7.1 Hz, 3H), 0.85 (t, J = 7.2 Hz, 3H); 13C NMR (151 MHz, CDCl3) δ 198.1 (C=O), 167.9 (C=O), 166.4 (C=O), 160.2 (Cq), 137.4 (CH), 136.9 (Cq), 128.9 (CH, 2C), 128.2 (CH), 125.5 (CH), 125.4 (CH, 2C), 119.6 (Cq), 119.2 (CH), 111.5 (CH), 70.4 (Cq), 62.0 (CH2), 61.7 (CH2), 58.8 (CH), 13.8 (CH3), 13.4 (CH3); HR-ESIMS m/z calcd for C21H22NO5 [M + H]+ 368.1492, found 368.1494.
Diethyl 2-(5-chloro-3-oxo-2-phenylindolin-2-yl)malonate (3b). According to procedure A, 3b was obtained as a yellow solid in 90% yield (36.1 mg; flash chromatographic condition: petroleum ether-acetone 90:10). 1H NMR (600 MHz, CDCl3) δ 7.53 (d, J = 2.2 Hz, 1H), 7.50 (t, J = 1.7 Hz, 1H), 7.49 (t, J = 1.7 Hz, 1H), 7.42 (dd, J = 8.7, 2.2 Hz, 1H), 7.33–7.30 (m, 2H), 7.27 (dt, J = 14.4, 1.1 Hz, 1H), 6.94 (d, J = 8.6 Hz, 1H), 6.13 (s, 1H), 4.70 (s, 1H), 4.09–4.01 (m, 3H), 3.96 (dq, J = 10.8, 7.1 Hz, 1H), 1.02 (t, J = 7.1 Hz, 3H), 0.95 (t, J = 7.1 Hz, 3H); 13C NMR (151 MHz, CDCl3) δ 196.0 (C=O), 166.8 (C=O), 165.2 (C=O), 157.4 (Cq), 136.3 (CH), 135.4 (Cq), 128.1 (CH, 2C), 127.5 (CH), 124.4 (CH, 2C), 123.8 (CH), 123.4 (Cq), 119.8 (Cq), 111.7 (CH), 70.0 (Cq), 61.2 (CH2), 60.9 (CH2), 57.8 (CH), 12.9 (CH3), 12.6 (CH3); HR-ESIMS m/z calcd for C21H21ClNO5 [M + H]+ 402.1103, found 402.1103.
Diethyl 2-(5-methyl-3-oxo-2-phenylindolin-2-yl)malonate (3c). According to procedure A, 3c was obtained as a yellow solid in 95% yield (36.2 mg; flash chromatographic condition: petroleum ether-acetone 90:10). 1H NMR (600 MHz, CDCl3) δ 7.52–7.48 (m, 2H), 7.36 (s, 1H), 7.32–7.28 (m, 3H), 7.25 (d, J = 7.3 Hz, 1H), 6.90 (d, J = 8.3 Hz, 1H), 5.92 (s, 1H), 4.71 (s, 1H), 4.10–3.99 (m, 3H), 3.92 (dq, J = 10.8, 7.1 Hz, 1H), 2.27 (s, 3H), 1.02 (t, J = 7.1 Hz, 3H), 0.89 (t, J = 7.1 Hz, 3H); 13C NMR (151 MHz, CDCl3) δ 198.2 (C=O), 167.9 (C=O), 166.5 (C=O), 158.7 (Cq), 138.8 (CH), 137.2 (Cq), 128.9 (CH, 2C), 128.8 (Cq), 128.1 (CH), 125.4 (CH, 2C), 124.9 (CH), 119.8 (Cq), 111.5 (CH), 70.8 (Cq), 62.0 (CH2), 61.7 (CH2), 58.9 (CH), 20.6 (CH3), 13.9 (CH3), 13.5 (CH3); HR-ESIMS m/z calcd for C22H24NO5 [M + H]+ 382.1649, found 382.1650.
Diethyl 2-(5-methoxy-3-oxo-2-phenylindolin-2-yl)malonate (3d). According to procedure A, 3d was obtained as a yellow solid in 98% yield (38.9 mg; flash chromatographic condition: petroleum ether-acetone 90:10). 1H NMR (600 MHz, CDCl3) δ 7.52–7.48 (m, 2H), 7.36 (s, 1H), 7.32–7.28 (m, 3H), 7.25 (d, J = 7.3 Hz, 1H), 6.90 (d, J = 8.3 Hz, 1H), 5.92 (s, 1H), 4.71 (s, 1H), 4.10–3.99 (m, 3H), 3.92 (dq, J = 10.8, 7.1 Hz, 1H), 2.27 (s, 3H), 1.02 (t, J = 7.1 Hz, 3H), 0.89 (t, J = 7.1 Hz, 3H); 13C NMR (151 MHz, CDCl3) δ 198.1 (C=O), 167.6 (C=O), 166.2 (C=O), 155.8 (Cq), 153.4 (Cq), 136.9 (Cq), 128.7 (CH, 2C), 127.9 (CH), 127.6 (CH), 125.2 (CH, 2C), 119.6 (Cq), 112.9 (CH), 105.3 (CH), 71.1 (Cq), 61.8 (CH2), 61.5 (CH2), 58.7 (CH3), 55.6 (CH), 13.6 (CH3), 13.4 (CH3); HR-ESIMS m/z calcd for C22H24NO6 [M + H]+ 398.1598, found 398.1600.
Diethyl 2-(6-methyl-3-oxo-2-phenylindolin-2-yl)malonate (3e). According to procedure A, 3e was obtained as a yellow solid in 94% yield (35.8 mg; flash chromatographic condition: petroleum ether-acetone 90:10). 1H NMR (600 MHz, CDCl3) δ 7.51 (d, J = 7.6 Hz, 2H), 7.45 (d, J = 7.9 Hz, 1H), 7.29 (t, J = 7.6 Hz, 2H), 7.24 (t, J = 7.3 Hz, 1H), 6.78 (s, 1H), 6.64 (d, J = 7.9 Hz, 1H), 6.00 (s, 1H), 4.70 (s, 1H), 4.09–3.98 (m, 3H), 3.92 (dq, J = 10.8, 7.1 Hz, 1H), 2.38 (s, 3H), 1.02 (t, J = 7.1 Hz, 3H), 0.89 (t, J = 7.1 Hz, 3H); 13C NMR (151 MHz, CDCl3) δ 197.4 (C=O), 167.9 (C=O), 166.4 (C=O), 160.7 (Cq), 149.1 (Cq), 137.3 (Cq), 128.9 (CH, 2C), 128.1 (CH), 125.4 (CH, 2C), 125.3 (CH), 121.0 (CH), 117.4 (Cq), 111.6 (CH), 70.6 (Cq), 62.0 (CH2), 61.7 (CH2), 58.7 (CH), 22.6 (CH3), 13.8 (CH3), 13.5 (CH3); HR-ESIMS m/z calcd for C22H24NO5 [M + H]+ 382.1649, found 382.1648.
Diethyl 2-(7-methyl-3-oxo-2-phenylindolin-2-yl)malonate (3f). According to procedure A, 3f was obtained as a yellow solid in 91% yield (34.6 mg; flash chromatographic condition: petroleum ether-acetone 90:10). 1H NMR (600 MHz, CDCl3) δ 7.51 (d, J = 7.5 Hz, 2H), 7.43 (d, J = 7.7 Hz, 1H), 7.34–7.29 (m, 3H), 7.25 (t, J = 7.3 Hz, 1H), 6.76 (t, J = 7.4 Hz, 1H), 5.87 (s, 1H), 4.72 (s, 1H), 4.04 (m, 3H), 3.88 (dq, J = 10.7, 7.1 Hz, 1H), 2.35 (s, 3H), 1.05 (t, J = 7.1 Hz, 3H), 0.84 (t, J = 7.1 Hz, 3H); 13C NMR (151 MHz, CDCl3) δ 198.4 (C=O), 168.0 (C=O), 166.3 (C=O), 159.4 (Cq), 137.4 (CH), 137.1 (Cq), 128.9 (CH, 2C), 128.2 (CH), 125.4 (CH, 2C), 122.9 (CH), 120.7 (Cq), 119.4 (CH), 119.1 (Cq), 70.6 (Cq), 62.0 (CH2), 61.8 (CH2), 58.8 (CH), 15.9 (CH3), 13.9 (CH3), 13.4 (CH3); HR-ESIMS m/z calcd for C22H24NO5 [M + H]+ 382.1649, found 382.1649.
Diethyl 2-(2-(4-fluorophenyl)-3-oxoindolin-2-yl)malonate (3g). According to procedure A, 3g was obtained as a yellow solid in 90% yield (34,6 mg; flash chromatographic condition: petroleum ether-acetone 90:10). 1H NMR (600 MHz, CDCl3) δ 7.57 (d, J = 7.7 Hz, 1H), 7.56–7.52 (m, 2H), 7.50–7.46 (m, 1H), 7.03–6.95 (m, 3H), 6.83 (t, J = 7.4 Hz, 1H), 6.10 (s, 1H), 4.64 (s, 1H), 4.12–3.97 (m, 3H), 3.91 (dq, J = 10.8, 7.1 Hz, 1H), 1.06 (t, J = 7.1 Hz, 3H), 0.86 (t, J = 7.1 Hz, 4H); 13C NMR (151 MHz, CDCl3) δ 198.1 (C=O), 167.8 (C=O), 166.1 (C=O), 163.6 (Cq), 161.9 (Cq), 160.1 (Cq), 137.6 (CH), 132.8 (Cq), 132.8 (Cq), 127.5 (CH, 2C), 127.4 (CH, 2C), 125.6 (CH), 119.6 (Cq), 119.4 (CH), 115.9 (CH, 2C), 115.8 (CH, 2C), 111.6 (CH), 69.8 (Cq), 62.2 (CH2), 61.9 (CH2), 58.9 (CH), 13.9 (CH3), 13.4 (CH3); HR-ESIMS m/z calcd for C21H21FNO5 [M + H]+ 386.1398, found 386.1402.
Diethyl 2-(3-oxo-2-(p-tolyl)indolin-2-yl)malonate (3h). According to procedure A, 3h was obtained as a yellow solid in 99% yield (37.7 mg; flash chromatographic condition: petroleum ether-acetone 90:10). 1H NMR (600 MHz, CDCl3) δ 7.56 (d, J = 7.6 Hz, 1H), 7.46 (t, J = 7.6 Hz, 1H), 7.38 (d, J = 8.2 Hz, 2H), 7.11 (d, J = 8.1 Hz, 2H), 6.96 (d, J = 8.2 Hz, 1H), 6.80 (t, J = 7.4 Hz, 1H), 6.03 (s, 1H), 4.70 (s, 1H), 4.12–3.98 (m, 3H), 3.90 (dq, J = 10.8, 7.1 Hz, 1H), 2.28 (s, 3H), 1.06 (t, J = 7.1 Hz, 3H), 0.85 (t, J = 7.1 Hz, 3H); 13C NMR (151 MHz, CDCl3) δ 198.2 (C=O), 167.9 (C=O), 166.5 (C=O), 160.2 (Cq), 137.9 (Cq), 137.3 (CH), 133.9 (Cq), 129.7 (CH, 2C), 125.6 (CH), 125.2 (CH, 2C), 119.7 (Cq), 119.2 (CH), 111.5 (CH), 70.3 (Cq), 62.0 (CH2), 61.7 (CH2), 58.7 (CH), 21.0 (CH3), 13.9 (CH3), 13.4 (CH3); HR-ESIMS m/z calcd for C22H24NO5 [M + H]+ 382.1649, found 382.1651.
Diethyl 2-(3-oxo-2-(4-(trifluoromethoxy)phenyl)indolin-2-yl)malonate (3i). According to procedure A, 3i was obtained as a yellow solid in 83% yield (37.4 mg; flash chromatographic condition: petroleum ether-acetone 90:10). 1H NMR (600 MHz, CDCl3) δ 7.63–7.60 (m, 2H), 7.58 (d, J = 7.7 Hz, 1H), 7.51–7.47 (m, 1H), 7.16 (d, J = 8.3 Hz, 2H), 6.98 (d, J = 8.2 Hz, 1H), 6.84 (t, J = 7.4 Hz, 1H), 6.11 (s, 1H), 4.64 (s, 1H), 4.09–4.03 (m, 2H), 4.00 (ddd, J = 14.3, 9.0, 5.4 Hz, 1H), 3.91 (dq, J = 10.8, 7.1 Hz, 1H), 1.03 (t, J = 7.1 Hz, 3H), 0.87 (t, J = 7.1 Hz, 3H); 13C NMR (151 MHz, CDCl3) δ 197.9 (C=O), 167.8 (C=O), 165.9 (C=O), 160.1 (Cq), 149.2 (Cq) 137.7 (CH), 135.9 (Cq), 127.3 (CH, 2C), 125.6 (CH), 121.3 (Cq), 121.2 (CH, 2C), 119.6 (Cq), 119.5 (CH), 119.5 (Cq), 111.7 (CH), 69.8 (Cq), 62.2 (CH2), 61.9 (CH2), 58.9 (CH), 13.8 (CH3), 13.5 (CH3); HR-ESIMS m/z calcd for C22H21F3NO6 [M + H]+ 452.1315, found 452.1314.
Diethyl 2-(2-(3-methoxyphenyl)-3-oxoindolin-2-yl)malonate (3j). According to procedure A, 3j was obtained as a yellow solid in 99% yield (39.3 mg; flash chromatographic condition: petroleum ether-acetone 90:10). 1H NMR (600 MHz, CDCl3) δ 7.56 (d, J = 7.7 Hz, 1H), 7.47 (ddd, J = 8.3, 7.2, 1.3 Hz, 1H), 7.22 (t, J = 8.0 Hz, 1H), 7.09 (ddd, J = 7.9, 1.8, 0.8 Hz, 1H), 7.07–7.05 (m, 1H), 6.96 (d, J = 8.2 Hz, 1H), 6.83–6.77 (m, 2H), 6.04 (s, 1H), 4.70 (s, 1H), 4.13–3.98 (m, 3H), 3.90 (dq, J = 10.7, 7.2 Hz, 1H), 3.77 (s, 3H), 1.06 (t, J = 7.1 Hz, 3H), 0.85 (t, J = 7.1 Hz, 3H); 13C NMR (151 MHz, CDCl3) δ 197.9 (C=O), 167.9 (C=O), 166.4 (C=O), 160.2 (Cq), 159.9 (Cq), 138.6 (Cq), 137.4 (CH), 129.9 (CH), 125.5 (CH), 119.6 (Cq), 119.3 (CH), 117.7 (CH), 113.3 (CH), 111.6 (CH), 111.5 (CH), 70.3 (Cq), 62.0 (CH2), 61.8 (CH2), 58.7 (CH3), 55.3 (CH), 13.9 (CH3), 13.4 (CH3); HR-ESIMS m/z calcd for C22H24NO6 [M + H]+ 398.1598, found 398.1599.
Diethyl 2-(2-methyl-3-oxoindolin-2-yl)malonate (3k). According to procedure A, 3k was obtained as a yellow solid in 84% yield (25.6 mg; flash chromatographic condition: petroleum ether-acetone 90:10). 1H NMR (600 MHz, CDCl3) δ 7.63 (d, J = 7.7 Hz, 1H), 7.45–7.41 (m, 1H), 6.85–6.79 (m, 2H), 5.45 (s, 1H), 4.36–4.27 (m, 2H), 3.98 (s, 1H), 3.97–3.93 (m, 1H), 3.89–3.83 (m, 1H), 1.35 (s, 3H), 1.33 (t, J = 7.1 Hz, 3H), 0.85 (t, J = 7.1 Hz, 3H); 13C NMR (151 MHz, CDCl3) δ 201.6 (C=O), 168.4 (C=O), 166.5 (C=O), 159.8 (Cq), 137.2 (CH), 125.0 (CH), 120.1 (Cq), 119.0 (CH), 112.3 (CH), 65.3 (Cq), 61.9 (CH2), 61.9 (CH2), 57.8 (CH), 22.3 (CH3), 14.2 (CH3), 13.4 (CH3); HR-ESIMS m/z calcd for C16H20NO5 [M + H]+ 306.1336, found 306.1335.
Dimethyl 2-(3-oxo-2-phenylindolin-2-yl)malonate (3l). According to procedure A, 3l was obtained as a yellow solid in 97% yield (32.8 mg; flash chromatographic condition: petroleum ether-acetone 90:10). 1H NMR (600 MHz, CDCl3) δ 7.57 (d, J = 7.7 Hz, 1H), 7.53–7.50 (m, 2H), 7.50–7.46 (m, 1H), 7.31 (t, J = 7.6 Hz, 2H), 7.26 (dd, J = 7.9, 5.9 Hz, 1H), 6.98 (d, J = 8.2 Hz, 1H), 6.82 (t, J = 7.4 Hz, 1H), 6.08 (s, 1H), 4.76 (s, 1H), 3.58 (s, 3H), 3.49 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 198.1 (C=O), 168.4 (C=O), 166.6 (C=O), 160.2 (Cq), 137.5 (CH), 136.8 (Cq), 129.0 (CH, 2C), 128.3 (CH), 125.6 (CH), 125.3 (CH, 2C), 119.4 (Cq), 119.4 (CH), 111.6 (CH), 70.4 (Cq), 58.6 (CH), 52.8 (CH3, 2C); HR-ESIMS m/z calcd for C19H18NO5 [M + H]+ 340.1179, found 340.1181.
Diisopropyl 2-(3-oxo-2-phenylindolin-2-yl)malonate (3m). According to procedure A, 3m was obtained as a yellow solid in 95% yield (37.5 mg; flash chromatographic condition: petroleum ether-acetone 90:10). 1H NMR (600 MHz, CDCl3) δ 7.56 (d, J = 7.7 Hz, 1H), 7.53–7.50 (m, 2H), 7.46 (t, J = 7.7 Hz, 1H), 7.29 (t, J = 7.6 Hz, 2H), 7.24 (t, J = 7.3 Hz, 1H), 6.96 (d, J = 8.2 Hz, 1H), 6.80 (t, J = 7.4 Hz, 1H), 6.09 (s, 1H), 4.89–4.82 (m, 2H), 4.66 (s, 1H), 1.09 (d, J = 6.3 Hz, 3H), 1.06 (d, J = 6.3 Hz, 3H), 0.98 (d, J = 6.3 Hz, 3H), 0.72 (d, J = 6.3 Hz, 3H); 13C NMR (151 MHz, CDCl3) δ 198.0 (C=O), 167.5 (C=O), 165.9 (C=O), 160.2 (Cq), 137.3 (CH), 137.2 (Cq), 128.9 (CH, 2C), 128.1 (CH), 125.5 (CH), 125.4 (CH, 2C), 119.8 (Cq), 119.2 (CH), 111.5 (CH), 70.4 (CH), 70.1 (CH), 69.4 (Cq), 59.1 (CH), 21.5 (CH3), 21.4 (CH3), 21.3 (CH3), 20.7 (CH3); HR-ESIMS m/z calcd for C23H26NO5 [M + H]+ 396.1805, found 396.1803.
Di-tert-butyl 2-(3-oxo-2-phenylindolin-2-yl)malonate (3n). According to procedure A, 3n was obtained as a yellow solid in 96% yield (32.5 mg; flash chromatographic condition: petroleum ether-acetone 90:10). 1H NMR (600 MHz, CDCl3) δ 7.56 (d, J = 7.6 Hz, 1H), 7.53 (d, J = 7.6 Hz, 2H), 7.48 (ddd, J = 8.3, 7.2, 1.3 Hz, 1H), 7.31 (t, J = 7.6 Hz, 2H), 7.26–7.23 (m, 1H), 6.96 (d, J = 8.2 Hz, 1H), 6.81 (t, J = 7.3 Hz, 1H), 6.07 (s, 1H), 4.56 (s, 1H), 1.23 (s, 9H), 1.14 (s, 9H); 13C NMR (151 MHz, CDCl3) δ 197.9 (C=O), 167.0 (C=O), 165.6 (C=O), 160.1 (Cq), 137.4 (Cq), 137.2 (CH), 128.6 (2C, CH), 127.8 (CH), 125.5 (CH), 125.4 (2C, CH), 119.6 (Cq), 118.9 (CH), 111.2 (CH), 83.1 (Cq), 82.3(Cq), 70.5(Cq), 60.4 (CH), 27.5 (CH3, 3C), 27.4 (CH3, 3C); HR-ESIMS m/z calcd for C25H30NO5 [M + H]+ 424.2118, found 424.2122.
Dibutyl 2-(3-oxo-2-phenylindolin-2-yl)malonate (3o). According to procedure A, 3o was obtained as a yellow solid in 99% yield (41.9 mg; flash chromatographic condition: petroleum ether-acetone 90:10). 1H NMR (600 MHz, CDCl3) δ 7.55 (d, J = 7.7 Hz, 1H), 7.53 (dd, J = 8.2, 0.9 Hz, 2H), 7.48–7.45 (m, 1H), 7.30 (t, J = 7.6 Hz, 2H), 7.25 (t, J = 7.3 Hz, 1H), 6.97 (d, J = 8.2 Hz, 1H), 6.81 (t, J = 7.4 Hz, 1H), 6.09 (s, 1H), 4.74 (s, 1H), 4.02–3.96 (m, 2H), 3.96–3.87 (m, 2H), 1.45–1.35 (m, 2H), 1.27–1.10 (m, 6H), 0.83 (t, J = 7.4 Hz, 3H), 0.78 (t, J = 7.2 Hz, 3H); 13C NMR (151 MHz, CDCl3) δ 198.0 (C=O), 168.1 (C=O), 166.5 (C=O), 160.2 (Cq), 137.4 (CH), 137.0 (Cq), 128.9 (CH, 2C), 128.2 (CH), 125.6 (CH), 125.4 (CH, 2C), 119.6 (Cq), 119.2 (CH), 111.6 (CH), 70.3 (Cq), 65.9 (CH2), 65.5 (CH2), 58.8 (CH), 30.4 (CH2), 30.1 (CH2), 18.9 (CH2, 2C), 13.7 (CH3), 13.6 (CH3); HR-ESIMS m/z calcd for C25H30NO5 [M + H]+ 424.2118, found 424.2120.
Dibenzyl 2-(3-oxo-2-phenylindolin-2-yl)malonate (3p). According to procedure A, 3p was obtained as a yellow solid in 99% yield (48.6 mg; flash chromatographic condition: petroleum ether-acetone 90:10). 1H NMR (600 MHz, CDCl3) δ 7.46 (dd, J = 7.8, 1.6 Hz, 2H), 7.43 (d, J = 7.7 Hz, 1H), 7.40 (ddd, J = 8.3, 7.2, 1.3 Hz, 1H), 7.29–7.18 (m, 9H), 7.01 (t, J = 6.9 Hz, 4H), 6.85 (d, J = 8.2 Hz, 1H), 6.75–6.71 (m, 1H), 6.01 (s, 1H), 4.97 (s, 2H), 4.93 (d, J = 12.2 Hz, 1H), 4.89 (d, J = 12.2 Hz, 1H), 4.84 (s, 1H); 13C NMR (151 MHz, CDCl3) δ 197.7 (C=O), 167.8 (C=O), 166.1 (C=O), 160.0 (Cq), 137.3 (CH), 136.8 (Cq), 134.9 (Cq), 134.6 (Cq), 129.0 (CH, 2C), 128.6 (CH, 2C), 128.5 (CH, 2C), 128.4 (CH), 128.3 (CH, 2C), 128.3 (CH), 128.2 (CH), 128.2 (CH, 2C), 125.6 (CH), 125.4 (CH, 2C), 119.4 (Cq), 119.3 (CH), 111.5 (CH), 70.4 (Cq), 67.8 (CH2), 67.5 (CH2), 58.8 (CH); HR-ESIMS m/z calcd for C31H26NO5 [M + H]+ 492.1805, found 492.1807.
3-(3-oxo-2-phenylindolin-2-yl)pentane-2,4-dione (3q). According to procedure A, 3q was obtained as a yellow solid in 80% yield (24.6 mg; flash chromatographic condition: petroleum ether-acetone 90:10). 1H NMR (600 MHz, CDCl3) δ 7.61 (d, J = 7.4 Hz, 2H), 7.53 (d, J = 7.7 Hz, 1H), 7.48 (ddd, J = 8.3, 7.2, 1.2 Hz, 1H), 7.32 (t, J = 7.7 Hz, 2H), 7.25 (d, J = 7.3 Hz, 1H), 6.99 (d, J = 8.3 Hz, 1H), 6.80 (t, J = 7.2 Hz, 1H), 6.28 (s, 1H), 5.08 (s, 1H), 2.14 (s, 3H), 2.05 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 203.6 (C=O), 200.1 (C=O), 199.6 (C=O), 160.8 (Cq), 138.1 (CH), 137.5 (Cq), 129.0 (CH, 2C), 128.2 (CH), 125.5 (CH, 2C), 125.4 (CH), 119.4 (CH), 119.2 (Cq), 112.3 (CH), 71.2 (Cq), 71.1 (CH), 33.1 (CH3), 31.3 (CH3); HR-ESIMS m/z calcd for C19H18NO3 [M + H]+ 308.1281, found 308.1280.
2-(1H-Indol-3-yl)-2-phenylindolin-3-one (5a). According to procedure B, 5a was obtained as a yellow solid in 98% yield (31.8 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, acetone-d6) δ 10.28 (s, 1H), 7.61 (d, J = 7.6 Hz, 2H), 7.57 (d, J = 7.7 Hz, 1H), 7.53 (ddd, J = 8.4, 7.1, 1.4 Hz, 1H), 7.43 (d, J = 8.2 Hz, 1H), 7.36–7.23 (m, 4H), 7.21 (s, 1H), 7.16 (d, J = 8.0 Hz, 1H), 7.08 (t, J = 9.0 Hz, 2H), 6.90–6.79 (m, 2H); 13C NMR (151 MHz, acetone-d6) δ 200.9(C=O), 161.9(Cq), 141.4(Cq), 138.3(CH), 138.2(Cq), 128.9(CH, 2C), 128.2(CH), 127.7(CH, 2C), 126.8(Cq), 125.5(CH), 124.9(CH), 122.5(CH), 121.0(CH), 119.8(CH), 119.6(Cq), 119.0(CH), 116.3(Cq), 113.3(CH), 112.6(CH), 71.9(Cq); HR-ESIMS m/z calcd for C22H17N2O [M + H]+ 325.1335, found 325.1337.
5-Chloro-2-(1H-indol-3-yl)-2-phenylindolin-3-one (5b). According to procedure B, 5b was obtained as a yellow solid in 90% yield (32.3 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, CDCl3) δ 8.25 (s, 1H), 7.64 (d, J = 2.2 Hz, 1H), 7.57–7.51 (m, 2H), 7.44 (dd, J = 8.7, 2.2 Hz, 1H), 7.36 (d, J = 8.2 Hz, 1H), 7.33–7.28 (m, 3H), 7.18 (t, J = 7.6 Hz, 1H), 7.13 (d, J = 8.0 Hz, 1H), 7.09 (d, J = 2.5 Hz, 1H), 6.99 (t, J = 7.6 Hz, 1H), 6.86 (d, J = 8.7 Hz, 1H), 5.43 (s, 1H); 13C NMR (151 MHz, CDCl3) δ 199.6 (C=O), 158.9 (Cq), 139.1 (Cq), 137.6 (CH), 137.1 (Cq), 128.7 (CH, 2C), 128.1 (CH), 126.8 (CH, 2C), 125.6 (Cq), 124.9 (CH), 124.9 (Cq), 123.9 (CH), 122.8 (CH), 120.7 (Cq), 120.3 (CH), 119.7 (CH), 115.3 (Cq), 114.2 (CH), 111.9 (CH), 72.3 (Cq); HR-ESIMS m/z calcd for C22H16ClN2O [M + H]+ 359.0946, found 359.0950.
2-(1H-Indol-3-yl)-5-methyl-2-phenylindolin-3-one (5c). According to procedure B, 5c was obtained as a yellow solid in 95% yield (32.2 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, CDCl3) δ 8.16 (s, 1H), 7.56 (dt, J = 3.8, 2.1 Hz, 2H), 7.50 (s, 1H), 7.38 (d, J = 8.2 Hz, 1H), 7.35 (dd, J = 8.3, 1.7 Hz, 1H), 7.32–7.27 (m, 3H), 7.20–7.15 (m, 3H), 6.99 (dd, J = 11.2, 4.0 Hz, 1H), 6.87 (d, J = 8.3 Hz, 1H), 5.23 (s, 1H), 2.33 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 200.9 (C=O), 159.2 (Cq), 139.8(Cq), 139.1 (CH), 137.0 (Cq), 129.4 (Cq), 128.5 (CH, 2C), 127.8 (CH), 126.9 (CH, 2C), 125.8 (Cq), 125.0 (CH), 123.9 (CH), 122.6 (CH), 120.1(CH), 119.9 (Cq), 119.9 (CH), 115.8 (Cq), 113.1 (CH), 111.8 (CH), 71.8 (Cq), 20.7 (CH3); HR-ESIMS m/z calcd for C23H19N2O [M + H]+ 339.1492, found 339.1495.
2-(1H-Indol-3-yl)-5-methoxy-2-phenylindolin-3-one (5d). According to procedure B, 5d was obtained as a yellow solid in 98% yield (34.6 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, CDCl3) δ 8.27 (s, 1H), 7.56 (dd, J = 8.1, 1.7 Hz, 2H), 7.34 (d, J = 8.3 Hz, 1H), 7.32–7.24 (m, 3H), 7.21–7.09 (m, 5H), 6.97 (t, J = 7.5 Hz, 1H), 6.89 (d, J = 8.8 Hz, 1H), 5.13 (s, 1H), 3.77 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 201.2 (C=O), 156.6 (Cq), 154.0 (Cq), 139.8 (Cq), 137.0 (Cq), 128.5 (CH, 2C), 128.3 (CH), 127.8 (CH), 126.9 (CH, 2C), 125.7 (Cq), 123.8 (Cq), 122.6 (CH), 120.1 (CH), 120.0 (CH), 119.8(CH), 115.8 (Cq), 114.8 (CH), 111.8 (CH), 105.2 (CH), 72.4 (Cq), 55.9 (CH3); HR-ESIMS m/z calcd for C23H19N2O2 [M + H]+ 355.1441, found 355.1443.
2-(1H-Indol-3-yl)-6-methyl-2-phenylindolin-3-one (5e). According to procedure B, 5e was obtained as a yellow solid in 94% yield (31.8 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, acetone-d6) δ 10.24 (s, 1H), 7.61–7.56 (m, 2H), 7.43 (d, J = 8.1 Hz, 1H), 7.41 (d, J = 8.2 Hz, 1H), 7.33–7.24 (m, 3H), 7.19–7.12 (m, 3H), 7.07 (ddd, J = 8.2, 7.0, 1.1 Hz, 1H), 6.89–6.81 (m, 2H), 6.65 (dd, J = 7.9, 1.1 Hz, 1H), 2.34 (s, 3H); 13C NMR (151 MHz, acetone-d6) δ 200.1 (C=O), 162.4 (Cq), 149.6 (Cq), 141.8 (Cq), 138.3 (Cq), 128.9 (CH, 2C), 128.2 (CH), 127.8 (CH, 2C), 126.9 (Cq), 125.4 (CH), 125.0 (CH), 122.5 (CH), 121,1 (CH), 120.9 (CH), 119.8 (CH), 117.5 (Cq), 116.7 (Cq), 113.2 (CH), 112.5 (CH), 72.2 (Cq), 22.5 (CH3); HR-ESIMS m/z calcd for C23H19N2O [M + H]+ 339.1492, found 339.1494.
2-(1H-Indol-3-yl)-7-methyl-2-phenylindolin-3-one (5f). According to procedure B, 5f was obtained as a yellow solid in 91% yield (30.8 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, CDCl3) δ 8.19 (s, 1H), 7.62–7.53 (m, 3H), 7.38–7.25 (m, 5H), 7.21–7.13 (m, 3H), 6.99 (t, J = 7.5 Hz, 1H), 6.85 (t, J = 7.5 Hz, 1H), 5.12 (s, 1H), 2.27 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 201.1(C=O), 159.8 (Cq), 139.7 (Cq), 137.5 (CH), 137.0 (Cq), 128.5 (CH, 2C), 127.8 (CH), 126.9 (CH, 2C), 125.8 (Cq), 124.0 (CH), 123.0 (CH), 122.5 (CH), 122.1 (Cq), 120.1 (CH), 119.9 (CH), 119.9 (CH), 119.3 (Cq), 115.8 (Cq), 111.7 (CH), 71.4 (Cq), 15.9 (CH3); HR-ESIMS m/z calcd for C23H19N2O [M + H]+ 339.1492, found 339.1496.
2-(4-Fluorophenyl)-2-(1H-indol-3-yl)indolin-3-one (5g). According to procedure B, 5g was obtained as a yellow solid in 97% yield (33.2 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, CDCl3) δ 8.29 (s, 1H), 7.70 (dd, J = 7.7, 1.3 Hz, 1H), 7.56–7.48 (m, 3H), 7.26 (s, 1H), 7.18 (ddd, J = 8.2, 7.0, 1.1 Hz, 1H), 7.16–7.09 (m, 2H), 7.03–6.94 (m, 3H), 6.94–6.88 (m, 2H), 5.37 (s, 1H); 13C NMR (151 MHz, CDCl3 ) δ 200.7 (C=O), 163.5 (Cq), 161.8 (Cq), 160.7 (Cq), 137.8 (CH), 137.1 (Cq), 135.4 (Cq), 135.4 (Cq), 128.8 (CH, 2C), 128.7 (CH, 2C), 125.7 (CH), 125.6 (Cq), 123.8 (CH), 122.8 (CH), 120.2 (CH), 120.0 (CH), 119.7 (CH), 119.6 (Cq), 115.5 (Cq), 115.4 (CH, 2C), 115.3 (CH, 2C), 113.2 (CH), 111.9 (CH), 70.9 (Cq); HR-ESIMS m/z calcd for C22H16FN2O [M + H]+ 343.1241, found 343.1238.
2-(1H-Indol-3-yl)-2-methylindolin-3-one (5h). According to procedure B, 5h was obtained as a yellow solid in 95% yield (24.9 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, acetone-d6) δ 10.20 (s, 1H), 7.57–7.49 (m, 2H), 7.43–7.34 (m, 3H), 7.06 (ddd, J = 8.0, 6.9, 1.1 Hz, 1H), 7.00 (d, J = 8.1 Hz, 1H), 6.87 (t, J = 7.5 Hz, 1H), 6.84–6.75 (m, 2H), 1.75 (s, 3H); 13C NMR (151 MHz, acetone-d6) δ 203.6 (C=O), 161.5 (Cq), 138.2(Cq), 138.0 (CH), 126.3 (Cq), 125.3 (CH), 123.8 (CH), 122.2 (CH), 121.0 (CH), 119.9 (Cq), 119.6 (CH), 118.5 (CH), 116.4 (Cq), 113.1 (CH), 112.3 (CH), 66.4(Cq), 24.1 (CH3); HR-ESIMS m/z calcd for C17H15N2O [M + H]+ 263.1179, found 263.1176.
2-Ethyl-2-(1H-indol-3-yl)indolin-3-one (5i). According to procedure B, 5i was obtained as a yellow solid in 92% yield (25.3 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, acetone-d6) δ 10.19 (s, 1H), 7.65 (d, J = 8.1 Hz, 1H), 7.55–7.45 (m, 2H), 7.41–7.33 (m, 2H), 7.11–6.99 (m, 2H), 6.92 (ddd, J = 8.1, 7.0, 1.1 Hz, 1H), 6.85 (s, 1H), 6.76 (ddd, J = 7.9, 7.1, 0.9 Hz, 1H), 2.35–2.30 (m, 1H), 2.26–2.21 (m, 1H), 0.89 (t, J = 7.4 Hz, 3H); 13C NMR (151 MHz, acetone-d6) δ 202.8 (C=O), 161.9 (Cq), 137.9 (Cq), 137.5 (CH), 126.0 (Cq), 124.6 (CH), 123.3 (CH), 121.9 (CH), 121.1 (CH), 120.6 (Cq), 119.3 (CH), 118.0 (CH), 115.2 (Cq), 112.4 (CH), 112.0 (CH), 70.1 (Cq), 30.5 (CH2), 8.1 (CH3); HR-ESIMS m/z calcd for C18H17N2O [M + H]+ 277.1335, found 277.1333.
2-(Cyclopropylmethyl)-2-(1H-indol-3-yl)indolin-3-one (5j). According to procedure B, 5j was obtained as a yellow solid in 90% yield (27.2 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, CDCl3) δ 8.25 (s, 1H), 7.66 (dd, J = 7.7, 1.3 Hz, 1H), 7.55 (d, J = 8.1 Hz, 1H), 7.51 (ddd, J = 8.4, 7.1, 1.4 Hz, 1H), 7.32 (d, J = 8.2 Hz, 1H), 7.18–7.13 (m, 2H), 7.03 (ddd, J = 8.1, 7.0, 1.0 Hz, 1H), 6.94 (d, J = 8.2 Hz, 1H), 6.87–6.83 (m, 1H), 5.20 (s, 1H), 2.57 (dd, J = 14.0, 4.7 Hz, 1H), 1.82 (dd, J = 14.0, 8.7 Hz, 1H), 0.81–0.72 (m, 1H), 0.41–0.29 (m, 2H), 0.19 (dq, J = 9.6, 4.9 Hz, 1H), 0.13–0.07 (m, 1H); 13C NMR (151 MHz, CDCl3) δ 203.6 (C=O), 160.8 (Cq), 137.5 (CH), 137.0 (Cq), 125.3 (Cq), 125.2 (CH), 122.7 (CH), 122.4 (CH), 120.9 (Cq), 120.3 (CH), 120.0 (CH), 119.0 (CH), 115.2 (Cq), 112.3 (CH), 111.6 (CH), 70.0 (Cq), 42.3 (CH2), 6.1 (CH2), 5.3 (CH2), 4.0 (CH); HR-ESIMS m/z calcd for C20H19N2O [M + H]+ 303.1492, found 303.1493.
Ethyl 5-(2-(1H-indol-3-yl)-3-oxoindolin-2-yl)pentanoate (5k). According to procedure B, 5k was obtained as a yellow solid in 96% yield (36.1 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, CDCl3) δ 8.49 (s, 1H), 7.65–7.61 (m, 1H), 7.49 (ddd, J = 8.3, 7.1, 1.4 Hz, 1H), 7.44 (d, J = 8.1 Hz, 1H), 7.30 (d, J = 9.0 Hz, 1H), 7.14 (ddd, J = 8.2, 7.0, 1.1 Hz, 1H), 7.05–6.98 (m, 2H), 6.90–6.81 (m, 2H), 5.10 (s, 1H), 4.09 (q, J = 7.1 Hz, 2H), 2.23 (m, 4H), 1.61 (p, J = 7.6 Hz, 2H), 1.45 (m, 1H), 1.25 (m, 1H), 1.21 (t, J = 7.1 Hz, 3H); 13C NMR (151 MHz, CDCl3 ) δ 203.5 (C=O), 173.8 (C=O), 160.9 (Cq), 137.7 (CH), 137.0 (Cq), 125.1 (CH), 125.0 (Cq), 122.8 (CH), 122.3 (CH), 120.7 (Cq), 120.0 (CH), 120.0 (CH), 119.0 (CH), 114.6 (Cq), 112.4 (CH), 111.8 (CH), 69.3(Cq), 60.4 (CH2), 37.0 (CH2), 34.2 (CH2), 25.2 (CH2), 23.1 (CH2), 14.3 (CH3); HR-ESIMS m/z calcd for C23H25N2O3 [M + H]+ 377.1860, found 377.1862.
2-(4-Methyl-1H-indol-3-yl)-2-phenylindolin-3-one (5l). According to procedure B, 5l was obtained as a yellow solid in 98% yield (33.2 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, CDCl3) δ 8.44 (s, 1H), 7.72 (dd, J = 7.8, 1.3 Hz, 1H), 7.53 (ddd, J = 8.4, 7.1, 1.4 Hz, 1H), 7.41–7.35 (m, 2H), 7.31–7.23 (m, 5H), 7.15–7.10 (m, 1H), 7.00–6.91 (m, 2H), 6.86 (dt, J = 7.2, 1.0 Hz, 1H), 5.33 (s, 1H), 2.09 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 201.2 (C=O), 160.6 (Cq), 141.6 (Cq), 138.1 (Cq), 137.7 (CH), 129.7 (Cq), 128.7 (CH, 2C), 127.7 (CH), 126.6 (CH), 125.8 (CH, 2C), 125.1 (CH), 124.6 (Cq), 122.8 (CH), 122.5 (CH), 120.1 (CH), 119.8 (Cq), 114.3 (Cq), 113.5 (CH), 109.6 (CH), 72.4 (Cq), 21.9 (CH3); HR-ESIMS m/z calcd for C23H19N2O [M + H]+ 339.1492, found 339.1496.
2-(5-Chloro-1H-indol-3-yl)-2-phenylindolin-3-one (5m). According to procedure B, 5m was obtained as a yellow solid in 90% yield (32.3 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, CDCl3) δ 8.54 (s, 1H), 7.67 (d, J = 7.8 Hz, 1H), 7.57–7.47 (m, 3H), 7.32–7.27 (m, 3H), 7.22 (d, J = 8.6 Hz, 1H), 7.12 (s, 2H), 7.09 (dd, J = 8.5, 2.0 Hz, 1H), 6.94 (d, J = 8.3 Hz, 1H), 6.89 (t, J = 7.4 Hz, 1H), 5.43 (s, 1H); 13C NMR (151 MHz, CDCl3) δ 200.8 (C=O), 160.6 (Cq), 139.3 (CH), 137.9 (Cq), 135.5 (Cq), 128.7 (CH, 2C), 128.1 (CH), 126.8 (CH, 2C), 125.7 (CH), 125.3 (Cq), 123.0(CH), 119.9 (CH), 119.5 (Cq), 119.3 (CH), 115.3 (Cq), 113.0 (CH), 112.9 (Cq), 71.3 (Cq); HR-ESIMS m/z calcd for C22H16ClN2O [M + H]+ 359.0946, found 359.0949.
2-(5-Methyl-1H-indol-3-yl)-2-phenylindolin-3-one (5n). According to procedure B, 5n was obtained as a yellow solid in 95% yield (32.2 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, CDCl3) δ 8.21 (s, 1H), 7.70 (d, J = 7.8 Hz, 1H), 7.59–7.54 (m, 2H), 7.51 (ddd, J = 8.3, 7.1, 1.4 Hz, 1H), 7.34–7.28 (m, 3H), 7.25 (dd, J = 8.3, 2.9 Hz, 1H), 7.10 (d, J = 5.1 Hz, 1H), 7.01 (d, J = 8.3 Hz, 1H), 6.96 (s, 1H), 6.93 (d, J = 8.2 Hz, 1H), 6.90 (t, J = 7.4 Hz, 1H), 5.45 (s, 1H), 2.32 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 200.8 (C=O), 160.8 (Cq), 139.6 (Cq), 137.6 (CH), 135.4 (Cq), 129.4 (CH), 128.5 (CH, 2C), 127.8 (CH), 126.9 (CH, 2C), 125.9 (Cq), 125.7 (CH), 124.2 (CH), 124.0 (Cq), 119.7 (CH), 119.6 (Cq), 119.3 (CH), 114.7 (Cq), 113.0 (CH), 111.5 (CH), 71.5 (Cq), 21.6 (CH3),; HR-ESIMS m/z calcd for C23H19N2O [M + H]+ 339.1492, found 339.1494.
2-(5-Methoxy-1H-indol-3-yl)-2-phenylindolin-3-one (5o). According to procedure B, 5o was obtained as a yellow solid in 98% yield (34.8mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, CDCl3) 1H NMR (600 MHz, CDCl3) δ 8.14 (s, 1H), 7.70 (dd, J = 7.8, 1.3 Hz, 1H), 7.65–7.57 (m, 2H), 7.52 (ddd, J = 8.3, 7.1, 1.3 Hz, 1H), 7.36–7.23 (m, 4H), 7.08 (d, J = 5.8 Hz, 1H), 6.94 (d, J = 8.2 Hz, 1H), 6.90 (t, J = 7.4 Hz, 1H), 6.83 (dd, J = 8.6, 2.4 Hz, 1H), 6.57 (d, J = 2.4 Hz, 1H), 5.40 (s, 1H), 3.61 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 200.8 (C=O), 160.5 (Cq), 153.9 (CH), 139.3 (CH), 137.5 (Cq), 132.0 (Cq), 128.3 (CH, 2C), 127.7 (CH), 126.8 (CH, 2C), 126.0 (CH), 125.4 (CH), 124.6 (Cq), 119.6 (Cq), 119.6 (CH), 115.4 (Cq), 112.8 (CH), 112.3 (Cq), 112.2 (CH), 101.8 (CH), 71.2 (Cq), 55.5 (CH3); HR-ESIMS m/z calcd for C23H19N2O2 [M + H]+ 355.1441, found 355.1444.
2-(6-Methyl-1H-indol-3-yl)-2-phenylindolin-3-one (5p). According to procedure B, 5p was obtained as a yellow solid in 94% yield (31.9 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, CDCl3) δ 8.11 (s, 1H), 7.70 (d, J = 7.8 Hz, 1H), 7.59–7.54 (m, 2H), 7.51 (ddd, J = 8.3, 7.1, 1.4 Hz, 1H), 7.33–7.27 (m, 3H), 7.16 (s, 1H), 7.08–7.02 (m, 2H), 6.94–6.87 (m, 2H), 6.83 (dd, J = 8.3, 1.3 Hz, 1H), 5.38 (s, 1H), 2.42 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 200.8 (C=O), 160.7 (Cq), 139.7 (Cq), 137.6 (CH), 137.5 (Cq), 132.5 (Cq), 128.5 (CH, 2C), 127.8 (CH), 126.9 (CH, 2C), 125.7 (CH), 123.5 (Cq), 123.2 (Cq), 121.9 (CH), 119.7 (CH), 119.7 (CH), 119.4 (CH), 115.4 (Cq), 113.0 (CH), 111.7 (CH), 71.5 (Cq), 21.7 (CH3); HRMS m/z calcd for C23H19N2O [M + H]+ 339.1492, found 339.1494.
2-(7-Methyl-1H-indol-3-yl)-2-phenylindolin-3-one (5q). According to procedure B, 5q was obtained as a yellow solid in 91% yield (30.8 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, CDCl3) δ 8.28 (s, 1H), 7.71 (d, J = 7.7 Hz, 1H), 7.61–7.55 (m, 2H), 7.51 (ddd, J = 8.4, 7.1, 1.4 Hz, 1H), 7.32–7.27 (m, 3H), 7.13 (s, 1H), 7.01 (dd, J = 19.3, 7.5 Hz, 2H), 6.95–6.87 (m, 3H), 5.45 (s, 1H), 2.46 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 200.8 (C=O), 160.7 (Cq), 139.6 (CH), 137.6 (Cq), 136.6 (Cq), 128.5 (CH, 2C), 127.8 (CH), 126.9 (CH, 2C), 125.7 (CH), 125.3 (CH), 123.6 (Cq), 123.1 (CH), 121.1 (Cq), 120.3 (CH), 119.7 (CH), 119.6 (Cq), 117.4 (CH), 115.9 (Cq), 113.0 (CH), 71.5 (Cq), 16.7 (CH3); HR-ESIMS m/z calcd for C23H19N2O [M + H]+ 339.1492, found 339.1495.
2-(3-Methyl-1H-indol-2-yl)-2-phenylindolin-3-one (5r). According to procedure C, 5r was obtained as a yellow solid in 90% yield (30.4 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, CDCl3) δ 8.84 (s, 1H), 7.69 (d, J = 7.7 Hz, 1H), 7.59–7.53 (m, 2H), 7.36–7.29 (m, 6H), 7.20 (ddd, J = 8.2, 7.0, 1.2 Hz, 1H), 7.13 (t, J = 7.4 Hz, 1H), 7.01 (d, J = 8.3 Hz, 1H), 6.93 (t, J = 7.4 Hz, 1H), 5.43 (s, 1H), 2.22 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 201.1 (C=O), 161.0 (Cq), 139.6 (Cq), 138.3 (CH), 134.6 (Cq), 131.0 (Cq), 129.6 (Cq), 129.0 (CH, 2C), 128.5 (CH), 126.5 (CH, 2C), 125.8 (CH), 122.4 (CH), 120.3 (CH), 119.6 (Cq), 119.5 (CH), 118.6 (CH), 112.9 (CH), 111.2 (CH), 109.7 (Cq), 71.6 (Cq), 9.6 (CH3); HR-ESIMS m/z calcd for C23H19N2O [M + H]+ 339.1492, found 339.1490.
N-(2-(5-Methoxy-2-(3-oxo-2-phenylindolin-2-yl)-1H-indol-3-yl)ethyl)acetamide (5s). According to procedure C, 5s was obtained as a yellow solid in 92% yield (40.3 mg; flash chromatographic condition: petroleum ether-acetone 60:40). 1H NMR (600 MHz, CDCl3) δ 9.35 (s, 1H), 8.07 (s, 1H), 7.53 (d, J = 7.8 Hz, 1H), 7.43 (ddd, J = 8.3, 7.0, 1.3 Hz, 1H), 7.26–7.14 (m, 6H), 7.07 (d, J = 8.3 Hz, 1H), 6.89 (d, J = 2.4 Hz, 1H), 6.80 (dd, J = 8.8, 2.4 Hz, 1H), 6.72 (ddd, J = 7.8, 7.0, 0.8 Hz, 1H), 6.13 (s, 1H), 3.78 (s, 3H), 3.47–3.31 (m, 1H), 3.19–3.13 (m, 1H), 2.84–2.79 (m, 1H), 2.75–2.70 (m, 1H), 1.90 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 201.4 (C=O), 171.7 (C=O), 162.3 (Cq), 154.2 (Cq), 140.0 (Cq), 138.6 (CH), 133.1 (Cq), 129.7 (Cq), 129.0 (Cq), 128.9(CH, 2C), 128.2 (CH), 126.0(CH, 2C), 125.6 (CH), 118.8 (CH), 117.3 (Cq), 112.4 (CH), 112.3 (CH), 112.2 (CH), 110.0 (Cq), 100.2 (CH), 71.1 (Cq), 56.1 (CH3), 41.2 (CH2), 24.6 (CH2), 23.3 (CH3); HR-ESIMS m/z calcd for C27H26N3O3 [M + H]+ 440.1969, found 440.1965.
Methyl (2-(2-(3-oxo-2-phenylindolin-2-yl)-1H-indol-3-yl)ethyl)carbamate (5t). According to procedure C, 5t was obtained as a yellow solid in 90% yield (38.2 mg; flash chromatographic condition: petroleum ether-acetone 60:40). 1H NMR (600 MHz, CDCl3) δ 9.46 (s, 1H), 7.74 (s, 1H), 7.61 (d, J = 7.8 Hz, 1H), 7.56–7.46 (m, 2H), 7.37 (d, J = 8.1 Hz, 1H), 7.30–7.16 (m, 6H), 7.15–7.08 (m, 2H), 6.80 (t, J = 7.4 Hz, 1H), 5.13 (s, 1H), 3.71 (s, 3H), 3.40–3.34 (m, 1H), 3.24–3.19 (m, 1H), 3.03–2.98 (m, 1H), 2.85–2.81 (m, 1H); 13C NMR (151 MHz, CDCl3) δ 201.5 (C=O), 162.2 (Cq), 158.2 (C=O), 140.1 (Cq), 138.6 (CH), 134.6 (Cq), 132.1 (Cq), 128.9 (CH, 2C), 128.6 (Cq), 128.3 (CH), 126.2 (CH, 2C), 125.7 (CH), 122.4 (CH), 119.7 (CH), 118.9 (CH), 118.1 (CH), 117.6 (Cq), 112.4 (CH), 111.6 (CH), 110.5 (Cq), 71.2 (Cq), 52.5 (CH3), 42.2 (CH2), 25.3 (CH2); HR-ESIMS m/z calcd for C26H24N3O3 [M + H]+ 426.1812, found 426.1815.
2-Phenyl-2-(2-phenyl-1H-indol-3-yl)indolin-3-one (6a). According to procedure D, 6a was obtained as a yellow solid in 98% yield (19.2 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, acetone-d6) δ 10.38 (s, 1H), 7.58–7.53 (m, 2H), 7.51 (ddd, J = 8.3, 7.1, 1.3 Hz, 1H), 7.40 (d, J = 8.1 Hz, 1H), 7.28 (d, J = 7.6 Hz, 1H), 7.22–7.14 (m, 4H), 7.14–7.04 (m, 6H), 7.02 (d, J = 8.2 Hz, 1H), 6.81–6.74 (m, 3H); 13C NMR (151 MHz, acetone-d6) δ 200.9 (C=O), 160.9 (Cq), 141.7 (Cq), 138.8 (Cq), 138.0 (CH), 137.2 (Cq), 134.6 (Cq), 130.6 (CH, 2C), 128.8 (Cq), 128.6 (CH, 2C), 128.4 (CH), 128.5 (CH, 2C), 128.1 (CH, 2C), 127.9 (CH), 125.4 (CH), 122.3 (CH), 121.9 (CH), 120.8 (Cq), 119.8 (CH), 119.0 (Cq), 118.9 (CH), 113.1 (CH), 111.9 (CH), 72.5 (Cq); HR-ESIMS m/z calcd for C28H21N2O [M + H]+ 401.1648, found 401.1652.
2-Methyl-2-(2-methyl-1H-indol-3-yl)indolin-3-one (6b). According to procedure D, 6b was obtained as a yellow solid in 90% yield (12.5 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, CDCl3) δ 7.86 (s, 1H), 7.72–7.69 (m, 1H), 7.51 (d, J = 0.9 Hz, 1H), 7.40 (d, J = 8.1 Hz, 1H), 7.24–7.22 (m, 1H), 7.08–7.04 (m, 1H), 6.96 (s, 1H), 6.93–6.85 (m, 3H), 2.42 (s, 3H), 1.92 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 204.4 (C=O), 159.7 (Cq), 137.6 (CH), 135.0 (Cq), 132.7 (Cq), 127.6 (Cq), 125.5 (CH), 121.4 (CH), 119.9 (CH), 119.7 (CH), 119.2 (CH), 112.6 (CH), 110.6 (CH), 110.3 (Cq), 109.7 (Cq), 67.3 (Cq), 25.2 (CH3), 14.8 (CH3); HR-ESIMS m/z calcd for C18H17N2O [M + H]+ 277.1335, found 277.1336.
4-Fluoro-2-(4-fluoro-2-phenyl-1H-indol-3-yl)-2-phenylindolin-3-one (6c). According to procedure D, 6c was obtained as a yellow solid in 88% yield (19.2 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, DMSO-d6) δ 11.64 (s, 1H), 8.62 (s, 1H), 7.48 (d, J = 5.6 Hz, 1H), 7.28–7.23 (m, 2H), 7.20 (d, J = 8.1 Hz, 1H), 7.18–7.04 (m, 6H), 6.96 (d, J = 2.5 Hz, 3H), 6.83 (d, J = 8.3 Hz, 1H), 6.65–6.60 (m, 1H), 6.37 (dd, J = 9.5, 8.0 Hz, 1H); 13C NMR (151 MHz, DMSO-d6) δ 196.4, 160.9, 160.9, 160.0, 158.3, 155.9, 154.3, 139.4, 139.3, 138.7, 138.6, 138.5, 138.4, 132.7, 129.7, 127.6, 127.4, 127.1, 126.9, 122.2, 122.1, 115.8, 108.2, 107.7, 107.7, 107.6, 107.6, 104.6, 104.5, 102.9, 102.8, 79.2, 71.4; HR-ESIMS m/z calcd for C28H19F2N2O [M + H]+ 437.1460, found 437.1461.
5-Chloro-2-(5-chloro-2-phenyl-1H-indol-3-yl)-2-phenylindolin-3-one (6d). According to procedure D, 6d was obtained as a yellow solid in 91% yield (21.4 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, CDCl3) δ 8.17 (s, 1H), 7.44–7.40 (m, 2H), 7.37 (dd, J = 8.6, 2.2 Hz, 1H), 7.32 (d, J = 2.2 Hz, 1H), 7.32–7.28 (m, 1H), 7.25–7.21 (m, 4H), 7.20–7.16 (m, 2H), 7.15–7.12 (m, 2H), 7.10 (dd, J = 8.6, 2.0 Hz, 1H), 6.90 (d, J = 2.0 Hz, 1H), 6.62 (d, J = 8.6 Hz, 1H), 5.12 (s, 1H); 13C NMR (151 MHz, CDCl3) δ 199.1 (C=O), 157.4 (Cq), 139.7 (Cq), 138.5 (Cq), 137.3 (CH), 133.9 (Cq), 132.9 (Cq), 129.8 (CH, 2C), 128.8 (CH), 128.7 (CH, 2C), 128.4 (Cq), 128.2 (CH), 128.0 (CH, 2C), 127.2 (CH, 2C), 125.8 (Cq), 124.7 (CH), 124.5 (Cq), 123.0 (CH), 121.3 (Cq), 121.1 (CH), 113.5 (CH), 111.9 (CH), 111.6 (Cq), 72.9 (Cq); HR-ESIMS m/z calcd for C28H19Cl2N2O [M + H]+ 469.0869, found 469.0869.
5-Methyl-2-(5-methyl-2-phenyl-1H-indol-3-yl)-2-phenylindolin-3-one (6e). According to procedure D, 6e was obtained as a yellow solid in 96% yield (20.6 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, CDCl3) δ 7.99 (s, 1H), 7.54–7.42 (m, 2H), 7.28 (dd, J = 8.3, 1.9 Hz, 1H), 7.25–7.22 (m, 1H), 7.22–7.21 (m, 1H), 7.19 (d, J = 8.2 Hz, 1H), 7.18–7.14 (m, 3H), 7.15–7.11 (m, 4H), 6.97 (dd, J = 8.3, 1.6 Hz, 1H), 6.82–6.80 (m, 1H), 6.65 (d, J = 8.2 Hz, 1H), 5.02 (s, 1H), 2.29 (s, 3H), 2.25(s, 3H); 13C NMR (151 MHz, CDCl3) δ 200.7 (C=O), 158.0 (Cq), 140.8 (Cq), 138.6 (CH), 137.3 (Cq), 134.0 (Cq), 133.6 (Cq), 129.9 (CH, 2C), 129.2 (Cq), 128.9 (Cq), 128.3 (CH, 2C), 128.2 (CH), 127.8 (Cq), 127.7 (CH, 2C), 127.5 (CH), 127.4 (CH, 2C), 124.8 (CH), 124.1 (CH), 121.3 (CH), 120.9 (Cq), 112.5 (CH), 111.9 (Cq), 110.5 (CH), 72.7 (Cq), 21.8(CH3), 20.7(CH3); HR-ESIMS m/z calcd for C30H25N2O [M + H]+ 429.1961, found 429.1963.
5-Methoxy-2-(5-methoxy-2-phenyl-1H-indol-3-yl)-2-phenylindolin-3-one (6f). According to procedure D, 6f was obtained as a yellow solid in 98% yield (22.6 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, CDCl3) δ 8.05 (s, 1H), 7.61–7.49 (m, 2H), 7.25–7.19 (m, 2H), 7.20–7.16 (m, 3H), 7.15–7.12 (m, 2H), 7.12–7.10 (m, 3H), 6.82 (d, J = 2.7 Hz, 1H), 6.78 (dd, J = 8.8, 2.4 Hz, 1H), 6.72 (dd, J = 8.8, 0.5 Hz, 1H), 6.36 (d, J = 2.4 Hz, 1H), 4.96 (s, 1H), 3.72 (s, 3H), 3.51 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 200.9 (C=O), 155.2 (Cq), 153.9 (Cq), 153.8 (Cq), 140.6 (Cq), 137.7 (Cq), 133.4 (Cq), 130.7 (Cq), 129.8 (CH. 2C), 128.3 (CH, 2C), 128.3 (CH), 128.0 (Cq), 127.7 (CH), 127.7(CH, 2C), 127.6 (CH), 127.5(CH, 2C), 121.2 (Cq), 114.2 (CH), 112.7 (CH), 112.3 (Cq), 111.5 (CH), 105.3 (CH), 103.3 (CH), 73.1 (Cq), 55.9(CH3), 55.5(CH3); HR-ESIMS m/z calcd for C30H25N2O3 [M + H]+ 461.1860, found 461.1860.
6-Methyl-2-(6-methyl-2-phenyl-1H-indol-3-yl)-2-phenylindolin-3-one (6g). According to procedure D, 6g was obtained as a yellow solid in 95% yield (20.3 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, acetone-d6) δ 10.19 (s, 1H), 7.51 (d, J = 7.7 Hz, 2H), 7.20–7.16 (m, 4H), 7.14 (s, 1H), 7.09–7.04 (m, 6H), 6.82 (s, 1H), 6.67 (d, J = 8.3 Hz, 1H), 6.61 (d, J = 8.2 Hz, 2H), 2.37 (s, 3H), 2.35 (s, 3H); 13C NMR (151 MHz, acetone-d6) δ 200.1 (C=O), 161.3 (Cq), 149.2 (Cq), 142.0 (Cq), 138.1 (Cq), 137.6 (Cq), 134.8 (Cq), 131.7 (CH), 130.6 (CH, 2C), 128.4 (CH, 2C), 128.2 (CH, 2C), 128.1 (CH), 128.0 (CH, 2C), 127.7 (CH), 126.8 (Cq), 125.1 (CH), 121.7 (CH), 121.5 (CH), 120.6 (Cq), 120.6 (Cq), 118.7 (Cq), 112.9 (CH), 111.7 (CH), 72.7 (Cq), 22.5(CH3), 21.6(CH3); HR-ESIMS m/z calcd for C30H25N2O [M + H]+ 429.1961, found 429.1962.
7-Methyl-2-(7-methyl-2-phenyl-1H-indol-3-yl)-2-phenylindolin-3-one (6h). According to procedure D, 6h was obtained as a yellow solid in 92% yield (19.8 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, CDCl3) δ 7.97 (s, 1H), 7.57–7.43 (m, 2H), 7.33–7.28 (m, 2H), 7.24 (dt, J = 7.1, 1.1 Hz, 1H), 7.23–7.19 (m, 3H), 7.19–7.15 (m, 3H), 6.99–6.91 (m, 2H), 6.87 (dd, J = 8.2, 7.1 Hz, 1H), 6.74 (t, J = 7.4 Hz, 1H), 4.89 (s, 1H), 2.45 (s, 3H), 1.94 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 200.9 (C=O), 158.5 (Cq), 141.0 (Cq), 137.1 (CH), 136.7 (Cq), 135.3 (Cq), 134.0 (Cq), 133.6 (Cq), 129.8 (CH, 2C), 129.2 (Cq), 128.4 (CH, 2C), 128.0 (CH, 2C), 127.7 (CH), 127.5 (CH, 2C), 127.0 (Cq), 123.1 (CH), 122.8 (CH), 121.4 (CH), 120.4 (CH), 119.9 (CH), 119.9 (Cq), 119.4 (CH), 112.6 (Cq), 72.4 (Cq), 16.7 (CH3), 15.6 (CH3); HR-ESIMS m/z calcd for C30H25N2O [M + H]+ 429.1961, found 429.1965.
[3,2′:2′,3″-Terindolin]-3′-one (7a). According to procedure D, 7a was obtained as a yellow solid in 75% yield (18.1 mg; flash chromatographic condition: petroleum ether-acetone 80:20). 1H NMR (600 MHz, acetone-d6) δ 10.16 (s, 2H), 7.56 (d, J = 7.7 Hz, 1H), 7.53–7.49 (m, 1H), 7.46 (d, J = 8.1 Hz, 2H), 7.38 (d, J = 8.2 Hz, 2H), 7.26–7.22 (m, 2H), 7.15 (s, 1H), 7.07–7.01 (m, 3H), 6.84 (ddd, J = 8.1, 7.0, 1.1 Hz, 2H), 6.82–6.77 (m, 1H); 13C NMR (151 MHz, acetone-d6) δ 201.4 (C=O), 161.6 (Cq), 138.4 (Cq, 2C), 138.0 (CH), 127.1 (CH), 125.4 (Cq, 2C), 125.0 (CH, 2C), 122.2 (CH, 2C), 121.8 (CH, 2C), 120.1 (Cq), 119.5 (CH, 2C), 118.6 (CH), 116.0 (CH), 113.1 (Cq, 2C), 112.3 (CH, 2C), 69.0 (Cq); HR-ESIMS m/z calcd for C24H18N3O [M + H]+ 364.1444, found 364.1445.
4,4′,4″-Trifluoro-[3,2′:2′,3″-terindolin]-3′-one (7b). According to procedure D, 7b was obtained as a yellow solid in 74% yield (20.5 mg; flash chromatographic condition: petroleum ether-acetone 80:20). 1H NMR (600 MHz, DMSO-d6) δ 11.29 (s, 2H), 7.69 (s, 1H), 7.43–7.37 (m, 1H), 7.24 (d, J = 8.1 Hz, 2H), 7.10–7.05 (m, 2H), 6.90 (s, 2H), 6.76 (d, J = 8.2 Hz, 1H), 6.71–6.66 (m, 2H), 6.40–6.36 (m, 1H); 13C NMR (151 MHz, DMSO-d6) δ 196.8, 161.6, 161.5, 160.0, 158.3, 156.2, 154.5, 140.0, 140.0, 138.4, 138.4, 125.6, 122.1, 122.0, 114.2, 114.1, 112.1, 112.1, 108.7, 108.7, 108.3, 108.3, 107.0, 106.9, 104.3, 104.2, 102.3, 102.2, 67.2; HR-ESIMS m/z calcd for C24H15F3N3O [M + H]+ 418.1162, found 418.1162.
5,5′,5″-Trifluoro-[3,2′:2′,3″-terindolin]-3′-one (7c). According to procedure D, 7c was obtained as a yellow solid in 73% yield (20.3 mg; flash chromatographic condition: petroleum ether-acetone 80:20).1H NMR (600 MHz, CDCl3) δ 8.08 (s, 2H), 7.38 (dd, J = 7.2, 2.7 Hz, 1H), 7.34–7.26 (m, 2H), 7.25 (s, 1H), 7.18 (s, 2H), 7.06–7.02 (m, 2H), 6.93–6.87 (m, 3H), 5.25 (s, 1H); 13C NMR (151 MHz, CDCl3) δ 200.7, 200.7, 158.5, 157.8, 156.9, 156.8, 156.2, 133.5, 126.0, 125.9, 125.8, 125.5, 120.5, 120.4, 114.9, 114.8, 114.4, 114.4, 112.3, 112.3, 111.1, 110.9, 110.3, 110.2, 105.4, 105.3, 69.0; HR-ESIMS m/z calcd for C24H15F3N3O [M + H]+ 418.1162, found 418.1163.
5,5′,5″-Trimethyl-[3,2′:2′,3″-terindolin]-3′-one (7d). According to procedure D, 7d was obtained as a yellow solid in 72% yield (19.4 mg; flash chromatographic condition: petroleum ether-acetone 80:20). 1H NMR (600 MHz, acetone-d6) δ 10.00 (s, 2H), 7.38–7.34 (m, 2H), 7.28–7.24 (m, 4H), 7.16 (d, J = 2.6 Hz, 2H), 6.96 (dd, J = 8.9, 1.7 Hz, 1H), 6.90–6.84 (m, 3H), 2.29 (s, 3H), 2.22 (s, 6H); 13C NMR (151 MHz, acetone-d6) δ 201.6 (C=O), 160.3 (Cq), 139.3 (CH), 136.9 (Cq, 2C), 128.1 (Cq, 2C), 127.5 (Cq, 2C), 125.1 (CH, 2C), 125.0 (Cq), 124.8 (CH), 123.9 (CH, 2C), 121.6 (CH, 2C), 120.6 (Cq), 115.8 (Cq, 2C), 113.3 (CH), 112.1 (CH, 2C), 69.6 (Cq), 21.9 (CH3, 2C), 20.7 (CH3); HR-ESIMS m/z calcd for C27H24N3O [M + H]+ 406.1914, found 406.1915.
5,5′,5″-Trimethoxy-[3,2′:2′,3″-terindolin]-3′-one (7e). According to procedure D, 7e was obtained as a yellow solid in 66% yield (19.9 mg; flash chromatographic condition: petroleum ether-acetone 80:20). 1H NMR (600 MHz, DMSO-d6) δ 10.79 (s, 2H), 7.79 (s, 1H), 7.27–7.22 (m, 3H), 7.05 (d, J = 2.5 Hz, 2H), 6.99 (s, 1H), 6.95 (d, J = 8.8 Hz, 1H), 6.82 (d, J = 2.5 Hz, 2H), 6.72–6.70 (m, 2H), 3.73 (s, 3H), 3.54 (s, 6H). 13C NMR (151 MHz, DMSO-d6) δ 201.2 (C=O), 156.9 (Cq), 152.6 (Cq, 2C), 151.8 (Cq), 132.1 (Cq, 2C), 127.8 (Cq), 126.1 (Cq, 2C), 124.7 (CH, 2C), 118.0 (CH), 113.7 (Cq, 2C), 113.5 (CH), 112.1 (CH, 2C), 110.6 (CH, 2C), 104.6 (CH), 103.1 (CH, 2C), 68.5 (Cq), 55.6 (CH3), 55.1 (CH3, 2C); HR-ESIMS m/z calcd for C27H24N3O4 [M + H]+ 454.1761, found 454.1761.
6,6′,6″-Trimethyl-[3,2′:2′,3″-terindolin]-3′-one (7f). According to procedure D, 7f was obtained as a yellow solid in 64% yield (17.3 mg; flash chromatographic condition: petroleum ether-acetone 80:20). 1H NMR (600 MHz, acetone-d6) δ 9.97 (s, 2H), 7.43 (d, J = 7.9 Hz, 1H), 7.32 (d, J = 8.1 Hz, 2H), 7.16 (s, 2H), 7.15–7.12 (m, 2H), 6.95 (s, 1H), 6.68 (dd, J = 8.2, 1.5 Hz, 2H), 6.63 (dt, J = 7.9, 1.5 Hz, 1H), 2.35 (s, 3H), 2.34 (s, 6H); 13C NMR (151 MHz, acetone-d6) δ 199.7 (C=O), 161.1 (Cq), 148.1 (Cq), 137.9 (Cq, 2C), 130.6 (Cq, 2C), 124.3 (CH, 2C), 124.2 (CH), 123.3 (Cq, 2C), 120.7 (CH, 2C), 120.3 (CH, 2C), 119.4 (CH), 117.2 (Cq), 115.3 (CH, 2C), 112.1 (CH), 111.2 (Cq, 2C), 68.4 (Cq), 21.6 (CH3), 20.8 (CH3, 2C); HR-ESIMS m/z calcd for C27H24N3O [M + H]+406.1914, found 406.1916.
7,7′,7″-Trimethyl-[3,2′:2′,3″-terindolin]-3′-one (7g). According to procedure D, 7g was obtained as a yellow solid in 65% yield (17.6 mg; flash chromatographic condition: petroleum ether-acetone 80:20). 1H NMR (600 MHz, acetone-d6) δ 8.01 (s, 2H), 7.63–7.58 (m, 1H), 7.35 (dt, J = 7.1, 1.2 Hz, 1H), 7.27 (s, 1H), 7.08 (d, J = 2.3 Hz, 2H), 6.97 (dt, J = 7.1, 1.1 Hz, 2H), 6.91 (dd, J = 8.0, 7.1 Hz, 2H), 6.85 (t, J = 7.5 Hz, 1H), 5.28 (s, 1H), 2.45 (s, 6H), 2.22 (s, 3H); 13C NMR (151 MHz, acetone-d6) δ 201.7 (C=O), 159.7 (Cq), 137.6 (CH), 136.7 (CH), 125.4 (Cq, 2C), 124.1 (Cq, 2C), 122.9 (CH), 122.8 (CH, 2C), 122.1 (Cq), 120.8 (Cq, 2C), 120.2 (CH, 2C), 119.8 (Cq), 119.6 (CH, 2C), 118.2 (CH, 2C), 115.8 (Cq, 2C), 68.6 (Cq), 16.7 (CH3, 2C), 15.9 (CH3); HR-ESIMS m/z calcd for C27H24N3O [M + H]+ 406.1914, found 406.1911.
2-Phenyl-2-(1H-pyrrol-2-yl)indolin-3-one (8). According to procedure B, 8 was obtained as a yellow solid in 94% yield (25.9 mg; flash chromatographic condition: petroleum ether-acetone 80:20). 1H NMR (600 MHz, CDCl3) δ 8.84 (s, 1H), 7.65 (d, J = 7.8 Hz, 1H), 7.51 (ddd, J = 8.4, 7.1, 1.4 Hz, 1H), 7.32–7.21 (m, 5H), 6.94 (d, J = 8.3 Hz, 1H), 6.91–6.86 (m, 1H), 6.79 (td, J = 2.7, 1.5 Hz, 1H), 6.27 (ddd, J = 3.9, 2.6, 1.5 Hz, 1H), 6.21 (dt, J = 3.5, 2.7 Hz, 1H), 5.38 (s, 1H); 13C NMR (151 MHz, CDCl3) δ 201.2 (C=O), 161.0 (Cq), 140.8 (Cq), 138.0 (CH), 129.1 (Cq), 128.9 (CH, 2C), 128.4 (CH), 126.8 (CH, 2C), 125.7 (CH), 119.9 (CH), 119.6 (Cq), 118.7 (CH), 112.8 (CH), 108.5 (CH), 107.2 (CH), 71.0 (Cq); HR-ESIMS m/z calcd for C18H15N2O [M + H]+ 275.1179, found 275.1177.
2-(1-Methyl-1H-pyrrol-3-yl)-2-phenylindolin-3-one(9). According to procedure B, 9 was obtained as a yellow solid in 90% yield (25.9 mg; flash chromatographic condition: petroleum ether-acetone 85:15). 1H NMR (600 MHz, CDCl3) δ 7.62 (d, J = 7.6 Hz, 1H), 7.52 (dd, J = 5.3, 3.4 Hz, 2H), 7.45 (ddd, J = 8.3, 7.1, 1.3 Hz, 1H), 7.31–7.26 (m, 2H), 7.24 (ddd, J = 7.2, 4.3, 1.3 Hz, 1H), 6.90 (d, J = 8.2 Hz, 1H), 6.84–6.80 (m, 1H), 6.56 (dt, J = 5.0, 2.2 Hz, 2H), 5.98 (dd, J = 2.6, 1.9 Hz, 1H), 5.15 (s, 1H), 3.56 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 201.3 (C=O), 160.4 (Cq), 140.9 (Cq), 137.4 (CH), 128.3 (CH, 2C), 127.6 (CH), 126.9 (CH, 2C), 125.6 (CH), 123.9 (Cq), 122.6 (CH), 120.8 (CH), 119.7 (Cq), 119.4 (CH), 112.7 (CH), 107.3 (CH), 71.3 (CH), 36.3 (CH3); HR-ESIMS m/z calcd for C19H17N2O [M + H]+ 289.1335, found 289.1333.
2-Phenyl-2-(thiophen-2-yl)indolin-3-one (10). According to procedure B, 10 was obtained as a yellow solid in 75% yield (21.8 mg; flash chromatographic condition: petroleum ether-acetone 80:20). 1H NMR (600 MHz, CDCl3) δ 7.67 (d, J = 7.7 Hz, 1H), 7.56–7.45 (m, 3H), 7.39–7.29 (m, 3H), 7.25 (d, J = 5.2 Hz, 1H), 7.12 (dd, J = 3.7, 1.2 Hz, 1H), 7.00 (dd, J = 5.1, 3.6 Hz, 1H), 6.96 (d, J = 8.2 Hz, 1H), 6.91 (t, J = 7.4 Hz, 1H), 5.35 (s, 1H); 13C NMR (151 MHz, CDCl3) δ 199.4 (C=O), 160.0 (Cq), 144.6 (Cq), 140.5 (Cq), 137.9 (CH), 128.7 (CH, 2C), 128.4 (CH), 127.3 (CH), 126.9 (CH, 2C), 126.4 (CH), 125.9 (CH), 125.4 (CH), 120.2 (CH), 119.4 (Cq), 112.8 (CH), 72.4 (Cq); HR-ESIMS m/z calcd for C18H14NOS [M + H]+ 292.0791, found 292.0791.
2-(3-Oxo-2-phenylindolin-2-yl)acetaldehyde (11). According to procedure B, 11 was obtained as a yellow solid in 72% yield (18.1 mg; flash chromatographic condition: petroleum ether-acetone 90:10). 1H NMR (600 MHz, CDCl3) δ 9.70 (d, J = 1.7 Hz, 1H), 7.59 (d, J = 7.7 Hz, 1H), 7.53–7.49 (m, 3H), 7.36–7.32 (m, 2H), 7.30–7.28 (m, 1H), 6.97 (d, J = 8.3 Hz, 1H), 6.86 (t, J = 7.4 Hz, 1H), 5.70 (s, 1H), 3.64 (dd, J = 17.6, 1.9 Hz, 1H), 2.98 (d, J = 17.5 Hz, 1H); 13C NMR (151 MHz, CDCl3) δ 199.9 (C=O), 199.8 (C=O), 160.2 (Cq) 138.0 (CH), 137.7 (Cq), 129.1 (CH, 2C), 128.1 (CH), 125.8 (CH), 125.4 (CH, 2C), 119.6 (CH), 118.5 (Cq), 112.1 (CH), 68.7 (Cq), 50.4 (CH2); HR-ESIMS m/z calcd for C16H14NO2 [M + H]+ 252.1019, found 252.1021.
2-(2-Oxopropyl)-2-phenylindolin-3-one (12). According to procedure B using 5 equiv of MsOH as additive, 12 was obtained as a yellow solid in 70% yield (18.5 mg; flash chromatographic condition: petroleum ether-acetone 90:10). 1H NMR (600 MHz, CDCl3) δ 7.57–7.52 (m, 3H), 7.48 (ddd, J = 8.3, 7.0, 1.3 Hz, 1H), 7.34–7.30 (m, 2H), 7.28–7.22 (m, 1H), 6.95 (d, J = 8.3 Hz, 1H), 6.80 (t, J = 7.4 Hz, 1H), 6.13 (s, 1H), 3.73 (d, J = 17.4 Hz, 1H), 2.73 (d, J = 17.4 Hz, 1H), 2.10 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 206.9 (C=O), 200.4 (C=O), 160.2 (Cq), 137.9 (CH), 137.8 (Cq), 128.8 (CH, 2C), 127.8 (CH), 125.6 (CH), 125.5 (CH, 2C), 119.1 (CH), 118.3 (Cq), 112.0 (CH), 69.1 (Cq), 49.6 (CH2), 31.6 (CH3); HR-ESIMS m/z calcd for C17H16NO2 [M + H]+ 265.1103, found 2665.1101.