A General and Scalable Synthesis of Polysubstituted Indoles
Abstract
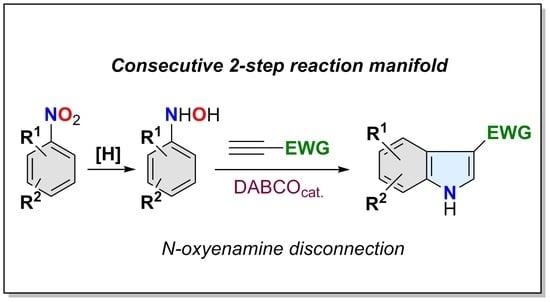
:1. Introduction
2. Results and Discussion
2.1. Initial Experiments and Optimization of the Reaction Conditions for a Consecutive 2-Step Protocol
2.2. Scope of the Consecutive 2-Step Protocol
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of Indoles 6 Directly from N-phenylhydroxylamine (4a). Preparation of Methyl Indol-3-carboxylate (6a)
3.3. General Procedure for the Consecutive 2-Step Synthesis of Indoles 6 from Nitroarenes 3. Preparation of Methyl Indol-3-carboxylate (6a)
3.4. Characterization Data for Indoles 6 and 3,3a-Dihydro-2H-Indoles 10
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Note
- Barluenga, J.; Valdés, C. Five-membered heterocycles: Indole and related systems. In Modern Heterocyclic Chemistry; Alvarez-Builla, J., Vaquero, J.J., Barluenga, J., Eds.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2011; pp. 377–531. [Google Scholar]
- Dhuguru, J.; Skouta, R. Role of indole scafolds as pharmacophores in the development of anti-lung cancer agents. Molecules 2020, 25, 1615. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Li, Y.; Yan, C.; Yan, M.; Tang, Z. Indole: A privileged scaffold for the design of anti-cancer agents. Eur. J. Med. Chem. 2019, 183, 111691–111709. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.; Kaur, A.; Goyal, B. Benzofuran and indole: Promising scaffolds for drug development in Alzheimer’s disease. ChemMedChem 2018, 13, 1275–1299. [Google Scholar] [CrossRef] [PubMed]
- Chadha, N.; Silakari, O. Indoles as therapeutics of interest in medicinal chemistry: Bird’s eye view. Eur. J. Med. Chem. 2017, 134, 159–184. [Google Scholar] [CrossRef] [PubMed]
- Gribble, G.W. Indole Ring Synthesis: From Natural Products to Drug Discovery; John Wiley & Sons: Chichester, UK, 2016. [Google Scholar]
- Owczarczyk, Z.R.; Braunecker, W.A.; Garcia, A.; Larsen, R.; Nardes, A.M.; Kopidakis, N.; Ginley, D.S.; Olson, D.C. 5,10-Dihydroindolo[3,2-b]indole-based copolymers with alternating donor and acceptor moieties for organic photovoltaics. Macromolecules 2013, 46, 1350–1360. [Google Scholar] [CrossRef]
- Nie, G.M.; Bai, Z.M.; Yu, W.Y.; Zhang, L. Electrochemiluminescence biosensor for ramos cells based on a nanostructured conducting polymer composite material (PICA-MWNTs). J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2385–2392. [Google Scholar] [CrossRef]
- Manickam, M.; Iqbal, P.; Belloni, M.; Kumar, S.; Preece, J.A. A brief review of carbazole-based photorefractive liquid crystalline materials. Isr. J. Chem. 2012, 52, 917–934. [Google Scholar] [CrossRef]
- Li, T.Z.; Liu, S.J.; Tan, W.; Shi, F. For a recent review on the current state of the field, see: Catalytic asymmetric construction of axially chiral indole-based frameworks: An emerging area. Chem. Eur. J. 2020, 26. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Jiang, F.; Shi, F. Organocatalytic asymmetric synthesis of indole-based chiral heterocycles: Strategies, reactions, and outreach. Acc. Chem. Res. 2020, 53, 425–446. [Google Scholar] [CrossRef]
- Ma, C.; Sheng, F.T.; Wang, H.Q.; Deng, S.; Zhang, Y.C.; Jiao, Y.; Tan, W.; Shi, F. Atroposelective access to oxindole-based axially chiral styrenes via the strategy of catalytic kinetic resolution. J. Am. Chem. Soc. 2020, 142, 15686–15696. [Google Scholar] [CrossRef]
- Lai, Y.T.; Wang, T.; O’Dell, S.; Louder, M.K.; Schön, A.; Cheung, C.S.; Chuang, G.Y.; Druz, A.; Lin, B.; McKee, K.; et al. Lattice engineering enables definition of molecular features allowing for potent small-molecule inhibition of HIV-1 entry. Nat. Commun. 2019, 10, 47–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Huang, B.; Zhan, P.; De Clercq, E.; Liu, X. Discovery of small molecular inhibitors targeting HIV-1 gp120eCD4 interaction drived from BMS-378806. J. Eur. Med. Chem. 2014, 86, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Guo, M.; Li, X.; Jia, F.; Li, C.; Yang, Y.; Cao, M.; Jiang, N.; Ma, E.; Zhai, X.; et al. Discovery of novel indole-based allosteric highly potent ATX inhibitors with great in vivo efficacy in a mouse lung fibrosis model. J. Med. Chem. 2020, 63, 7326–7346. [Google Scholar] [CrossRef] [PubMed]
- Apirakkan, O.; Gavrilović, I.; Cowan, D.A.; Abbate, V. In vitro phase i metabolic profiling of the synthetic cannabinoids AM-694, 5F-NNEI, FUB-APINACA, MFUBINAC, and AMB-FUBINACA. Chem. Res. Toxicol. 2020, 33, 1653–1664. [Google Scholar] [CrossRef]
- Liou, J.P. 3-Aroylindoles display antitumor activity in vitro and in vivo: Effects of N1-substituents on biological activity. J. Med. Chem. 2017, 125, 1268–1278. [Google Scholar]
- Smith, A.C.; Kung, D.W.; Shavnya, A.; Brandt, T.A.; Dent, P.D.; Genung, N.E.; Cabral, S.; Panteleev, J.; Herr, M.; Yip, K.N.; et al. Evolution of the synthesis of AMPK activators for the treatment of diabetic nephropathy: From three preclinical candidates to the investigational new drug PF-06409577. Org. Process. Res. Dev. 2018, 22, 681–696. [Google Scholar] [CrossRef]
- Wu, Y.S.; Coumar, M.S.; Chang, J.Y.; Sun, H.Y.; Kuo, F.M.; Kuo, C.C.; Chen, Y.J.; Chang, C.Y.; Hsiao, C.L.; Liou, J.P.; et al. Synthesis and evaluation of 3-aroylindoles as anticancer agents: Metabolite approach. J. Med. Chem. 2009, 52, 4941–4945. [Google Scholar] [CrossRef]
- Liou, J.P.; Chang, Y.L.; Kuo, F.M.; Chang, C.W.; Tseng, H.Y.; Wang, C.C.; Yang, Y.N.; Chang, J.Y.; Lee, S.J.; Hsieh, H.P.; et al. Concise synthesis and structure-activity relationships of combretastatin A-4 analogues, 1-aroylindoles and 3-aroylindoles, as novel classes of potent antitubulin agents. J. Med. Chem. 2004, 47, 4247–4257. [Google Scholar] [CrossRef]
- Kuo, C.C.; Hsieh, H.P.; Pan, W.Y.; Chen, C.P.; Liou, J.P.; Lee, S.J.; Chang, Y.L.; Chen, L.T.; Chen, C.T.; Chang, J.Y.; et al. BPR0L075, a novel synthetic indole compound with antimitotic activity in human cancer cells, exerts effective antitumoral activity in vivo. Cancer Res. 2004, 64, 4621–4628. [Google Scholar] [CrossRef] [Green Version]
- Huck, C.J.; Sarlah, D. Shaping Molecular Landscapes: Recent Advances, Opportunities, and Challenges in Dearomatization. Chem 2020, 6, 1589–1603. [Google Scholar] [CrossRef]
- Li, K.; Gonçalves, T.P.; Huang, K.W.; Lu, Y. Dearomatization of 3-nitroindoles by a phosphine-catalyzed enantioselective [3 + 2] annulation reaction. Angew. Chem. Int. Ed. 2019, 58, 5427–5431. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, J.; Tu, Y.; Zhang, J. Phosphine-catalyzed enantioselective dearomative [3 + 2]-cycloaddition of 3-nitroindoles and 2-nitrobenzofurans. Angew. Chem. Int. Ed. 2019, 58, 5422–5426. [Google Scholar] [CrossRef] [PubMed]
- Suo, J.J.; Liu, W.; Du, J.; Ding, C.H.; Hou, X.L. Diastereo-and enantioselective palladium-catalyzed dearomative [3 + 2] cycloaddition of 3-nitroindoles. Chem. Asian J. 2018, 13, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Zhang, F.; Cai, Y.; Guo, Y.L.; You, S.L. Stereodivergent synthesis of tetrahydrofuroindoles through Pd-catalyzed asymmetric dearomative formal [3 + 2] cycloaddition. Angew. Chem. Int. Ed. 2018, 57, 2134–2138. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhu, Z.Q.; Gu, L.; Wan, X.; Mei, G.J.; Shi, F. Catalytic asymmetric dearomative [3 + 2] cycloaddition of electron-deficient indoles with all-carbon 1,3-dipoles. J. Org. Chem. 2018, 83, 2341–2348. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Tong, F.; Sun, B.B.; Fan, W.T.; Chen, J.B.; Hu, D.; Wang, X.W. Pd-catalyzed asymmetric dearomative cycloaddition for construction of optically active pyrroloindoline and cyclopentaindoline derivatives: Access to 3a-aminopyrroloindolines. J. Org. Chem. 2018, 83, 2882–2891. [Google Scholar] [CrossRef]
- Yue, D.F.; Zhao, J.Q.; Chen, X.Z.; Zhou, Y.; Zhang, X.M.; Xu, X.Y.; Yuan, W.C. Multiple hydrogen-bonding bifunctional thiourea-catalyzed asymmetric dearomative [4 + 2] annulation of 3-nitroindoles: Highly enantioselective access to hydrocarbazole skeletons. Org. Lett. 2017, 19, 4508–4511. [Google Scholar] [CrossRef]
- Gerten, L.; Stanley, L.M. Enantioselective dearomative [3 + 2] cycloadditions of indoles with azomethine ylides derived from alanine imino esters. Org. Chem. Front. 2016, 3, 339–343. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.Q.; Wu, Z.J.; Zhou, M.Q.; Xu, X.Y.; Zhang, X.M.; Yuan, W.C. Zn-catalyzed diastereo-and enantioselective cascade reaction of 3-isothiocyanato oxindoles and 3-nitroindoles: Stereocontrolled syntheses of polycyclic spirooxindoles. Org. Lett. 2015, 17, 5020–5023. [Google Scholar] [CrossRef]
- Awata, A.; Arai, T. PyBidine/Copper catalyst: Asymmetric exo’-selective [3 + 2] cycloaddition using imino ester and electrophilic indole. Angew. Chem. Int. Ed. 2014, 53, 10462–10465. [Google Scholar] [CrossRef]
- Li, Y.; Tur, F.; Nielsen, R.P.; Jiang, H.; Jensen, F.; Jørgensen, K.A. Enantioselective formal [4 + 2] cycloadditions to 3-nitroindoles by trienamine catalysis: Synthesis of chiral dihydrocarbazoles. Angew. Chem. Int. Ed. 2016, 55, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Roche, S.P.; Tendoung, J.J.Y.; Treguier, B. Advances in dearomatization strategies of indoles. Tetrahedron 2015, 71, 3549–3591. [Google Scholar] [CrossRef]
- Kumar, N.; Parle, A. 3-Substituted indole: A review. Int. J. Chem. Stud. 2019, 72, 848–856. [Google Scholar]
- Norwood, V.M.; Huigens, R.W. Harnessing the chemistry of the indole heterocycle to drive discoveries in biology and medicine. ChemBioChem 2019, 20, 2273–2297. [Google Scholar] [CrossRef]
- Joule, J.A. Indole and its derivatives. Chapter 10.13. In Science of Synthesis: Houben-Weyl Methods of Molecular Transformations; Thomas, E.J., Ed.; George Thieme Verlag: Stuttgart, Germany, 2000; Volume 10, pp. 361–652. [Google Scholar]
- Li, L.H.; Niu, Z.J.; Liang, Y.M. New Friedel–Crafts strategy for preparing 3-acylindoles. Org. Biomol. Chem. 2018, 16, 7792–7796. [Google Scholar] [CrossRef]
- Wu, W.; Su, W. Mild and selective Ru-catalyzed formylation and Fe-catalyzed acylation of free (NH) indoles using anilines as the carbonyl source. J. Am. Chem. Soc. 2011, 133, 11924–11927. [Google Scholar] [CrossRef]
- Guchhait, S.K.; Kashyap, M.; Kamble, H. ZrCl4-Mediated regio-and chemoselective friedel–crafts acylation of indole. J. Org. Chem. 2011, 76, 4753–4758. [Google Scholar] [CrossRef]
- Katritzky, R.; Suzuki, K.; Singh, S.K.; He, H.Y. Regiospecific C-acylation of pyrroles and indoles using N-acylbenzotriazoles. J. Org. Chem. 2003, 58, 5720–5723. [Google Scholar] [CrossRef]
- Okauchi, T.; Itonaga, M.; Minami, T.; Owa, T.; Kitoh, K.; Yoshino, H. A general method for acylation of indoles at the 3-position with acyl chlorides in the presence of dialkylaluminum chloride. Org. Lett. 2000, 2, 1485–1487. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, J.; Yu, W.; Huang, Q.; Fu, Y.; Kuang, G.; Pan, C.; Yu, G. Visible light-driven C-3 functionalization of indoles over conjugated microporous polymers. ACS Catal. 2018, 8, 8084–8091. [Google Scholar] [CrossRef]
- Wu, C.J.; Meng, Q.Y.; Lei, T.; Zhong, J.J.; Liu, W.Q.; Zhao, L.M.; Li, Z.J.; Chen, B.; Tung, C.H.; Wu, L.Z.; et al. An oxidant-free strategy for indole synthesis via intramolecular C−C bond construction under visible light irradiation: Cross-coupling hydrogen evolution reaction. ACS Catal. 2016, 6, 4635–4639. [Google Scholar] [CrossRef]
- Li, X.; Gu, X.; Li, Y.; Li, P. Aerobic transition-metal-free visible-light photoredox indole C-3 formylation reaction. ACS Catal. 2014, 4, 1897–1900. [Google Scholar] [CrossRef]
- Zhang, P.; Xiao, T.; Xiong, S.; Dong, X.; Zhou, L. Synthesis of 3-acylindoles by visible-light induced intramolecular oxidative cyclization of o-alkynylated N,N-dialkylamines. Org. Lett. 2014, 16, 3264–3267. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, G.R.; Kuethe, J.T. Practical methodologies for the synthesis of indoles. Chem. Rev. 2006, 106, 2875–2911. [Google Scholar] [CrossRef]
- Neto, J.S.S.; Zeni, G. Recent advances in the synthesis of indoles from alkynes and nitrogen sources. Org. Chem. Front. 2020, 7, 155–210. [Google Scholar] [CrossRef]
- Inman, M.; Moody, C.J. Indole synthesis-something old, something new. Chem. Sci. 2013, 4, 29–41. [Google Scholar] [CrossRef]
- Tabolin, A.A.; Ioffe, S.L. Rearrangement of N-oxyenamines and related reactions. Chem. Rev. 2014, 114, 5426–5476. [Google Scholar] [CrossRef]
- Heravi, M.M.; Rohani, S.; Zadsirjan, V.; Zahedi, N. Fischer indole synthesis applied to the total synthesis of natural products. RSC Adv. 2017, 7, 52852–52887. [Google Scholar] [CrossRef] [Green Version]
- Hughes, D.L. Progress in the Fischer indole reaction. A review. Org. Prep. Proced. Int. 1993, 25, 607–632. [Google Scholar] [CrossRef]
- Bartoli, G.; Dalpozzo, R.; Nardib, M. Applications of Bartoli indole synthesis. Chem. Soc. Rev. 2014, 43, 4728–4750. [Google Scholar] [CrossRef]
- Dalpozzo, R.; Bartoli, G. Bartoli indole synthesis. Curr. Org. Chem. 2005, 9, 163–178. [Google Scholar] [CrossRef]
- Fischer, E.E.; Jourdan, F. Ueber die hydrazine der brenztraubensäure. Eur. J. Inorg. Chem. 1883, 16, 2241–2245. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, L.; Zhang, L. Combining Zn ion catalysis with homogeneous gold catalysis: An efficient annulation approach to N-protected indoles. Chem. Sci. 2013, 4, 739–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, H.; Guo, L.; Liu, F.; Miao, Z.; Feng, L.; Gao, H. A copper-catalyzed synthesis of polysubstituted indoles using N-arylhydroxylamines and vinyliodonium salts using the same disconnection pattern has been recently described, although its application to 3-EWG-indoles was not reported. Copper-catalyzed tandem o-vinylation of arylhydroxylamines/[3,3]-rearrangement/cyclization: Synthesis of highly substituted indoles and benzoindoles. ACS Catal. 2019, 9, 3906–3912. [Google Scholar]
- Tejedor, D.; Delgado-Hernández, S.; Colella, L.; García-Tellado, F. Catalytic hydrocyanation of activated terminal alkynes. Chem. Eur. J. 2019, 25, 15046–15049. [Google Scholar] [CrossRef]
- Tejedor, D.; Álvarez-Méndez, S.J.; López-Soria, J.M.; Martín, V.S.; García-Tellado, F. A robust and general protocol for the Lewis-base-catalysed reaction of alcohols and alkyl propiolates. Eur. J. Org. Chem. 2014, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Mola, L.; Font, J.; Bosch, L.; Caner, J.; Costa, A.M.; Etxebarría-Jardí, G.; Pineda, O.; De Vicente, D.; Vilarrasa, J. Nucleophile-catalyzed additions to activated triple bonds. Protection of lactams, imides, and nucleosides with MocVinyl and related groups. J. Org. Chem. 2013, 78, 5832–5842. [Google Scholar] [CrossRef] [Green Version]
- Tejedor, D.; Santos-Expósito, A.; Méndez-Abt, G.; Ruiz-Pérez, C.; García-Tellado, F. Trialkylamine versus trialkylphosphine: Catalytic conjugate addition of alcohols to alkyl propiolates. Synlett 2009, 1223–1226. [Google Scholar] [CrossRef] [Green Version]
- Feierfeil, J.; Magauer, T. De novo synthesis of benzannelated heterocycles. Chem. Eur. J. 2018, 24, 1455–1458. [Google Scholar] [CrossRef] [Green Version]
- Pereira, M.M.A.; Prabhakar, S.; Lobo, A.M. A Synthesis of the amaryllidaceae alkaloid pratosine. J. Nat. Prod. 1996, 59, 744–747. [Google Scholar] [CrossRef]
- Hwu, J.R.; Patel, H.V.; Lin, R.J.; Gray, M.O. Novel methods for the synthesis of functionalized indoles from arylhydroxylamines and activated acetylenes. J. Org. Chem. 1994, 59, 1577–1582. [Google Scholar] [CrossRef]
- Toyota, M.; Fukumoto, K. Tandem Michael addition-[3,3]sigmatropic rearrangement processes. Part 2. Construction of cyclopropa[3,4]pyrrolo [3,2-e]indol-4-one (CPI) unit of antitumour antibiotic CC-1065. J. Chem. Soc. Perkin Trans. 1992, 1, 547–552. [Google Scholar] [CrossRef]
- Blechert, S. Hetero-Cope-rearrangements, region-controlled synthesis of indoles. Helv. Chim. Acta 1985, 68, 1835–1843. [Google Scholar] [CrossRef]
- Chehardoli, G.; Bahmani, A. Synthetic strategies, SAR studies, and computer modeling of indole 2 and 3-carboxamides as the strong enzyme inhibitors: A review. Mol. Divers. 2020, 1–16. [Google Scholar] [CrossRef]
- Grandberg, I.; Belyaeva, L.D.; Dmitriev, L.B. Ratio of indole isomers formed during the fischer cyclization of meta-substituted diethyl ketone phenylhydrazones. Chem. Het. Compd. 1971, 7, 1131. [Google Scholar] [CrossRef]
- Krenske, E.H.; Burns, J.M.; McGeary, R.P. Claisen rearrangements of benzyl vinyl ethers: Theoretical investigation of mechanism, substituent effects, and regioselectivity. Org. Biomol. Chem. 2017, 15, 7887–7893. [Google Scholar] [CrossRef]
- An ortho-methyl group dropped the yield of the reaction to 25%.
- Lee, K.N.; Lei, Z.; Morales-Rivera, C.A.; Liu, P.; Ngai, M.Y. Mechanistic studies on intramolecular C–H trifluoromethoxylation of (hetero)arenes via OCF3-migration. Org. Biomol. Chem. 2016, 14, 5599. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.Q.; Marchini, M.; Xiao, W.J.; Ceroni, P.; Bandini, M. Visible-light-induced direct photocatalytic carboxylation of indoles with CBr4/MeOH. Chem. Eur. J. 2015, 50, 18052–18056. [Google Scholar] [CrossRef]
- Abe, T.; Yamada, K. Amination/cyclization cascade by acid-catalyzed activation of indolenine for the one-pot synthesis of phaitanthrin E. Org. Lett. 2016, 18, 6504–6507. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, C.; Yang, X.; Su, W. Decarboxylative acylation of N-free indoles enabled by a catalytic amount of copper catalyst and liquid-assisted grinding. Org. Biomol. Chem. 2019, 17, 4446–4451. [Google Scholar] [CrossRef]
- Wynne, J.H.; Lloyd, C.T.; Jensen, S.D.; Boson, S.; Stalick, W.M. 3-Acylindoles via a one-pot, regioselective Friedel-Crafts reaction. Synthesis 2004, 2277–2282. [Google Scholar] [CrossRef]
- Peng, J.; Liu, L.; Hu, Z.; Huang, J.; Zhu, Q. Direct carboxamidation of indoles by palladium-catalyzed C–H activation and isocyanide insertion. Chem. Commun. 2012, 48, 3772–3774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velavan, A.; Sumathi, S.; Balasubramanian, K.K. AlMe3-mediated region- and chemoselective reactions of indole with carbamoyl electrophiles. Eur. J. Org. Chem. 2013, 3148–3157. [Google Scholar] [CrossRef]
- Yamazaki, K.; Nakamura, Y.; Kondo, Y. Solid-phase synthesis of indolecarboxylates using palladium-catalyzed reactions. J. Org. Chem. 2003, 68, 6011–6019. [Google Scholar] [CrossRef]
- Linton, E.C.; Kozlowski, M.C. Catalytic enantioselective Meerwein−Eschenmoser Claisen rearrangement: Asymmetric synthesis of allyl oxindoles. J. Am. Chem. Soc. 2008, 130, 16162–16163. [Google Scholar] [CrossRef]
- Fisher, M.J.; McMurray, L.; Lu, S.; Morse, C.L.; Liow, J.S.; Zoghbi, S.S.; Kowalski, A.; Tye, G.L.; Innis, R.B.; Aigbirhio, F.I.; et al. [Carboxyl-11 C]labelling of four high-affinity cPLA2α inhibitors and their evaluation as radioligands in mice by positron emission tomography. ChemMedChem 2018, 13, 138–146. [Google Scholar] [CrossRef]
- Natarajan, R.; Rappa, J.P.; Unnikrishnan, P.A.; Radhaman, S.; Prathapan, S. A new method for the synthesis of 3-substituted indoles. Synlett 2015, 26, 2467–2471. [Google Scholar]
- Zhang, Z.W.; Xue, H.; Li, H.; Kang, H.; Feng, J.; Lin, A.; Liu, S. Collective synthesis of 3-acylindoles, indole-3-carboxylic esters, indole-3-sulfinic acids, and 3-(methylsulfonyl)indoles from free (N-H) indoles via common N-indolyl triethylborate. Org. Lett. 2016, 18, 3918–3921. [Google Scholar] [CrossRef]
- Ji, X.; Huang, H.; Wu, W.; Li, X.; Jiang, H. Palladium-catalyzed oxidative coupling of aromatic primary amines and alkenes under molecular oxygen: Stereoselective assembly of (Z)-enamines. J. Org. Chem. 2013, 38, 11155–11162. [Google Scholar] [CrossRef]
- Rashad, M.; La Vecchia, L.; Prasad, K.; Repic, O. A convenient synthesis of 3-substituted 1H-indoles. Synth. Commun. 1995, 25, 95–100. [Google Scholar] [CrossRef]
- Yoo, W.J.; Capdevila, M.G.; Du, X.; Kobayashi, S. Base-mediated carboxylation of unprotected indole derivatives with carbon dioxide. Org. Lett. 2012, 14, 5326–5329. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 6a–ad are available from the authors. |







| entry | DBACO | Temperature | Time | Yield (6a) |
|---|---|---|---|---|
| 1 | 10 mol% | RT | 1 h | 68% |
| 2 | 10 mol% | 0 °C to RT | 1 h | 83% |
| 3 | 1 mol% | 0 °C to RT | Overnight | 75% |
| 4 | 5 mol% | 0 °C to RT | 1 h | 82% |
| 5 | 5 mol% | −25 °C to RT | 2 h | 86% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejedor, D.; Diana-Rivero, R.; García-Tellado, F. A General and Scalable Synthesis of Polysubstituted Indoles. Molecules 2020, 25, 5595. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25235595
Tejedor D, Diana-Rivero R, García-Tellado F. A General and Scalable Synthesis of Polysubstituted Indoles. Molecules. 2020; 25(23):5595. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25235595
Chicago/Turabian StyleTejedor, David, Raquel Diana-Rivero, and Fernando García-Tellado. 2020. "A General and Scalable Synthesis of Polysubstituted Indoles" Molecules 25, no. 23: 5595. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25235595