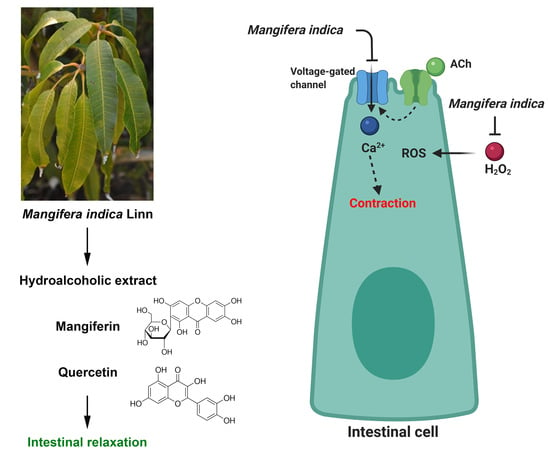
Metabolomic Profiling of Mango (Mangifera indica Linn) Leaf Extract and Its Intestinal Protective Effect and Antioxidant Activity in Different Biological Models
Abstract
:1. Introduction
2. Results
2.1. UHPLC-MS Metabolomic Analysis of MIE
2.1.1. Benzophenone Derivatives
2.1.2. Xanthones Derivatives
2.1.3. Phenolic Acids
2.1.4. Fatty Acids
2.1.5. Flavonoids
2.1.6. Procyanidins
2.2. Total Phenolic Content and Antioxidant Activity of MIE
2.3. Spasmolytic Activity of MIE on Rat Ileum
2.4. Antispasmodic Activity of MIE Reduced the Contractile Response to Acetylcholine in Rat Ileum: Role of Extracellular Calcium
2.5. MIE and Bioactive Molecules Attenuates the Acute Oxidative Stress Damage in Rat Ileum
2.6. Chronic Treatment with MIE Reduced Ex-Vivo the Contractile Response to Acetylcholine and Acute Oxidative Stress Damage in Rat Ileum
3. Discussion
4. Material and Methods
4.1. Chemicals
4.2. Plant Material
4.3. UHPLC-DAD-MS Instrument and LC Parameters and MS Parameters
4.4. Determination of Antioxidant Activity
4.5. Animals
4.6. Study of the Chronic Administration of MIE on the Cholinergic Response and Acute Oxidative Stress Induced by H2O2
- Group 1 (n = 5; Control) treated with vehicle (peanut butter; ConAgra Foods Export Company Inc., Omaha, NE, 68102, USA).
- Group 2 (n = 5; MIE 50 mg/kg) treated with MIE (50 mg/kg) plus vehicle for 28 days.
- Group 3 (n = 5; MIE 100 mg/kg) treated with MIE (100 mg/kg) plus vehicle for 28 days.
4.7. Intestinal Reactivity Experiments
4.8. Determination of Lipid Peroxidation in Ileum by TBARS
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kolle, S.R.; Shankarappa, T.H.; Reddy, T.B.M. Trends in Mango Research as Seen through Science Citation Expanded Index of Web of Science. Erwerbs-Obstbau 2018, 60, 261–270. [Google Scholar] [CrossRef]
- Ronchi, S.N.; Brasil, G.A.; Nascimento, A.M.D.; Lima, E.M.; Scherer, R.; Costa, H.B.; Romão, W.; Boëchat, G.A.P.; Lenz, D.; Fronza, M.; et al. Phytochemical and in vitro and in vivo biological investigation on the antihypertensive activity of mango leaves (Mangifera indica L.). Ther. Adv. Cardiovasc. Dis. 2015, 9, 244–256. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Olivo, G.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; Osuna-Enciso, T.; León-Félix, J.; Heredia, J.B. Cellular antioxidant activity and in vitro intestinal permeability of phenolic compounds from four varieties of mango bark (Mangifera indica L.). J. Sci. Food Agric. 2019, 99, 3481–3489. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef] [Green Version]
- Mahomoodally, M.F.; Protab, K.; Aumeeruddy, M.Z. Medicinal plants brought by Indian indentured immigrants: A comparative review of ethnopharmacological uses between Mauritius and India. J. Ethnopharmacol. 2019, 234, 245–289. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Sharon, D. Traditional medicinal plant use in Northern Peru: Tracking two thousand years of healing culture. J. Ethnobiol. Ethnomed. 2006, 2, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Banerjee, N.; Barnes, R.C.; Pfent, C.M.; Talcott, S.T.; Dashwood, R.H.; Mertens-Talcott, S.U. Mango polyphenolics reduce inflammation in intestinal colitis-involvement of the miR-126/PI3K/AKT/mTOR axis in vitro and in vivo. Mol. Carcinog. 2016, 56, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Ediriweera, M.K.; Tennekoon, K.; Samarakoon, S. A Review on Ethnopharmacological Applications, Pharmacological Activities, and Bioactive Compounds of Mangifera indica (Mango). Evidence-Based Complement. Altern. Med. 2017, 2017, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Tawaha, K.; Sadi, R.; Qa’Dan, F.; Matalka, K.Z.; Nahrstedt, A. A Bioactive Prodelphinidin from Mangifera indica Leaf Extract. Z. Nat. C J. Biosci. 2010, 65, 322–326. [Google Scholar] [CrossRef] [Green Version]
- Márquez, L.; Perez-Nievas, B.G.; Gárate, I.; Garcia-Bueno, B.; Madrigal, J.L.M.; Menchen, L.; Garrido, G.; Leza, J.C. Anti-inflammatory effects of Mangifera indica L. extract in a model of colitis. World J. Gastroenterol. 2010, 16, 4922–4931. [Google Scholar] [CrossRef]
- Kim, H.; Banerjee, N.; Ivanov, I.; Pfent, C.M.; Prudhomme, K.R.; Bisson, W.H.; Dashwood, R.H.; Talcott, S.T.; Mertens-Talcott, S.U. Comparison of anti-inflammatory mechanisms of mango (Mangifera Indica L.) and pomegranate (Punica Granatum L.) in a preclinical model of colitis. Mol. Nutr. Food Res. 2016, 60, 1912–1923. [Google Scholar] [CrossRef] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nat. Cell Biol. 2007, 448, 427–434. [Google Scholar] [CrossRef]
- Alañón, M.; Pimentel-Moral, S.; Arráez-Román, D.; Segura-Carretero, A. HPLC-DAD-Q-ToF-MS profiling of phenolic compounds from mango (Mangifera indica L.) seed kernel of different cultivars and maturation stages as a preliminary approach to determine functional and nutraceutical value. Food Chem. 2020, 337, 127764. [Google Scholar] [CrossRef] [PubMed]
- Burgueño, J.F.; Fritsch, J.; Santander, A.M.; Brito, N.; Fernández, I.; Pignac-Kobinger, J.; Conner, G.E.; Abreu, M.T. Intestinal Epithelial Cells Respond to Chronic Inflammation and Dysbiosis by Synthesizing H2O2. Front. Physiol. 2019, 10, 1484. [Google Scholar] [CrossRef]
- Fajardo, A.F.; Sobchak, C.; Shifrin, Y.; Pan, J.; Gonska, T.; Belik, J. Hydrogen peroxide promotes gastric motility in the newborn rat. Pediatr. Res. 2018, 84, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Linnane, A.W.; Kios, M.; Vitetta, L. Healthy aging: Regulation of the metabolome by cellular redox modulation and prooxidant signaling systems: The essential roles of superoxide anion and hydrogen peroxide. Biogerontology 2007, 8, 445–467. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Sarna, S.K. Different types of contractions in rat colon and their modulation by oxidative stress. Am. J. Physiol. Liver Physiol. 2001, 280, G546–G554. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; López-Cobo, A.; Verardo, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-DAD-q-TOF-MS as a powerful platform for the determination of phenolic and other polar compounds in the edible part of mango and its by-products (peel, seed, and seed husk). Electrophoresis 2016, 37, 1072–1084. [Google Scholar] [CrossRef]
- Medlicott, A.P.; Thompson, A.K. Analysis of sugars and organic acids in ripening mango fruits (Mangifera indica L. var Keitt) by high performance liquid chromatography. J. Sci. Food Agric. 1985, 36, 561–566. [Google Scholar] [CrossRef]
- Barreto, J.C.; Trevisan, M.T.S.; Hull, W.E.; Erben, G.; De Brito, E.S.; Pfundstein, B.; Würtele, G.; Spiegelhalder, B.; Owen, R.W. Characterization and Quantitation of Polyphenolic Compounds in Bark, Kernel, Leaves, and Peel of Mango (Mangifera indica L.). J. Agric. Food Chem. 2008, 56, 5599–5610. [Google Scholar] [CrossRef]
- Hu, K.; Dars, A.G.; Liu, Q.; Xie, B.; Sun, Z. Phytochemical profiling of the ripening of Chinese mango (Mangifera indica L.) cultivars by real-time monitoring using UPLC-ESI-QTOF-MS and its potential benefits as prebiotic ingredients. Food Chem. 2018, 256, 171–180. [Google Scholar] [CrossRef]
- Benites, J.; Asuncion-Alvarez, H.D.; Ybanez-Julca, R.O.; Ganoza-Yupanqui, M.L.; Jacinto-Fernandez, J.J.; Reyes-De la Vega, J.B.; Zavaleta-Cruz, H.J.; Pinedo-Alcantara, A.N.; Layado-Fonseca, C.M.; Medina-Mejia, C.A.; et al. Chemical composition by HPLC-ESI-QTOF-MS/MS: Estrogenic and antioxidant effects of Mangifera indica L. cv. “Kent” leave extracts on ovariectomized rats. Bol. Latinoam. Caribe Plantas Med. Aromat. 2019, 18, 336–346. [Google Scholar]
- Yannai, S. Dictionary of Food Compounds with CD-ROM; CRC Press: New York, NY, USA, 2004. [Google Scholar]
- Berardini, N.; Carle, R.; Schieber, A. Characterization of gallotannins and benzophenone derivatives from mango (Mangifera indica L. cv. ’Tommy Atkins’) peels, pulp and kernels by high-performance liquid chromatography electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 2208–2216. [Google Scholar] [CrossRef]
- Laulloo, S.J.; Bhowon, M.G.; Soyfoo, S.; Chua, L.S. Nutritional and Biological Evaluation of Leaves of Mangifera indica from Mauritius. J. Chem. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, J.E.; Zambrano, R.; Sepúlveda, B.; Simirgiotis, M.J. Antioxidant Properties and Hyphenated HPLC-PDA-MS Profiling of Chilean Pica Mango Fruits (Mangifera indica L. Cv. piqueño). Molecules 2013, 19, 438–458. [Google Scholar] [CrossRef] [Green Version]
- Baeza, G.; Sarriá, B.; Clemente, L.B.; Mateos, R.; Mateos, R. Exhaustive Qualitative LC-DAD-MS Analysis of Arabica Green Coffee Beans: Cinnamoyl-glycosides and Cinnamoylshikimic Acids as New Polyphenols in Green Coffee. J. Agric. Food Chem. 2016, 64, 9663–9674. [Google Scholar] [CrossRef] [Green Version]
- Simirgiotis, M.J.; Adachi, S.; To, S.; Yang, H.; Reynertson, K.A.; Basile, M.J.; Gil, R.R.; Weinstein, I.B.; Kennelly, E.J. Cytotoxic chalcones and antioxidants from the fruits of Syzygium samarangense (Wax Jambu). Food Chem. 2008, 107, 813–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persia, F.A.; Troncoso, M.E.; Rinaldini, E.; Simirgiotis, M.; Tapia, A.; Bórquez, J.; Mackern-Oberti, J.P.; Hapon, M.B.; Gamarra-Luques, C. UHPLC–Q/Orbitrap/MS/MS fingerprinting and antitumoral effects of Prosopis strombulifera (LAM.) BENTH. queous extract on allograft colorectal and melanoma cancer models. Heliyon 2020, 6, e03353. [Google Scholar] [CrossRef] [PubMed]
- Urbain, A.; Marston, A.; Queiroz, E.F.; Ndjoko, K.; Hostettmann, K. Xanthones from Gentiana campestrisas New Acetylcholinesterase Inhibitors. Planta Med. 2004, 70, 1011–1014. [Google Scholar] [CrossRef]
- Hajimehdipoor, H.; Dijoux-Franca, M.-G.; Mariotte, A.-M.; Amanzadeh, Y.; Sadat-Ebrahimi, S.-E.; Ghazi-Khansari, M. Two new xanthone diglycosides from Swertia longifolia Boiss. Nat. Prod. Res. 2006, 20, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Laub, A.; Sendatzki, A.-K.; Palfner, G.; Wessjohann, L.A.; Schmidt, J.; Arnold, N. HPTLC-DESI-HRMS-Based Profiling of Anthraquinones in Complex Mixtures—A Proof-of-Concept Study Using Crude Extracts of Chilean Mushrooms. Foods 2020, 9, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Bishop, K.S.; Zhang, J.; Chen, D.; Quek, S.-Y. Characterization of phenolic compounds and aroma active compounds in feijoa juice from four New Zealand grown cultivars by LC-MS and HS-SPME-GC-O-MS. Food Res. Int. 2020, 129, 108873. [Google Scholar] [CrossRef]
- Rivera-Chávez, J.; Bustos-Brito, C.; Aguilar-Ramírez, E.; Martínez-Otero, D.; Rosales-Vázquez, L.D.; Dorazco-González, A.; Cano-Sánchez, P. Hydroxy-neo-Clerodanes and 5,10-seco-neo-Clerodanes from Salvia decora. J. Nat. Prod. 2020, 83, 2212–2220. [Google Scholar] [CrossRef]
- Somani, S.; Zambad, S.; Modi, K. Mangiferin attenuates DSS colitis in mice: Molecular docking and in vivo approach. Chem. Interact. 2016, 253, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Hou, Q.; Lei, J.; Wolf, P.G.; Ayansola, H.; Zhang, B. Quercetin Alleviates Intestinal Oxidative Damage Induced by H2O2 via Modulation of GSH: In Vitro Screening and In Vivo Evaluation in a Colitis Model of Mice. ACS Omega 2020, 5, 8334–8346. [Google Scholar] [CrossRef] [Green Version]
- Sumaya-Martinez, M.T.; Medina-Carrillo, R.E.; Gonzalez-Ocegueda, E.; Jimenez-Ruiz, E.I.; Balois-Morales, R.; Sanchez-Herrera, L.M.; Lopez-Nahuatt, G. Mango (Mangifera indica L.) pulping byproducts: Antioxidant activity and bioactive compounds of three mango cultivars. Revista Bio Ciencias 2019, 6, 20. [Google Scholar] [CrossRef]
- Kulkarni, V.M.; Rathod, V.K. Exploring the potential of Mangifera indica leaves extract versus mangiferin for therapeutic application. Agric. Nat. Resour. 2018, 52, 155–161. [Google Scholar] [CrossRef]
- Khumpook, T.; Saenphet, S.; Tragoolpua, Y.; Saenphet, K. Anti-inflammatory and antioxidant activity of Thai mango (Mangifera indica Linn.) leaf extracts. Comp. Haematol. Int. 2018, 28, 157–164. [Google Scholar] [CrossRef]
- Corral-Aguayo, R.D.; Yahia, E.M.; Carrillo-López, A.; González-Aguilar, G. Correlation between Some Nutritional Components and the Total Antioxidant Capacity Measured with Six Different Assays in Eight Horticultural Crops. J. Agric. Food Chem. 2008, 56, 10498–10504. [Google Scholar] [CrossRef]
- Reid, M.; Spence, J.; Nwokocha, M.; Palacios, J.; Nwokocha, C.R. Chapter 2—The role of NADP(H) oxidase inhibition and its implications in cardiovascular disease management using natural plant products. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 58, pp. 43–59. [Google Scholar]
- Herson, P.S.; Lee, K.; Pinnock, R.D.; Hughes, J.; Ashford, M.L.J. Hydrogen Peroxide Induces Intracellular Calcium Overload by Activation of a Non-selective Cation Channel in an Insulin-secreting Cell Line. J. Biol. Chem. 1999, 274, 833–841. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Yi, X.; Zhang, S.; Cheng, J.; Wang, Y.; Liu, C.; He, X. Bioactive phenolics from mango leaves (Mangifera indica L.). Ind. Crop. Prod. 2018, 111, 400–406. [Google Scholar] [CrossRef]
- Morais, T.C.; Arruda, B.R.; Magalhães, H.D.S.; Trevisan, M.T.; Viana, D.D.A.; Rao, V.; Santos, F.A. Mangiferin ameliorates the intestinal inflammatory response and the impaired gastrointestinal motility in mouse model of postoperative ileus. Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 531–538. [Google Scholar] [CrossRef]
- Kammalla, A.K.; Ramasamy, M.K.; Inampudi, J.; Dubey, G.P.; Agrawal, A.; Kaliappan, I. Comparative Pharmacokinetic Study of Mangiferin After Oral Administration of Pure Mangiferin and US Patented Polyherbal Formulation to Rats. AAPS PharmSciTech 2015, 16, 250–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agbonon, A.; Eklu-Gadegbeku, K.; Aklikokou, K.; Essien, K.; Akpagana, K.; Gbeassor, M. The effect of Mangifera indica stem bark and Pluchea ovalis roots on tracheal smooth muscle in vitro. Fitoterapia 2002, 73, 619–622. [Google Scholar] [CrossRef]
- Agbonon, A.; Aklikokou, K.; Gbeassor, M. Mangifera indica. Stem Bark Effect on the Rat Trachea Contracted by Acetylcholine and Histamine. Pharm. Biol. 2005, 43, 475–479. [Google Scholar] [CrossRef]
- Morais, T.C.; Lopes, S.C.; Carvalho, K.M.M.B.; Arruda, B.R.; De Souza, F.T.C.; Trevisan, M.T.; Rao, V.; Santos, F.A. Mangiferin, a natural xanthone, accelerates gastrointestinal transit in mice involving cholinergic mechanism. World J. Gastroenterol. 2012, 18, 3207–3214. [Google Scholar]
- Vieira, A.B.; Coelho, L.P.; Insuela, D.B.R.; Carvalho, V.F.; Dos Santos, M.H.; Silva, P.M.; Martins, M.A. Mangiferin Prevents Guinea Pig Tracheal Contraction via Activation of the Nitric Oxide-Cyclic GMP Pathway. PLoS ONE 2013, 8, e71759. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, M.R.; Park, J.J.; Choi, J.Y.; Song, B.R.; Son, H.J.; Choi, Y.W.; Kim, K.M.; Hong, J.T.; Hwang, D.-Y. Quercetin promotes gastrointestinal motility and mucin secretion in loperamide-induced constipation of SD rats through regulation of the mAChRs downstream signal. Pharm. Biol. 2018, 56, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef]
- Hammad, H.M.; Abdalla, S.S. Pharmacological effects of selected flavonoids on rat isolated ileum: Structure-activity relationship. Gen. Pharmacol. Vasc. Syst. 1997, 28, 767–771. [Google Scholar] [CrossRef]
- Ma, R.; Seifi, M.; Papanikolaou, M.; Brown, J.F.; Swinny, J.D.; Lewis, A. TREK-1 Channel Expression in Smooth Muscle as a Target for Regulating Murine Intestinal Contractility: Therapeutic Implications for Motility Disorders. Front. Physiol. 2018, 9, 157. [Google Scholar] [CrossRef] [Green Version]
- Van Der Vliet, A.; Tuinstra, T.J.; Bast, A. Modulation of oxidative stress in the gastrointestinal tract and effect on rat intestinal motility. Biochem. Pharmacol. 1989, 38, 2807–2818. [Google Scholar] [CrossRef]
- Sellés, A.J.N.; Castro, H.T.V.; Agüero-Agüero, J.; González-González, J.; Naddeo, F.; De Simone, F.; Rastrelli, L. Isolation and Quantitative Analysis of Phenolic Antioxidants, Free Sugars, and Polyols from Mango (Mangifera indica L.) Stem Bark Aqueous Decoction Used in Cuba as a Nutritional Supplement. J. Agric. Food Chem. 2002, 50, 762–766. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Quispe, C.; Bórquez, J.; Schmeda-Hirschmann, G.; Avendaño, M.; Sepúlveda, B.; Winterhalter, P. Fast high resolution Orbitrap MS fingerprinting of the resin of Heliotropium taltalense Phil. from the Atacama Desert. Ind. Crop. Prod. 2016, 85, 159–166. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]







| Peak # | Retention Time (min.) | UV Max | Tentative Identification | Molecular Formula | Theoretical Mass (m/z) | Measured Mass (m/z) | Accuracy (δ ppm) | References | MS2 Ions (m/z) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.40 | unknown | 272.9586 | - | |||||
| 2 | 3.22 | - | Quinic acid | C7H11O6− | 191.05611 | 191.05547 | 5.10 | [19] | 127.03929, 85.02829 |
| 3 | 4.51 | 235 | Citric acid | C6H7O7− | 191.01973 | 191.01936 | 3.01 | [19,20] | 111.00771 C5H3O3− |
| 4 | 8.21 | 236–271 | Gallic acid | C7H5O5− | 169.01425 | 169.01372 | 3.20 | [19,21,22] | 125.02363 C6H5O3− [M− − CO2] |
| 5 | 8.70 | Gentisoyl glucoside | C13H16O9− | 315.07106 | 315.07227 | 3.83 | PubChem 101339724 | 287.05600, 153.08177 | |
| 6 | 11.25 | 236–294 | Iriflophenone-3-C-β-d-glucoside | C19H19O10− | 407.09837 | 407.09837 | 2.69 | [19,21,23] | 117.03393 C6H5O3− |
| 7 | 11.95 | 236–294 | Iriflophenone-3-C-β-d-galactoside | C19H19O10− | 407.09837 | 407.09836 | 2.66 | [21,23] | 117.03393 C6H5O3− |
| 8 | 12.32 | 236–294 | Iriflophenone-5-C-β-d-glucoside | C19H19O10− | 407.09837 | 407.09833 | 2.65 | [21,23] | 125.02370 C6H5O3− |
| 9 | 12.54 | 236–277 | Gallic acid derivative of iriflophenone | C26H23O14 | 559.10933 | 559.10883 | −0.89 | [21] | 421.07785 C19H17O11 |
| 10 | 13.43 | 280 | Cicerin 7 (6′-malonyl) glucoside | C26H25O15− | 577.11880 | 577.11981 | 1.75 | [24] | 179.05022, 151.00436 |
| 11 | 13.72 | 258–318 | Mangiferin | C19H17O11− | 421.07763 | 421.07773 | 2.82 | [21,22,23,25,26] | 258.01666 C13H6O6− [M− − glucose] |
| 12 | 14.02 | 236–274 | Dehidro-mangiferin-6-O-gallate | C26H21O14− | 557.09368 | 557.09387 | 2.53 | [27] | 303.09067 |
| 13 | 14.21 | 236–260 | Mangiferin-6-O-gallate | C26H21O15− | 573.08859 | 573.08820 | −0.68 | [21,25,26] | 421.07762 C19H17O11− |
| 14 | 14.35 | 236–279 | Iriflophenone-3-C-(2,3-di-O-galloyl)-β-d-glucoside | C33H27O18− | 711.12029 | 711.12140 | 4.65 | [21,25] | 245.21232 |
| 15 | 15.02 | 238–271 | Syringic acid | C9H9O5− | 197.04555 | 197.04507 | 3.16 | [19] | 124.01559 C6H4O3− [M− − CO2 − 2Me] |
| 16 | 16.32 | 258–318 | Iso mangiferin | C19H17O11− | 421.07763 | 421.07776 | 2.89 | [21,25,26] | 258.01666 C13H6O6− [M− − glucose] |
| 17 | 16.83 | 237–269 | Apigenin 7-O-glucuronide | C21H18O11 | 447.09329 | 447.09280 | 2.26 | [25] | 271.15491, 225.05186, 179.0765, 150.9982 |
| 18 | 17.35 | 238–271 | Sinapoyl-caffeoylshikimic acid | C27H25O12− | 541.13515 | 541.13489 | 1.43 | [28] | 507.06539, 463.25494 |
| 19 | 18.92 | 254–354 | Reynoutrin | C20H18O11− | 477.07654 | 433.07767 | 2.60 | [29] | 179.05022, 151.00436 |
| 20 | 20.55 | 220–280 | OMe-gallic acid/methyl gallate ester | C16H13O9− | 349.05651 | 349.05661 | 3.35 | [21] | 125.05342 |
| 21 | 21.37 | 220–265 | Salicylic acid | C7H5O3− | 137.02442 | 137.02379 | 3.25 | [22] | 93.03452, 59.01385 |
| 22 | 21.75 | 254–354 | Quercetin | C15H9O6− | 301.03538 | 301.03546 | 4.02 | [26] | 179.05012, 151.0023 |
| 23 | 23.23 | 215 | Trihydroxyoctadienoic acid (Trihydroxylinoleic acid) | C18H31O5− | 327.21770 | 327.21790 | 3.95 | [30] | 259.06116, 174.95134 |
| 24 | 23.76 | 209 | Trihydroxyoctaenoic acid | C18H33O5− | 329.23335 | 329.23349 | 3.85 | [30] | 293.17896, 239.09236, |
| 25 | 24.23 | 258–318 | Bellidin (1,3,5,8-tetrahydroxyxanthone) | C13H8O6− | 259.02371 | 259.02475 | 3.99 | [31,32] | 197.04517, 174.95560 |
| 26 | 25.12 | 258–318 | Skyrin | C30H17O10− | 537.08162 | 537.08264 | 1.89 | [33] | 387.07208, 325.20193 |
| 27 | 26.45 | 280 | Procyanidin B1 | C30H25O12− | 577.13405 | 577.13544 | 2.39 | [34] | 407.07702, 289.07120, 125.02291 |
| 28 | 27.20 | 280 | 5,8-dihydroxy-6,7,3-trimethoxy-3′,4′-methylenedioxyflavone | C19H15O9− | 387.07106 | 387.07236 | −1.25 | PubChem NSC678101 | 179.05012, 151.0023 |
| 29 | 28.80 | 254–354 | Eupatorin | C18H15O7− | 343.08123 | 343.08258 | 3.93 | [35] | 179.05026, 151.0045 |
Sample Availability: Samples of the compounds and the datasets generated during and/or analyzed during the current study are available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ybañez-Julca, R.O.; Asunción-Alvarez, D.; Quispe-Díaz, I.M.; Palacios, J.; Bórquez, J.; Simirgiotis, M.J.; Perveen, S.; Nwokocha, C.R.; Cifuentes, F.; Paredes, A. Metabolomic Profiling of Mango (Mangifera indica Linn) Leaf Extract and Its Intestinal Protective Effect and Antioxidant Activity in Different Biological Models. Molecules 2020, 25, 5149. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25215149
Ybañez-Julca RO, Asunción-Alvarez D, Quispe-Díaz IM, Palacios J, Bórquez J, Simirgiotis MJ, Perveen S, Nwokocha CR, Cifuentes F, Paredes A. Metabolomic Profiling of Mango (Mangifera indica Linn) Leaf Extract and Its Intestinal Protective Effect and Antioxidant Activity in Different Biological Models. Molecules. 2020; 25(21):5149. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25215149
Chicago/Turabian StyleYbañez-Julca, Roberto O., Daniel Asunción-Alvarez, Ivan M. Quispe-Díaz, Javier Palacios, Jorge Bórquez, Mario J. Simirgiotis, Shagufta Perveen, Chukwuemeka R. Nwokocha, Fredi Cifuentes, and Adrián Paredes. 2020. "Metabolomic Profiling of Mango (Mangifera indica Linn) Leaf Extract and Its Intestinal Protective Effect and Antioxidant Activity in Different Biological Models" Molecules 25, no. 21: 5149. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25215149