Recent Strategies and Applications for l-Asparaginase Confinement
Abstract
:1. Introduction
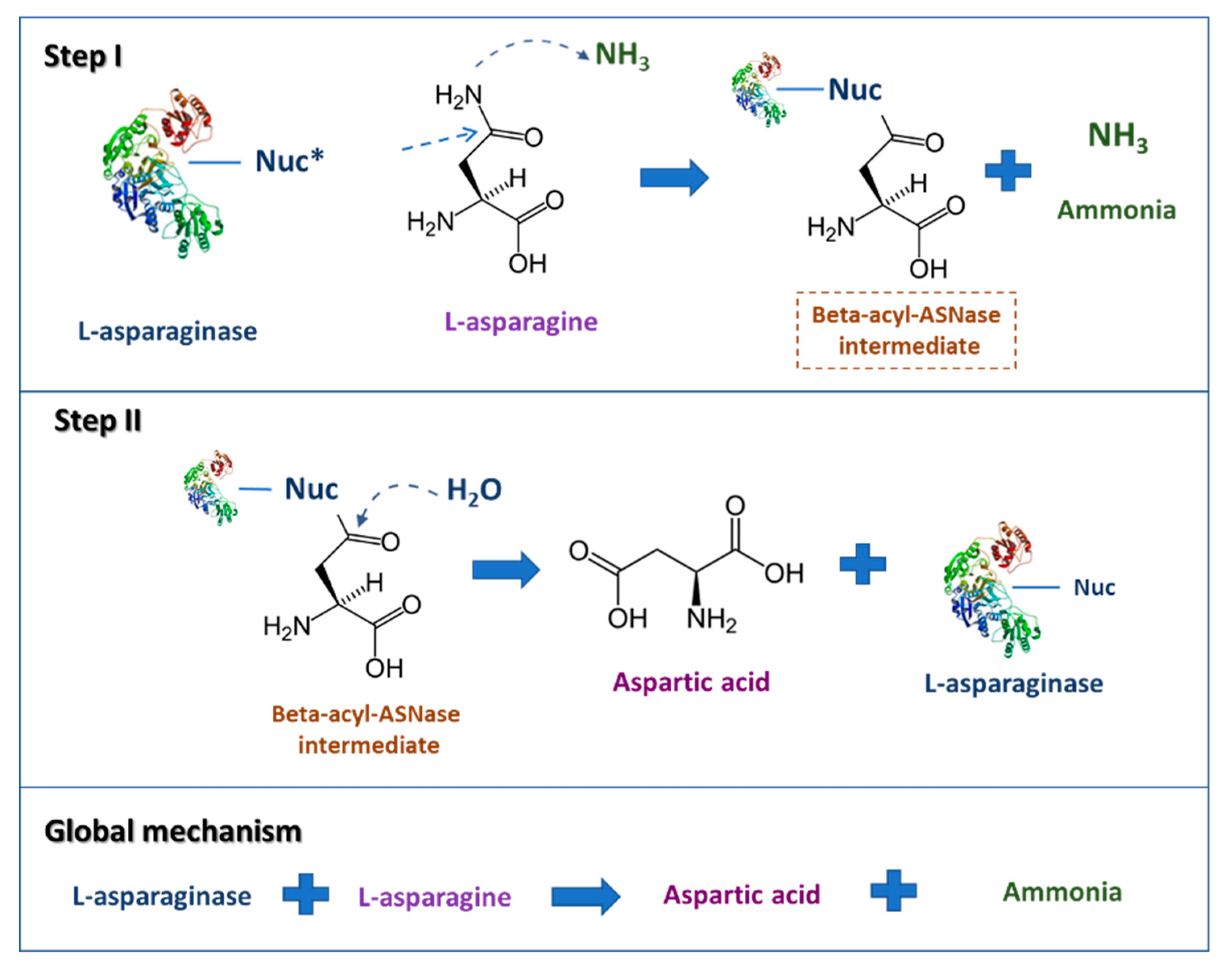
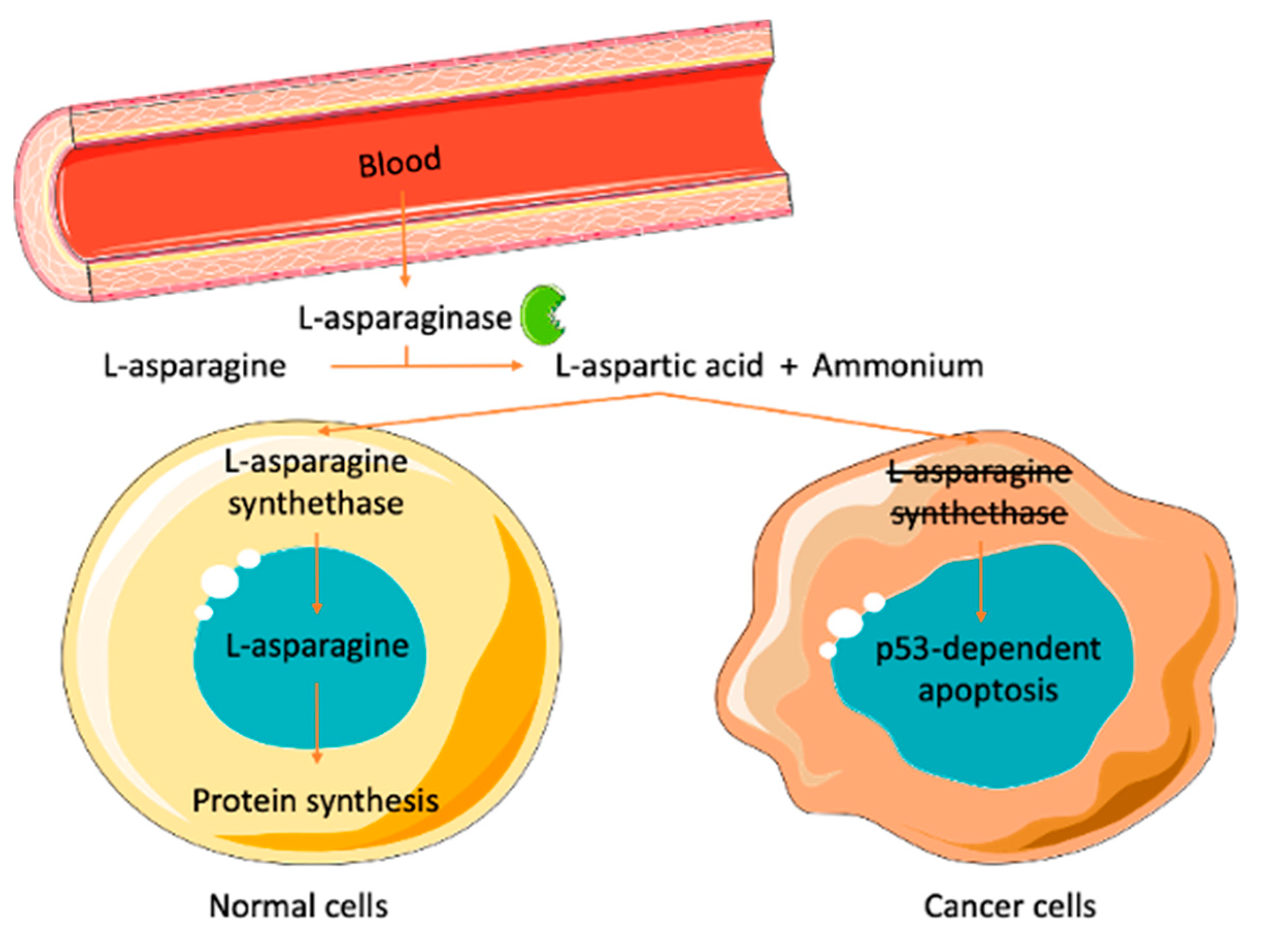
2. l-Asparaginase
Commercial ASNase
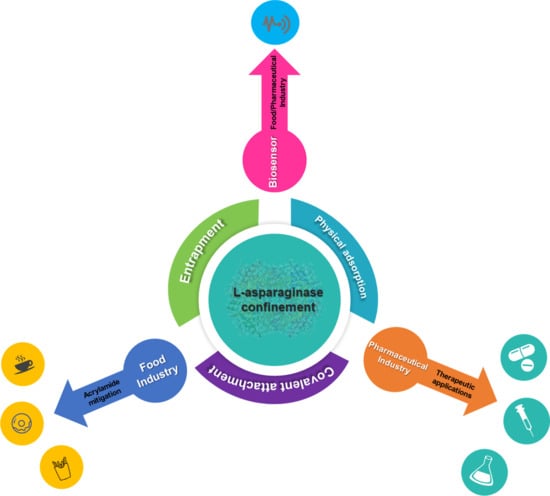
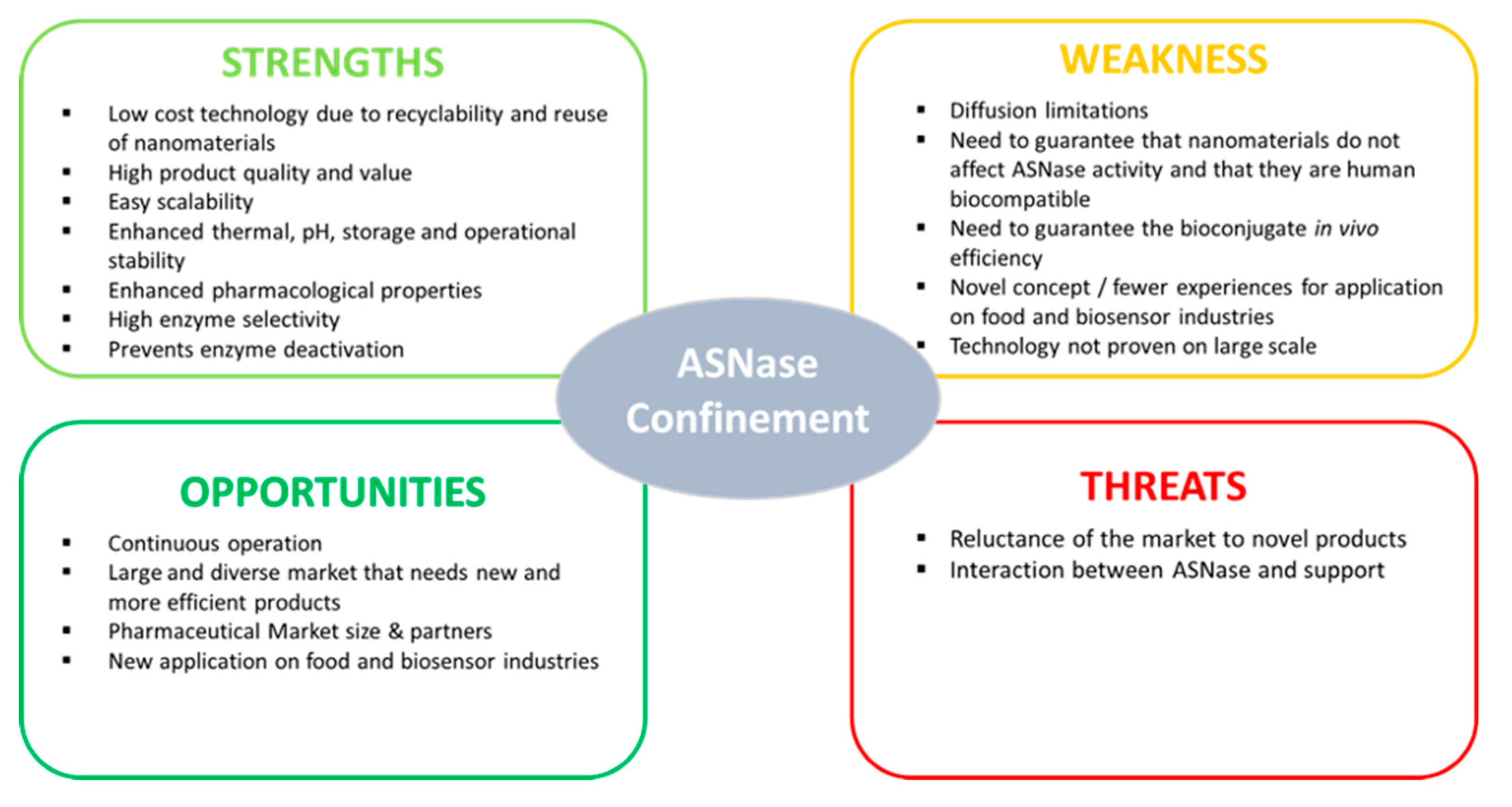
3. Types of ASNase Confinement
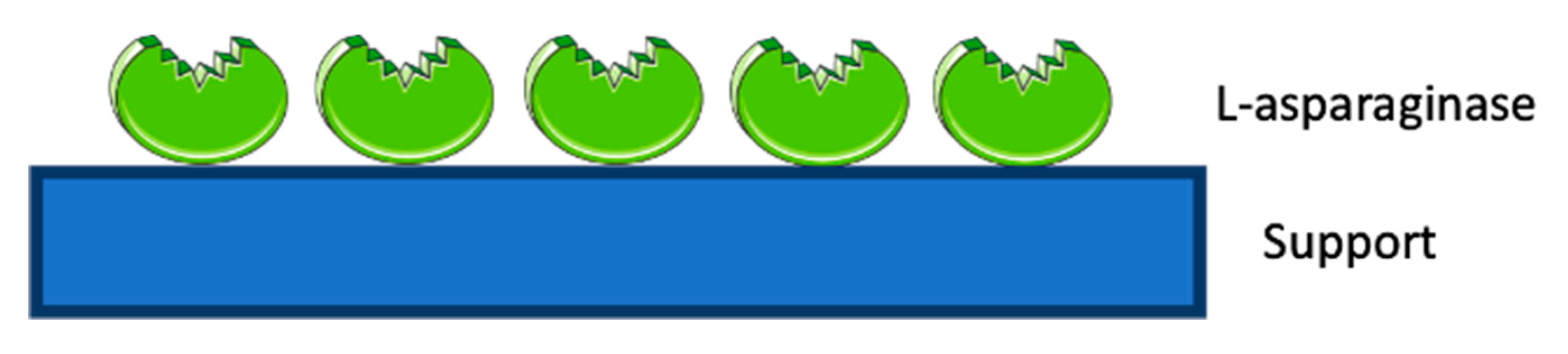
3.1. ASNase Confinement by Physical Adsorption
3.2. ASNase Confinement by Covalent Attachment
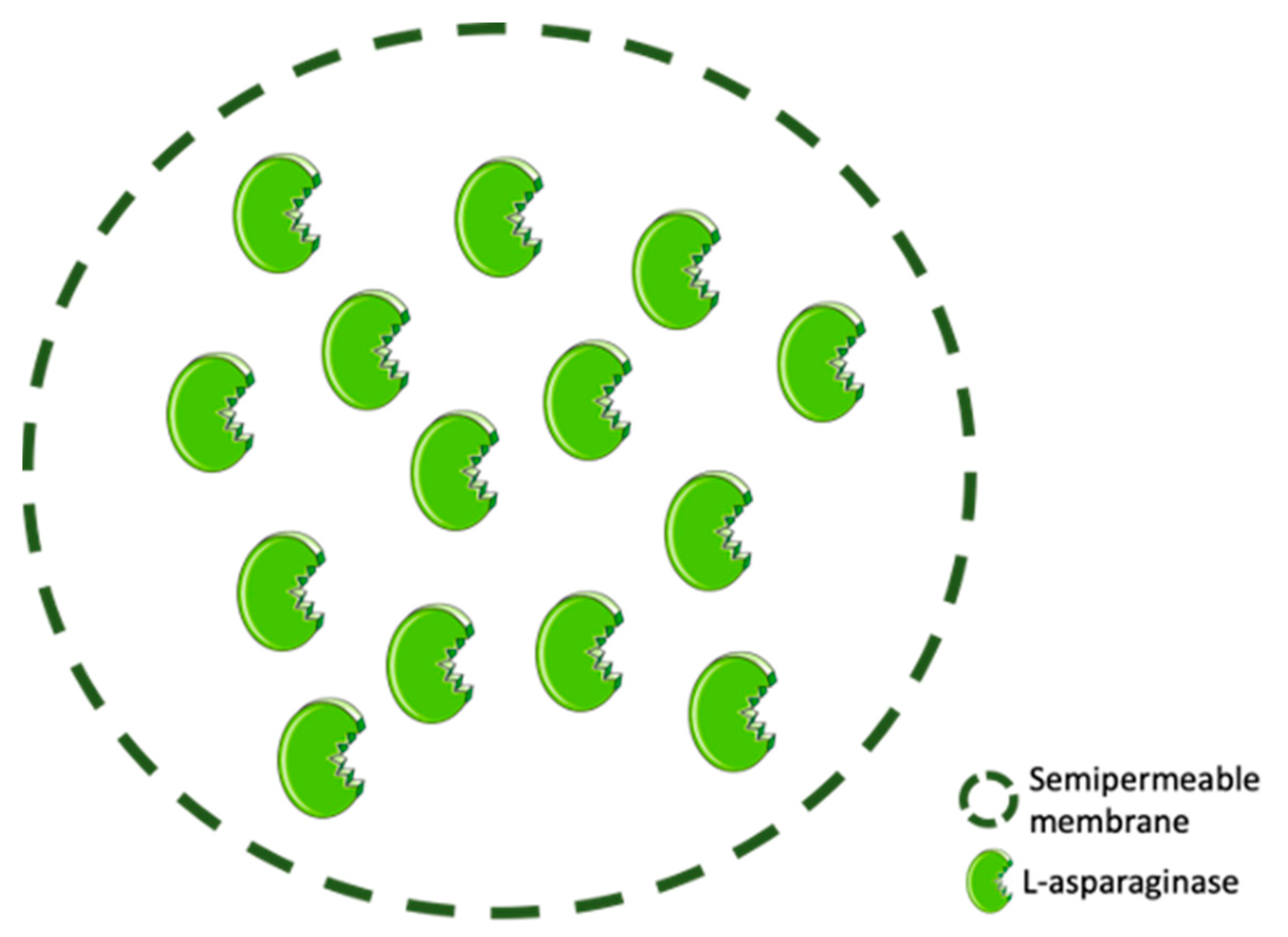
3.3. ASNase Confinement by Entrapment
4. Applications of Confined ASNase
4.1. Therapeutic Applications
4.2. Food Applications
4.3. Biosensor Applications
5. Conclusions and Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Radha, R.; Arumugam, N.; Gummadi, S.N. Glutaminase free l-asparaginase from Vibrio cholerae: Heterologous expression, purification and biochemical characterization. Int. J. Biol. Macromol. 2018, 111, 129–138. [Google Scholar] [CrossRef]
- Kumar, S.; Venkata Dasu, V.; Pakshirajan, K. Purification and characterization of glutaminase-free l-asparaginase from Pectobacterium carotovorum MTCC 1428. Bioresour. Technol. 2011, 102, 2077–2082. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Asad, S.; Kabiri, M.; Dabirmanesh, B. Cloning and characterization of Halomonas elongata l-asparaginase, a promising chemotherapeutic agent. Appl. Microbiol. Biotechnol. 2017, 101, 7227–7238. [Google Scholar] [CrossRef]
- Karpel-Massler, G.; Ramani, D.; Shu, C.; Halatsch, M.-E.; Westhoff, M.-A.; Bruce, J.N.; Canoll, P.; Siegelin, M.D. Metabolic reprogramming of glioblastoma cells by l-asparaginase sensitizes for apoptosis in vitro and in vivo. Oncotarget 2016, 7, 33512–33528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidd, J.G. Regression of transplanted lymphomas induced in vivo by means of normal guinea pig serum. I. Course of transplanted cancers of various kinds in mice and rats given guinea pig serum, horse serum, or rabbit serum. J. Exp. Med. 1953, 98, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Kidd, J.G. Regression of transplanted lymphomas induced in vivo by means of normal guinea pig serum. II. Studies on the nature of the active serum constituent: Histological mechanism of the regression: Tests for effects of guinea pig serum on lymphoma cells in vitro. J. Exp. Med. 1953, 98, 583–606. [Google Scholar] [CrossRef] [PubMed]
- Clementi, A. La Désamidation Enzymatique De L’asparagine Chez Les Différentes Espéces Animales Et La Signification Physio Logique De Sa Presence Dans L’organisme. Arch. Int. Physiol. 1922, 19, 369–398. (In French) [Google Scholar] [CrossRef]
- Broome, J.D. Evidence that the l-asparaginase activity of guinea pig serum is responsible for its antilymphoma effects. Nature 1961, 191, 1114–1115. [Google Scholar] [CrossRef]
- Panosyan, E.H.; Seibel, N.L.; Martin-Aragon, S.; Gaynon, P.S.; Avramis, I.A.; Sather, H.; Franklin, J.; Nachman, J.; Ettinger, L.J.; La, M.; et al. Asparaginase antibody and asparaginase activity in children with higher-risk acute lymphoblastic leukemia: Children’s cancer group study CCG-1961. J. Pediatr. Hematol. Oncol. 2004, 26, 217–226. [Google Scholar] [CrossRef]
- Van der Sluis, I.M.; Vrooman, L.M.; Pieters, R.; Baruchel, A.; Escherich, G.; Goulden, N.; Mondelaers, V.; Sanchez de Toledo, J.; Rizzari, C.; Silverman, L.B.; et al. Consensus expert recommendations for identification and management of asparaginase hypersensitivity and silent inactivation. Haematologica 2016, 101, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Danks, M.K.; Yoon, K.J.; Bush, R.A.; Remack, J.S.; Wierdl, M.; Tsurkan, L.; Kim, S.U.; Garcia, E.; Metz, M.Z.; Najbauer, J.; et al. Tumor-targeted enzyme/prodrug therapy mediates long-term disease-free survival of mice bearing disseminated neuroblastoma. Cancer Res. 2007, 67, 22–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.-Q.; Tao, M.-L.; Shen, W.-D.; Zhou, Y.-Z.; Ding, Y.; Ma, Y.; Zhou, W.-L. Immobilization of l-asparaginase on the microparticles of the natural silk sericin protein and its characters. Biomaterials 2004, 25, 3751–3759. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Oruna-Concha, M.-J.; Elmore, J.S. The use of asparaginase to reduce acrylamide levels in cooked food. Food Chem. 2016, 210, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Zhang, T.; Jiang, B.; Mu, W. Reduction of acrylamide level through blanching with treatment by an extremely thermostable l-asparaginase during French fries processing. Extremophiles 2015, 19, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Zyzak, D.V.; Sanders, R.A.; Stojanovic, M.; Tallmadge, D.H.; Eberhart, B.L.; Ewald, D.K.; Gruber, D.C.; Morsch, T.R.; Strothers, M.A.; Rizzi, G.P.; et al. Acrylamide formation mechanism in heated foods. J. Agric. Food Chem. 2003, 51, 4782–4787. [Google Scholar] [CrossRef] [PubMed]
- Safety Evaluation of Certain Food Additives and Contaminants, Who Food Additive Series; World Health Organization: Geneva, Switzerland, 2008.
- Safety Evaluation of Certain Food Additives and Contaminants, Who Food Additive Series; World Health Organization: Geneva, Switzerland, 2009.
- Friedman, M. Acrylamide: Inhibition of formation in processed food and mitigation of toxicity in cells, animals, and humans. Food Funct. 2015, 6, 1752–1772. [Google Scholar] [CrossRef] [PubMed]
- Batool, T.; Makky, E.A.; Jalal, M.; Yusoff, M.M. A comprehensive review on l-asparaginase and its applications. Appl. Biochem. Biotechnol. 2016, 178, 900–923. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.; Kataria, M.; Verma, N. Plant asparaginase-based asparagine biosensor for leukemia. Artif. Cells Nanomed. Biotechnol. 2013, 41, 184–188. [Google Scholar] [CrossRef]
- Anastasescu, C.; Preda, S.; Rusu, A.; Culita, D.; Plavan, G.; Strungaru, S.; Calderon-Moreno, J.M.; Munteanu, C.; Gifu, C.; Enache, M.; et al. Tubular and spherical SiO2 obtained by sol gel method for lipase immobilization and enzymatic activity. Molecules 2018, 23, 1362. [Google Scholar] [CrossRef] [Green Version]
- Bodakowska-Boczniewicz, J.; Garncarek, Z. Immobilization of naringinase from aspergillus niger on a magnetic polysaccharide carrier. Molecules 2020, 25, 2731. [Google Scholar] [CrossRef]
- Ansari, S.A.; Husain, Q. Potential applications of enzymes immobilized on/in nano materials: A review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Kushagri, S.; Abha, M.; Deepankar, S.; Kavita, S. Nanotechnology in enzyme immobilization: An overview on enzyme immobilization with nanoparticle matrix. Curr. Nanosci. 2019, 15, 234–241. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization-purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernal, C.; Rodríguez, K.; Martínez, R. Integrating enzyme immobilization and protein engineering: An alternative path for the development of novel and improved industrial biocatalysts. Biotechnol. Adv. 2018, 36, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Bosio, V.E.; Islan, G.A.; Martínez, Y.N.; Durán, N.; Castro, G.R. Nanodevices for the immobilization of therapeutic enzymes. Crit. Rev. Biotechnol. 2016, 36, 447–464. [Google Scholar] [CrossRef]
- Clark, D.S. Can immobilization be exploited to modify enzyme activity? Trends Biotechnol. 1994, 12, 439–443. [Google Scholar] [CrossRef]
- Brumano, L.P.; da Silva, F.V.S.; Costa-Silva, T.A.; Apolinário, A.C.; Santos, J.H.P.M.; Kleingesinds, E.K.; Monteiro, G.; de Rangel-Yagui, C.O.; Benyahia, B.; Junior, A.P. Development of l-asparaginase biobetters: Current research status and review of the desirable quality profiles. Front. Bioeng. Biotechnol. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Ulu, A.; Ates, B. Immobilization of l-asparaginase on carrier materials: A comprehensive review. Bioconjug. Chem. 2017, 28, 1598–1610. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Rasoul-Amini, S.; Ghasemi, Y. l-Asparaginase production by moderate halophilic bacteria isolated from maharloo salt lake. Indian J. Microbiol. 2011, 51, 307–311. [Google Scholar] [CrossRef] [Green Version]
- De Moura Sarquis, M.I.; Oliveira, E.M.M.; Santos, A.S.; da Costa, G.L. Production of l-asparaginase by filamentous fungi. Mem. Inst. Oswaldo Cruz 2004, 99, 489–492. [Google Scholar] [CrossRef] [Green Version]
- Peterson, R.E.; Ciegler, A. l-Asparaginase production by various bacteria. Appl. Microbiol. 1969, 17, 929–930. [Google Scholar] [CrossRef] [PubMed]
- El-Bessoumy, A.A.; Sarhan, M.; Mansour, J. Production, isolation, and purification of l-asparaginase from pseudomonas aeruginosa 50071 using solid-state fermentation. J. Biochem. Mol. Biol. 2004, 37, 387–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosa, T.; Sano, R.; Yamamoto, K.; Nakamura, M.; Ando, K. l-Asparaginase from proteus vulgaris. Appl. Microbiol. 1971, 22, 387–392. [Google Scholar] [CrossRef] [PubMed]
- DeJong, P.J. l-Asparaginase production by streptomyces griseus. Appl. Microbiol. 1972, 23, 1163–1164. [Google Scholar] [CrossRef]
- Kafkewitz, D.; Goodman, D. l-Asparaginase production by the rumen anaerobe vibrio succinogenes. Appl. Microbiol. 1974, 27, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.V.; Saran, S.; Kameswaran, K.; Kumar, V.; Saxena, R.K. Efficient production of l-asparaginase from Bacillus licheniformis with low-glutaminase activity: Optimization, scale up and acrylamide degradation studies. Bioresour. Technol. 2012, 125, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Kenari, S.L.D.; Alemzadeh, I.; Maghsodi, V. Production of l-asparaginase from Escherichia coli ATCC 11303: Optimization by response surface methodology. Food Bioprod. Process. 2011, 89, 315–321. [Google Scholar] [CrossRef]
- Schwartz, J.H.; Reeves, J.Y.; Broome, J.D. Two l-asparaginases from E. coli and their action against tumors. Proc. Natl. Acad. Sci. USA 1966, 56, 1516–1519. [Google Scholar] [CrossRef] [Green Version]
- Keating, G.M. Asparaginase erwinia chrysanthemi (Erwinaze®): A guide to its use in acute lymphoblastic leukemia in the USA. BioDrugs 2013, 27, 413–418. [Google Scholar] [CrossRef]
- Emadi, A.; Zokaee, H.; Sausville, E.A. Asparaginase in the treatment of non-ALL hematologic malignancies. Cancer Chemother. Pharmacol. 2014, 73, 875–883. [Google Scholar] [CrossRef]
- Avramis, V.I. Asparaginases: Biochemical pharmacology and modes of drug resistance. Anticancer Res. 2012, 32, 2423–2437. [Google Scholar] [PubMed]
- Covini, D.; Tardito, S.; Bussolati, O.; Chiarelli, L.R.; Pasquetto, M.V.; Digilio, R.; Valentini, G.; Scotti, C. Expanding targets for a metabolic therapy of cancer: l-Asparaginase. Recent Pat. Anticancer Drug Discov. 2011, 7, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Krasotkina, J.; Borisova, A.A.; Gervaziev, Y.V.; Sokolov, N.N. One-step purification and kinetic properties of the recombinant l-asparaginase from Erwinia carotovora. Biotechnol. Appl. Biochem. 2004, 39, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Campbell, H.A.; Mashburn, L.T.; Boyse, E.A.; Old, L.J. Two l-asparaginases from Escherichia coli B. Their separation, purification, and antitumor activity. Biochemistry 1967, 6, 721–730. [Google Scholar] [CrossRef]
- Verma, N.; Kumar, K.; Kaur, G.; Anand, S. l-Asparaginase: A promising chemotherapeutic agent. Crit. Rev. Biotechnol. 2007, 27, 45–62. [Google Scholar] [CrossRef]
- Yun, M.-K.; Nourse, A.; White, S.W.; Rock, C.O.; Heath, R.J. Crystal structure and allosteric regulation of the cytoplasmic Escherichia coli l-asparaginase I. J. Mol. Biol. 2007, 369, 794–811. [Google Scholar] [CrossRef] [Green Version]
- Aghaiypour, K.; Wlodawer, A.; Lubkowski, J. Structural basis for the activity and substrate specificity of erwinia chrysanthemi l-asparaginase. Biochemistry 2001, 40, 5655–5664. [Google Scholar] [CrossRef]
- Shakambari, G.; Ashokkumar, B.; Varalakshmi, P. l-Asparaginase—A promising biocatalyst for industrial and clinical applications. Biocatal. Agric. Biotechnol. 2019, 17, 213–224. [Google Scholar] [CrossRef]
- Hill, J.M.; Roberts, J.; Loeb, E.; Khan, A.; MacLellan, A.; Hill, R.W. l-Asparaginase therapy for leukemia and other malignant neoplasms. Remission in human leukemia. JAMA 1967, 202, 882–888. [Google Scholar] [CrossRef]
- Elspar® (Asparaginase); Merck & Co., Inc.: Riverside, PA, USA, 2000.
- Chand, S.; Mahajan, R.V.; Prasad, J.P.; Sahoo, D.K.; Mihooliya, K.N.; Dhar, M.S.; Sharma, G. A comprehensive review on microbial l-asparaginase: Bioprocessing, characterization, and industrial applications. Biotechnol. Appl. Biochem. 2020, 67, 619–647. [Google Scholar] [CrossRef]
- Assessment Report Oncaspar; European Medicines Agency: London, UK, 2016.
- Assessment Report Spectrila; European Medicines Agency: London, UK, 2015.
- Public Assessment Report Crisantaspase; Medicines Evaluation Board: Utrecht, The Netherlands, 2015.
- Dinndorf, P.A.; Gootenberg, J.; Cohen, M.H.; Keegan, P.; Pazdur, R. FDA drug approval summary: Pegaspargase (Oncaspar®) for the first-line treatment of children with acute Lymphoblastic Leukemia (ALL). Oncologist 2007, 12, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Costa-Silva, T.A.; Costa, I.M.; Biasoto, H.P.; Lima, G.M.; Silva, C.; Pessoa, A.; Monteiro, G. Critical overview of the main features and techniques used for the evaluation of the clinical applicability of L-asparaginase as a biopharmaceutical to treat blood cancer. Blood Rev. 2020, 43, 100651. [Google Scholar] [CrossRef] [PubMed]
- BC Cancer Drug Manual. Available online: http://www.cdha.nshealth.ca/nova-scotia-cancer-care-program-25 (accessed on 9 December 2020).
- Krishnakumar, T.; Visvanathan, R. Acrylamide in food products: A review. J. Food Process. Technol. 2014, 5, 344. [Google Scholar] [CrossRef] [Green Version]
- Morales, F.; Capuano, E.; Fogliano, V. Mitigation strategies to reduce acrylamide formation in fried potato products. Ann. N. Y. Acad. Sci. 2008, 1126, 89–100. [Google Scholar] [CrossRef] [PubMed]
- JECFA. Compendium of Food Additive Specifications; Food and Agriculture Organization of the United Nations: Rome, Italy, 2007. [Google Scholar]
- Arana-Peña, S.; Carballares, D.; Morellon-Sterlling, R.; Berenguer-Murcia, Á.; Alcántara, A.R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Enzyme co-immobilization: Always the biocatalyst designers’ choice or not? Biotechnol. Adv. 2020, 107584. [Google Scholar] [CrossRef] [PubMed]
- Dunker, A.K.; Fernández, A. Engineering productive enzyme confinement. Trends Biotechnol. 2007, 25, 189–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosa, T.; Mori, T.; Fuse, N.; Chibata, I. Studies on continuous enzyme reactions. I. Screening of carriers for preparation of water-insoluble aminoacylase. Agric. Biol. Chem. 1969, 33, 1047–1059. [Google Scholar] [CrossRef]
- Brena, B.; González-Pombo, P.; Batista-Viera, F. Immobilization of enzymes: A literature survey. Immobil. Enzym. Cells 2013, 1051, 15–31. [Google Scholar] [CrossRef]
- Da Silva, A.M.; Tavares, A.P.M.; Rocha, C.M.R.; Cristóvão, R.O.; Teixeira, J.A.; Macedo, E.A. Immobilization of commercial laccase on spent grain. Process. Biochem. 2012, 47, 1095–1101. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.N.; Mattiasson, B. Unique applications of immobilized proteins in bioanalytical systems. Methods Biochem. Anal. 1992, 36, 1–34. [Google Scholar] [CrossRef]
- Vahidnia, M.; Pazuki, G.; Abdolrahimi, S. Impact of polyethylene glycol as additive on the formation and extraction behavior of ionic-liquid based aqueous two-phase system. AIChE J. 2016, 62, 264–274. [Google Scholar] [CrossRef]
- Da Silva Barbosa, G.S.; Oliveira, M.E.P.S.; dos Santos, A.B.S.; Sánchez, O.C.; Soares, C.M.F.; Fricks, A.T. Immobilization of low-cost alternative vegetable peroxidase (Raphanus sativus L. peroxidase): Choice of support/technique and characterization. Molecules 2020, 25, 3668. [Google Scholar] [CrossRef]
- Monajati, M.; Borandeh, S.; Hesami, A.; Mansouri, D.; Tamaddon, A.M. Immobilization of l-asparaginase on aspartic acid functionalized graphene oxide nanosheet: Enzyme kinetics and stability studies. Chem. Eng. J. 2018, 354, 1153–1163. [Google Scholar] [CrossRef]
- Haroun, A.A.; Ahmed, H.M.; Mossa, A.-T.H.; Mohafrash, S.M.; Ahmed, E.F. Production, characterization and immobilization of Aspergillus versicolor l-asparaginase onto multi-walled carbon nanotubes. Biointerface Res. Appl. Chem. 2020, 10, 5733–5740. [Google Scholar] [CrossRef]
- Cristóvão, R.O.; Almeida, M.R.; Barros, M.A.; Nunes, J.C.F.; Boaventura, R.A.R.; Loureiro, J.M.; Faria, J.L.; Neves, M.C.; Freire, M.G.; Ebinuma-Santos, V.C.; et al. Development and characterization of a novel l-asparaginase/MWCNT nanobioconjugate. RSC Adv. 2020, 10, 31205–31213. [Google Scholar] [CrossRef]
- Tarhan, T.; Ulu, A.; Sariçam, M.; Çulha, M.; Ates, B. Maltose functionalized magnetic core/shell Fe3O4@Au nanoparticles for an efficient l-asparaginase immobilization. Int. J. Biol. Macromol. 2020, 142, 443–451. [Google Scholar] [CrossRef]
- Golestaneh, D.; Varshosaz, J. Enhancement in biological activity of l-asparginase by its conjugation on silica nanoparticles. Recent Pat. Nanotechnol. 2018, 12, 70–82. [Google Scholar] [CrossRef]
- Baskar, G.; Garrick, B.G.; Lalitha, K.; Chamundeeswari, M. Gold nanoparticle mediated delivery of fungal asparaginase against cancer cells. J. Drug Deliv. Sci. Technol. 2018, 44, 498–504. [Google Scholar] [CrossRef]
- Agrawal, S.; Kango, N. Development and catalytic characterization of l-asparaginase nano-bioconjugates. Int. J. Biol. Macromol. 2019, 135, 1142–1150. [Google Scholar] [CrossRef]
- Agrawal, S.; Sharma, I.; Prajapati, B.P.; Suryawanshi, R.K.; Kango, N. Catalytic characteristics and application of l-asparaginase immobilized on aluminum oxide pellets. Int. J. Biol. Macromol. 2018, 114, 504–511. [Google Scholar] [CrossRef]
- Ates, B.; Ulu, A.; Köytepe, S.; Ali Noma, S.A.; Kolat, V.S.; Izgi, T. Magnetic-propelled Fe3O4-chitosan carriers enhance l-asparaginase catalytic activity: A promising strategy for enzyme immobilization. RSC Adv. 2018, 8, 36063–36075. [Google Scholar] [CrossRef] [Green Version]
- Ulu, A.; Ozcan, I.; Koytepe, S.; Ates, B. Design of epoxy-functionalized Fe3O4@MCM-41 core-shell nanoparticles for enzyme immobilization. Int. J. Biol. Macromol. 2018, 115, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Ulu, A.; Noma, S.A.A.; Koytepe, S.; Ates, B. Chloro-modified magnetic Fe3O4 @MCM-41 core-shell nanoparticles for l-asparaginase immobilization with improved catalytic activity, reusability, and storage stability. Appl. Biochem. Biotechnol. 2018, 187, 938–956. [Google Scholar] [CrossRef] [PubMed]
- Orhan, H.; Aktaş Uygun, D. Immobilization of l-asparaginase on magnetic nanoparticles for cancer treatment. Appl. Biochem. Biotechnol. 2020, 191, 1432–1443. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Ahmad, R.; Pranaw, K.; Mishra, P.; Khare, S.K. Asparaginase conjugated magnetic nanoparticles used for reducing acrylamide formation in food model system. Bioresour. Technol. 2018, 269, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Baskar, G.; Lalitha, K.; Aiswarya, R.; Naveenkumar, R. Synthesis, characterization and synergistic activity of cerium-selenium nanobiocomposite of fungal l-asparaginase against lung cancer. Mater. Sci. Eng. C 2018, 93, 809–815. [Google Scholar] [CrossRef]
- Baskar, G.; Supria Sree, N. Synthesis, characterization and anticancer activity of β-cyclodextrin-asparaginase nanobiocomposite on prostate and lymphoma cancer cells. J. Drug Deliv. Sci. Technol. 2020, 55, 101417. [Google Scholar] [CrossRef]
- Baskar, G.; Supria Sree, N. Anticancer activity of gelatin-asparaginase nanobiocomposite against cervical and brain cancer cell lines. J. Drug Deliv. Sci. Technol. 2020, 57, 101689. [Google Scholar] [CrossRef]
- Abd El-Baky, H.H.; El-Baroty, G.S. Spirulina maxima l-asparaginase: Immobilization, antiviral and antiproliferation activities. Recent Pat. Biotechnol. 2020, 14, 154–163. [Google Scholar] [CrossRef]
- Ashok, A.; Devarai, S.K. l-Asparaginase production in rotating bed reactor from Rhizopus microsporus IBBL-2 using immobilized Ca-alginate beads. 3 Biotech. 2019, 9, 349. [Google Scholar] [CrossRef]
- De Brito, A.E.M.; Pessoa, A.; Converti, A.; de Rangel-Yagui, C.O.; da Silva, J.A.; Apolinário, A.C. Poly (lactic-co-glycolic acid) nanospheres allow for high l-asparaginase encapsulation yield and activity. Mater. Sci. Eng. C 2019, 98, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Tinoco, A.; Sárria, M.P.; Loureiro, A.; Parpot, P.; Espiña, B.; Gomes, A.C.; Cavaco-Paulo, A.; Ribeiro, A. BSA/ASN/Pol407 nanoparticles for acute lymphoblastic leukemia treatment. Biochem. Eng. J. 2019, 141, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Possarle, L.H.R.R.; Siqueira Junior, J.R.; Caseli, L. Insertion of carbon nanotubes in Langmuir-Blodgett films of stearic acid and asparaginase enhancing the catalytic performance. Colloids Surf. B Biointerfaces 2020, 192, 111032. [Google Scholar] [CrossRef] [PubMed]
- Ulu, A.; Karaman, M.; Yapıcı, F.; Naz, M.; Sayın, S.; Saygılı, E.İ.; Ateş, B. The carboxylated multi-walled carbon nanotubes/l-asparaginase doped calcium-alginate beads: Structural and biocatalytic characterization. Catal. Lett. 2020, 150, 1679–1691. [Google Scholar] [CrossRef]
- Cristóvão, R.O.; Tavares, A.P.M.; Brígida, A.I.; Loureiro, J.M.; Boaventura, R.A.R.; Macedo, E.A.; Coelho, M.A.Z. Immobilization of commercial laccase onto green coconut fiber by adsorption and its application for reactive textile dyes degradation. J. Mol. Catal. B Enzym. 2011, 72, 6–12. [Google Scholar] [CrossRef]
- Flickinger, M.C.; Drew, S.W. Fermentation, biocatalysis and bioseparation. Encycl. Bioprocess. Technol. 1999. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Azevedo, R.M.; Costa, J.B.; Serp, P.; Loureiro, J.M.; Faria, J.L.; Silva, C.G.; Tavares, A.P.M. A strategy for improving peroxidase stability via immobilization on surface modified multi-walled carbon nanotubes. J. Chem. Technol. Biotechnol. 2015, 90, 1570–1578. [Google Scholar] [CrossRef]
- Costa, J.B.; Lima, M.J.; Sampaio, M.J.; Neves, M.C.; Faria, J.L.; Morales-Torres, S.; Tavares, A.P.M.; Silva, C.G. Enhanced biocatalytic sustainability of laccase by immobilization on functionalized carbon nanotubes/polysulfone membranes. Chem. Eng. J. 2019, 355, 974–985. [Google Scholar] [CrossRef]
- Tavares, A.P.M.; Silva, C.G.; Dražić, G.; Silva, A.M.T.; Loureiro, J.M.; Faria, J.L. Laccase immobilization over multi-walled carbon nanotubes: Kinetic, thermodynamic and stability studies. J. Colloid Interface Sci. 2015, 454, 52–60. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Leng, J.; Yang, X.; Liao, L.; Liu, L.; Xiao, A. Enhanced performance of magnetic graphene oxide-immobilized laccase and its application for the decolorization of dyes. Molecules 2017, 22, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.P.; Herrera, C.E.; Singh, B.; Singh, S.; Singh, R.K.; Kumar, R. Graphene oxide: An efficient material and recent approach for biotechnological and biomedical applications. Mater. Sci. Eng. C 2018, 86, 173–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, F.; Yang, H.; Huang, X.; Liu, H.; Zhang, J.; Guo, S. Graphene oxide as a matrix for enzyme immobilization. Langmuir 2010, 26, 6083–6085. [Google Scholar] [CrossRef]
- Simon, J.; Flahaut, E.; Golzio, M. Overview of carbon nanotubes for biomedical applications. Materials 2019, 12, 624. [Google Scholar] [CrossRef] [Green Version]
- Neves, V.; Heister, E.; Costa, S.; Tîlmaciu, C.; Flahaut, E.; Soula, B.; Coley, H.M.; McFadden, J.; Silva, S.R.P. Design of double-walled carbon nanotubes for biomedical applications. Nanotechnology 2012, 23. [Google Scholar] [CrossRef] [Green Version]
- Madani, S.Y.; Mandel, A.; Seifalian, A.M. A concise review of carbon nanotube’s toxicology. Nano Rev. 2013, 4, 21521. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.F.; Vashist, S.K.; Al-Rubeaan, K.; Luong, J.H.T.; Sheu, F.S. Interfacing carbon nanotubes with living mammalian cells and cytotoxicity issues. Chem. Res. Toxicol. 2010, 23, 1131–1147. [Google Scholar] [CrossRef]
- Chen, X.; Tam, U.C.; Czlapinski, J.L.; Lee, G.S.; Rabuka, D.; Zettl, A.; Bertozzi, C.R. Interfacing carbon nanotubes with living cells. J. Am. Chem. Soc. 2006, 128, 6292–6293. [Google Scholar] [CrossRef]
- Dumortier, H.; Lacotte, S.; Pastorin, G.; Marega, R.; Wu, W.; Bonifazi, D.; Briand, J.-P.; Prato, M.; Muller, S.; Bianco, A. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett. 2006, 6, 1522–1528. [Google Scholar] [CrossRef]
- Sayes, C.M.; Liang, F.; Hudson, J.L.; Mendez, J.; Guo, W.; Beach, J.M.; Moore, V.C.; Doyle, C.D.; West, J.L.; Billups, W.E.; et al. Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol. Lett. 2006, 161, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.A.; Daniel-da-Silva, A.L.; Tavares, A.P.M.; Xavier, A.M.R.B. EDTA-Cu (II) chelating magnetic nanoparticles as a support for laccase immobilization. Chem. Eng. Sci. 2017, 158, 599–605. [Google Scholar] [CrossRef]
- Fortes, C.C.S.; Daniel-da-Silva, A.L.; Xavier, A.M.R.B.; Tavares, A.P.M. Optimization of enzyme immobilization on functionalized magnetic nanoparticles for laccase biocatalytic reactions. Chem. Eng. Process. Process. Intensif. 2017, 117, 1–8. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Altintas, B.; Yilmaz, M.; Arica, M.Y. Immobilization of chloroperoxidase onto highly hydrophilic polyethylene chains via bio-conjugation: Catalytic properties and stabilities. Bioresour. Technol. 2011, 102, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Cristóvão, R.O.; Silvério, S.C.; Tavares, A.P.M.; Brígida, A.I.S.; Loureiro, J.M.; Boaventura, R.A.R.; Macedo, E.A.; Coelho, M.A.Z. Green coconut fiber: A novel carrier for the immobilization of commercial laccase by covalent attachment for textile dyes decolourization. World J. Microbiol. Biotechnol. 2012, 28, 2827–2838. [Google Scholar] [CrossRef]
- Pereira, M.G.; Velasco-Lozano, S.; Moreno-Perez, S.; Polizeli, A.M.; Heinen, P.R.; Facchini, F.D.A.; Vici, A.C.; Cereia, M.; Pessela, B.C.; Fernandez-Lorente, G.; et al. Different covalent immobilizations modulate lipase activities of hypocrea pseudokoningii. Molecules 2017, 22, 1448. [Google Scholar] [CrossRef]
- Hussain, F.; Arana-Peña, S.; Morellon-Sterling, R.; Barbosa, O.; Braham, S.A.; Kamal, S.; Fernandez-Lafuente, R. Further stabilization of alcalase immobilized on glyoxyl supports: Amination plus modification with glutaraldehyde. Molecules 2018, 23, 3188. [Google Scholar] [CrossRef] [Green Version]
- Betancor, L.; Luckarift, H.R. Bioinspired enzyme encapsulation for biocatalysis. Trends Biotechnol. 2008, 26, 566–572. [Google Scholar] [CrossRef]
- Mureseanu, M.; Galarneau, A.; Renard, G.; Fajula, F. A New mesoporous micelle-templated silica route for enzyme encapsulation. Langmuir 2005, 21, 4648–4655. [Google Scholar] [CrossRef]
- Wang, Y.; Caruso, F. Enzyme encapsulation in nanoporous silica spheres. Chem. Commun. 2004, 1, 1528–1529. [Google Scholar] [CrossRef]
- Tang, L.; Cheng, J. Nonporous silica nanoparticles for nanomedicine application. Nano Today 2013, 8, 290–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.; Rashid, R.; Murtaza, G.; Zahra, A. Gold nanoparticles: Synthesis and applications in drug delivery. Trop. J. Pharm. Res. 2014, 13, 1169. [Google Scholar] [CrossRef]
- Kumar, A.; Zhang, X.; Liang, X.J. Gold nanoparticles: Emerging paradigm for targeted drug delivery system. Biotechnol. Adv. 2013, 31, 593–606. [Google Scholar] [CrossRef]
- Kumar, A.; Ma, H.; Zhang, X.; Huang, K.; Jin, S.; Liu, J.; Wei, T.; Cao, W.; Zou, G.; Liang, X. Gold nanoparticles functionalized with therapeutic and targeted peptides for cancer treatment. Biomaterials 2012, 33, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Schluesener, H.J.; Xu, S. Gold nanoparticle-based biosensors. Gold Bull. 2010, 43, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.B.; Dong, J.; Bischof, J.C. Cellular uptake and nanoscale localization of gold nanoparticles in cancer using label-free confocal raman microscopy. Mol. Pharm. 2011, 8, 176–184. [Google Scholar] [CrossRef]
- Shahriari, S.; Bakhshi, M.; Shahverdi, A.R.; Berahmeh, A.; Safavifar, F.; Khorramizadeh, M.R. Targeted intracellular heat transfer in cancer therapy: Assessment of asparagine-laminated gold nanoparticles in cell model of T cell leukemia. Iran. J. Public Health 2017, 46, 357–367. [Google Scholar]
- Tiwari, P.; Vig, K.; Dennis, V.; Singh, S. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef]
- Baskar, G.; Chandhuru, J.; Sheraz Fahad, K.; Praveen, A.S.; Chamundeeswari, M.; Muthukumar, T. Anticancer activity of fungal l-asparaginase conjugated with zinc oxide nanoparticles. J. Mater. Sci. Mater. Med. 2015, 26, 43. [Google Scholar] [CrossRef]
- Mozumder, M.S.; Mairpady, A.; Mourad, A.H.I. Polymeric nanobiocomposites for biomedical applications. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2016, 105, 1241–1259. [Google Scholar] [CrossRef]
- Escudero, A.; De Los Ríos, A.P.; Godínez, C.; Tomás, F.; Hernández-Fernández, F.J. Immobilization in ionogel: A new way to improve the activity and stability of Candida antarctica Lipase B. Molecules 2020, 25, 3233. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, V. Engineering aspects of membrane bioreactors. Handb. Membr. React. 2013, 2, 3–53. [Google Scholar] [CrossRef]
- Li, S. Fundamentals of biochemical reaction engineering. Chem. React. Eng. 2017, 491–539. [Google Scholar] [CrossRef]
- Grosová, Z.; Rosenberg, M.; Rebroš, M. Perspectives and applications of immobilised β-galactosidase in food industry—A review. Czech. J. Food Sci. 2008, 26, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.; Tamer, M.T.; Omer, M.A. Methods of enzyme immobilization. Int. J. Curr. Pharm. Rev. Res. 2016, 7, 385–392. [Google Scholar] [CrossRef]
- Giri, T.K. Alginate containing nanoarchitectonics for improved cancer therapy. Nanoarchitecton. Smart Deliv. Drug Target. 2016, 565–588. [Google Scholar] [CrossRef]
- Giri, T.K.; Choudhary, C.; Alexander, A.; Ajazuddin, A.; Badwaik, H.; Tripathy, M.; Tripathi, D.K. Sustained release of diltiazem hydrochloride from cross-linked biodegradable IPN hydrogel beads of pectin and modified xanthan gum. Indian J. Pharm. Sci. 2013, 75, 619–627. [Google Scholar] [CrossRef]
- Kim, C.-K.; Lee, E.-J. The controlled release of blue dextran from alginate beads. Int. J. Pharm. 1992, 79, 11–19. [Google Scholar] [CrossRef]
- Murano, E. Use of natural polysaccharides in the microencapsulation techniques. J. Appl. Ichthyol. 1998, 14, 245–249. [Google Scholar] [CrossRef]
- Singh, O.N.; Burgess, D.J. Characterization of Albumin-alginic acid complex coacervation. J. Pharm. Pharmacol. 1989, 41, 670–673. [Google Scholar] [CrossRef]
- Smidsrod, O.; Skjakbrk, G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990, 8, 71–78. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Caseli, L. Enzymes immobilized in langmuir-blodgett films: Why determining the surface properties in langmuir monolayer is important? An. Acad. Bras. Cienc. 2018, 90, 631–644. [Google Scholar] [CrossRef]
- Girard-Egrot, A.P.; Godoy, S.; Blum, L.J. Enzyme association with lipidic Langmuir-Blodgett films: Interests and applications in nanobioscience. Adv. Colloid Interface Sci. 2005, 116, 205–225. [Google Scholar] [CrossRef]
- El-Nagga, N.E.-A.; El-Ewasy, S.M.; El-Shweihy, N.M. Microbial l-asparaginase as a potential therapeutic agent for the treatment of acute lymphoblastic leukemia: The pros and cons. Int. J. Pharmacol. 2014, 10, 182–199. [Google Scholar] [CrossRef]
- Aiswarya, R.; Baskar, G. Enzymatic mitigation of acrylamide in fried potato chips using asparaginase from Aspergillus terreus. Int. J. Food Sci. Technol. 2018, 53, 491–498. [Google Scholar] [CrossRef]
- Tardito, S.; Uggeri, J.; Bozzetti, C.; Bianchi, M.G.; Rotoli, B.M.; Franchi-Gazzola, R.; Gazzola, G.C.; Gatti, R.; Bussolati, O. The inhibition of glutamine synthetase sensitizes human sarcoma cells to l-asparaginase. Cancer Chemother. Pharmacol. 2007, 60, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Tardito, S.; Chiu, M.; Uggeri, J.; Da Ros, F.; Zerbini, A.; Dall’Asta, V.; Missale, G.; Bussolati, O. l-Asparaginase and Inhibitors of glutamine synthetase disclose glutamine addiction of β-catenin-mutated human hepatocellular carcinoma cells. Curr. Cancer Drug Targets 2011, 11, 929–943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Dong, L.-W.; Tan, Y.-X.; Zhang, J.; Pan, Y.-F.; Yang, C.; Li, M.-H.; Ding, Z.-W.; Liu, L.-J.; Jiang, T.-Y.; et al. Asparagine synthetase is an independent predictor of surgical survival and a potential therapeutic target in hepatocellular carcinoma. Br. J. Cancer 2013, 109, 14–23. [Google Scholar] [CrossRef]
- Scotti, C.; Sommi, P.; Pasquetto, M.V.; Cappelletti, D.; Stivala, S.; Mignosi, P.; Savio, M.; Chiarelli, L.R.; Valentini, G.; Bolanos-Garcia, V.M.; et al. Cell-cycle inhibition by helicobacter pylori l-asparaginase. PLoS ONE 2010, 5, e13892. [Google Scholar] [CrossRef] [Green Version]
- Cappelletti, D.; Chiarelli, L.R.; Pasquetto, M.V.; Stivala, S.; Valentini, G.; Scotti, C. Helicobacter pyloril-asparaginase: A promising chemotherapeutic agent. Biochem. Biophys. Res. Commun. 2008, 377, 1222–1226. [Google Scholar] [CrossRef] [PubMed]
- Savitri; Asthana, N.; Azmi, W. Microbial L-asparaginase: A potent antitumour enzyme. Indian J. Biotechnol. 2003, 2, 184–194. [Google Scholar]
- Chiu, M.; Taurino, G.; Bianchi, M.G.; Kilberg, M.S.; Bussolati, O. Asparagine synthetase in cancer: Beyond acute lymphoblastic leukemia. Front. Oncol. 2020, 9, 1480. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Lorenzi, P.L.; Anishkin, A.; Purwaha, P.; Rogers, D.M.; Sukharev, S.; Rempe, S.B.; Weinstein, J.N. The glutaminase activity of l-asparaginase is not required for anticancer activity against ASNS-negative cells. Blood 2014, 123, 3596–3606. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.K.; Horvath, T.D.; Tan, L.; Link, T.; Harutyunyan, K.G.; Pontikos, M.A.; Anishkin, A.; Du, D.; Martin, L.A.; Yin, E.; et al. Glutaminase activity of l-asparaginase contributes to durable preclinical activity against acute lymphoblastic leukemia. Mol. Cancer Ther. 2019, 18, 1587–1592. [Google Scholar] [CrossRef] [Green Version]
- Marchese, L.; Nascimento, J.D.F.; Damasceno, F.S.; Bringaud, F.; Michels, P.A.M.; Silber, A.M. The uptake and metabolism of amino acids, and their unique role in the biology of pathogenic trypanosomatids. Pathogens 2018, 7, 36. [Google Scholar] [CrossRef] [Green Version]
- Cachumba, J.J.M.; Antunes, F.A.F.; Peres, G.F.D.; Brumano, L.P.; Dos Santos, J.C.; Da Silva, S.S. Current applications and different approaches for microbial L-asparaginase production. Braz. J. Microbiol. 2016, 47, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Narta, U.; Roy, S.; Kanwar, S.S.; Azmi, W. Improved production of l-asparaginase by bacillus brevis cultivated in the presence of oxygen-vectors. Bioresour. Technol. 2011, 102, 2083–2085. [Google Scholar] [CrossRef]
- Narta, U.K.; Kanwar, S.S.; Azmi, W. Pharmacological and clinical evaluation of l-asparaginase in the treatment of leukemia. Crit. Rev. Oncol. Hematol. 2007, 61, 208–221. [Google Scholar] [CrossRef]
- Shrivastava, A.; Khan, A.A.; Khurshid, M.; Kalam, M.A.; Jain, S.K.; Singhal, P.K. Recent developments in l-asparaginase discovery and its potential as anticancer agent. Crit. Rev. Oncol. Hematol. 2016, 100, 1–10. [Google Scholar] [CrossRef]
- Wang, B.; Relling, M.V.; Storm, M.C.; Woo, M.H.; Ribeiro, R.; Pui, C.H.; Hak, L.J. Evaluation of immunologic crossreaction of antiasparaginase antibodies in acute lymphoblastic leukemia (ALL) and lymphoma patients. Leukemia 2003, 17, 1583–1588. [Google Scholar] [CrossRef]
- Baran, E.T.; Özer, N.; Hasirci, V. In vivo half life of nanoencapsulated l-asparaginase. J. Mater. Sci. Mater. Med. 2002, 13, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Diaconeasa, Z.; Barbu-Tudoran, L.; Coman, C.; Leopold, L.; Mesaros, A.; Pop, O.; Rugină, D.; Ştefan, R.; Tăbăran, F.; Tripon, S.; et al. Cerium oxide nanoparticles and its cytotoxicity human lung cancer cells. Rom. Biotechnol. Lett. 2015, 20, 10679–10687. [Google Scholar]
- Skovgaard, N. Health implications of acrylamide in food. Int. J. Food Microbiol. 2004, 90, 116–117. [Google Scholar] [CrossRef]
- IARC IARC monographs on the evaluation of carcinogenic risks to humans. Anal. Chim. Acta 1996, 336, 229–230. [CrossRef] [Green Version]
- International Food Safety Authorities Network. Acrylamide in Food Is a Potential Health Hazard. Available online: https://www.who.int/foodsafety/fs_management/No_02_Acrylamide_Mar05_en_rev1.pdf?ua=1 (accessed on 9 December 2020).
- Abt, E.; Robin, L.P.; McGrath, S.; Srinivasan, J.; DiNovi, M.; Adachi, Y.; Chirtel, S. Acrylamide levels and dietary exposure from foods in the United States, an update based on 2011–2015 data. Food Addit. Contam. Part. A Chem. Anal. Control. Expo. Risk Assess. 2019, 36, 1475–1490. [Google Scholar] [CrossRef] [PubMed]
- Bedade, D.K.; Singhal, R.S. Biodegradation of acrylamide by a novel isolate, Cupriavidus oxalaticus ICTDB921: Identification and characterization of the acrylamidase produced. Bioresour. Technol. 2018, 261, 122–132. [Google Scholar] [CrossRef]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef]
- Gökmen, V.; Palazoǧlu, T.K.; Şenyuva, H.Z. Relation between the acrylamide formation and time-temperature history of surface and core regions of French fries. J. Food Eng. 2006, 77, 972–976. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, biochemistry, and safety of acrylamide. A review. J. Agric. Food Chem. 2003, 51, 4504–4526. [Google Scholar] [CrossRef]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef] [PubMed]
- Yaylayan, V.A.; Wnorowski, A.; Perez Locas, C. Why asparagine needs carbohydrates to generate acrylamide. J. Agric. Food Chem. 2003, 51, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- Amrein, T.M.; Schönbächler, B.; Escher, F.; Amadò, R. Acrylamide in gingerbread: Critical factors for formation and possible ways for reduction. J. Agric. Food Chem. 2004, 52, 4282–4288. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.K.; Balagiannis, D.P.; Higley, J.; Smith, G.; Wedzicha, B.L.; Mottram, D.S. Kinetic model for the formation of acrylamide during the finish-frying of commercial french fries. J. Agric. Food Chem. 2012, 60, 9321–9331. [Google Scholar] [CrossRef] [PubMed]
- Eisele, N.; Linke, D.; Bitzer, K.; Na’amnieh, S.; Nimtz, M.; Berger, R.G. The first characterized asparaginase from a basidiomycete, Flammulina velutipes. Bioresour. Technol. 2011, 102, 3316–3321. [Google Scholar] [CrossRef]
- Ciesarová, Z.; Kukurová, K.; Mikušová, L.; Basil, E.; Polakovičová, P.; Duchoňová, L.; Vlček, M.; Šturdík, E. Nutritionally enhanced wheat-oat bread with reduced acrylamide level. Qual. Assur. Saf. Crop. Foods 2014, 6, 327–334. [Google Scholar] [CrossRef]
- Mohan Kumar, N.S.; Shimray, C.A.; Indrani, D.; Manonmani, H.K. Reduction of acrylamide formation in sweet bread with l-asparaginase treatment. Food Bioprocess. Technol. 2014, 7, 741–748. [Google Scholar] [CrossRef]
- Pedreschi, F.; Kaack, K.; Granby, K. The effect of asparaginase on acrylamide formation in French fries. Food Chem. 2008, 109, 386–392. [Google Scholar] [CrossRef]
- Xu, F.; Khalid, P.; Oruna-Concha, M.J.; Elmore, J.S. Effect of Asparaginase on Flavour Formation in Roasted Coffee. In Flavour Science: Proceedings of the XIV Weurman Flavour Research Symposium, Cambridge, UK, 15–19 September 2014; Queen’s College Cambridge: Cambridge, UK, 2015; pp. 563–566. [Google Scholar]
- Hendriksen, H.V.; Kornbrust, B.A.; Ostergaard, P.R.; Stringer, M.A. Evaluating the potential for enzymatic acrylamide mitigation in a range of food products using an asparaginase from aspergillus oryzae. J. Agric. Food Chem. 2009, 57, 4168–4176. [Google Scholar] [CrossRef]
- Munir, N.; Zia, M.A.; Sharif, S.; Tahir, I.M.; Jahangeer, M.; Javed, I.; Riaz, M.; Sarwar, M.U.; Akram, M.; Shah, S.M.A. l-Asparaginase potential in acrylamide mitigation from foodstuff: A mini-review. Prog. Nutr. 2019, 21, 498–506. [Google Scholar] [CrossRef]
- Shi, R.; Liu, Y.; Mu, Q.; Jiang, Z.; Yang, S. Biochemical characterization of a novel l-asparaginase from Paenibacillus barengoltzii being suitable for acrylamide reduction in potato chips and mooncakes. Int. J. Biol. Macromol. 2017, 96, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Zhang, T.; Jiang, B.; Mu, W. Recent research progress on microbial l-asparaginases. Appl. Microbiol. Biotechnol. 2015, 99, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Vimal, A.; Kumar, A. Biotechnological production and practical application of l-asparaginase enzyme. Biotechnol. Genet. Eng. Rev. 2017, 33, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Bahadır, E.B.; Sezgintürk, M.K. Applications of commercial biosensors in clinical, food, environmental, and biothreat/biowarfare analyses. Anal. Biochem. 2015, 478, 107–120. [Google Scholar] [CrossRef]
- Verma, N.; Bansal, M.; Kumar, S. Whole cell based miniaturized fiber optic biosensor to monitor l-asparagine. J. Appl. Sci. Res. 2012, 3, 809–814. [Google Scholar]
- Kotzia, G.A.; Labrou, N.E. Engineering substrate specificity of E. carotovora l-asparaginase for the development of biosensor. J. Mol. Catal. B Enzym. 2011, 72, 95–101. [Google Scholar] [CrossRef]
- Labrou, N.E.; Muharram, M.M. Biochemical characterization and immobilization of Erwinia carotovora l-asparaginase in a microplate for high-throughput biosensing of l-asparagine. Enzym. Microb. Technol. 2016, 92, 86–93. [Google Scholar] [CrossRef]



| ASNase Application | ASNase Form | Microorganism | ASNase Commercial Name | ASNase Manufacturer |
|---|---|---|---|---|
| Therapeutic/Pharmaceutical | Native ASNase | E. coli | Elspar® | Ovation Pharmaceuticals |
| Leukanase® | Sanofi-aventis | |||
| Kidrolase® | EUSA Pharma | |||
| PEGylated ASNase | E. coli | Oncaspar® | Enzon Pharmaceuticals | |
| Native recombinant ASNase | E. coli | Spectrila® | Medac Gesellschaft | |
| E. chrysanthemi | Erwinase® | EUSA Pharma | ||
| Food Industry | Native ASNase | A. oryzae | Acrylaway® | Novozymes A/S |
| A. niger | PreventASeTM | DSM |
| ASNase Confinement Type | Support | ASNase Source | Results | Ref. |
|---|---|---|---|---|
| Physical Adsorption | Aspartic-acid-functionalised graphene oxide nanosheets | E. coli (Medac®, Wedel, Germany) | TS §: 24 h (60 °C)–25.1% * | [71] |
| OS §: 8 cycles 60 °C–29% * | ||||
| Multi-walled carbon nanotubes (MWCNTs) | Aspergillus versicolor | ηi§: 54.4% | [72] | |
| TS: 30 min 45 °C–100% * | ||||
| ASNase half-life: 1155 min (50 °C) | ||||
| Km§: 0.045 M | ||||
| ASNase toxicity: stable after 50 μg mL−1 (BHK-21 cell line) | ||||
| In vivo tests: eased ASNase harmful effect (biochemical biomarkers) | ||||
| E. coli (Deltaclon S.L., Madrid, Spain) | ηi: 100% | [73] | ||
| Relative recovered activity: >90% | ||||
| Km: 109 mM | ||||
| Vmax§: 0.029 mM min−1 | ||||
| Fe3O4@Au NPs | E. coli Pro-Spec, Ness-Ziona, Israel) | ηi: 77.2 % | [74] | |
| TS: 3 h 55 °C–90% * | ||||
| SS §: 28 days (25 °C)–64% * | ||||
| OS: 13 cycles–50% * | ||||
| Km: 1.59 mM | ||||
| Covalent attachment | Silica NPs | E. coli HAP (Kyowa Hakko Kirin Co, Ltd., Tokyo, Japan) | pH range: 6.5–7.5 1 §§; 5–8.5 2 §§ | [75] |
| TS (pH 7, 50 IU of trypsin): 1h 37 °C–80% 1; 72% 2 | ||||
| Stability half-lives of the bioconjugated ASNase increase | ||||
| Km: 2.29 ± 0.10 mM 1; 2.57 ± 0.08 mM 2 | ||||
| AuNPs | Aspergillus terreus (CSIR-IMTECH) | Protein concentration: 0.332 mg mL−1 | [76] | |
| IVtC §: 84.51% (1000 μg mL−1, A549 cell line); 18.51% (100 μg mL−1, A2780) | ||||
| AONP 3 TONP 4 | E. coli (Sun pharmaceutical Ltd., Mumbai, India) | SS: 23 days 37 °C–> 40 % 3 §§§; >35 % 4 §§§ | [77] | |
| OS: 9 cycles–91.8% 3; 95.1% 4 | ||||
| Km: 1.9 μM 3 | ||||
| IVtC: 61% 3 (10 μg mL−1, MOLT-4 cell line); 40% 4 (10 μg mL−1, MOLT-4 cell line) | ||||
| AlOPs | E. coli (Sigma-Aldrich, St. Louis, Missouri, USA) | ηi: 85 % | [78] | |
| SS: 30 days 4 °C–72.97% * | ||||
| OS: 9 cycles–83% * | ||||
| Km: 5.39 μM | ||||
| Aspartic-acid-functionalised graphene oxide nanosheets | E. coli (Medac®, Wedel, Germany) | ηi: 100 % | [71] | |
| TS: 24 h (60 °C)–40.6% * | ||||
| OS: 8 cycles 60 °C–42% * | ||||
| Magnetic Fe3O4–chitosan NPs | E. coli (Pro-Spec) | ηi: 73.2 % | [79] | |
| TS: 70 °C–>60% * | ||||
| SS: 28 days (4 °C; RT)–50% *; 48% * | ||||
| OS: 16 cycles–60.5% * | ||||
| Epoxy-functionalised Fe3O4@MCM-41 magnetic NPs | E. coli (Sigma-Aldrich, St. Louis, MI, USA) | ηi: 98% | [80] | |
| TS: 3 h 55 °C–>92% * | ||||
| SS: 30 days (4 °C; 25 °C)–54% *; 26% * | ||||
| OS: 12 cycles–56.3% * | ||||
| Chloro-modified magnetic Fe3O4@MCM-41 Core–Shell NPs | E. coli (Sigma-Aldrich) | ηi: 63 % | [81] | |
| TS: 3 h 55 °C–69.7% * | ||||
| SS: 28 days (4 °C; 25 °C)–47 % *; 32.5% * | ||||
| OS: 18 cycles–42.2% * | ||||
| pH range (7.0–9.0): >85% * | ||||
| Magnetic poly(HEMA-GMA) NPs | E. coli (Sigma-Aldrich) | TS: 10 h–50 % * | [82] | |
| SS: 40 days–30% *; OS: 8 cycles– 5% * | ||||
| IVtA §: 74.74% * | ||||
| APTES-modified magnetic NPs | B. aryabhattai | ηi: 62 % | [83] | |
| TS: 70 °C –3.3 folds increase | ||||
| OS: 5 cycles–90% * | ||||
| Better substrate affinity S-A §: >90% acrylamide mitigation (30 min) | ||||
| Cerium selenium nanobiocomposite | Aspergillus terreus MTCC 1782 (CSIR-IMTECH) | MTT assay §: 70.84% (1000 μg mL−1); 48.78% (IC50 125 μg mL−1) (A549 cell line) | [84] | |
| β-cyclodextrin- ASNase nanobiocomposite | A. terreus MTCC 1782 (CSIR-IMTECH) | IVtC: 64.79% at 1000 μg mL−1 (PC3 cell lines); 56.42% at 1000 μg mL−1 (U937 cell lines) | [85] | |
| IC50 §: 125 μg mL−1 (PC3 cell lines); 500 μg mL−1 (U937 cell lines) | ||||
| β-cyclodextrin-gelatin-ASNase nanobiocomposite | A. terreus MTCC 1782 (CSIR-IMTECH) | IVtC: 78.23% at 1000 μg mL−1 (HeLa cell lines); 82.74% at 1000 μg mL−1 (U87 cell lines) | [86] | |
| Entrapment | Agar cake beadsAgarose piecesGelatin blocks | Spirulina maxima | IC50: 22.54 μg mL−1 (A549 cell line); 24.65 μg mL−1 (Hep-G2 cell line); 56.61 μg mL−1 (PC3 cell lines) | [87] |
| Ca-alginate beads | Rhizopus microsporus IBBL-2 | TS: 48 h–17.68 U mL−1 | [88] | |
| PLGA NPs | (Changzhou Qianhong BioPharma Co. Ltd., Changzhou, China) | ηi: 80% | [89] | |
| Encapsulated ASNase activity: 265 ± 6 U mg−1 | ||||
| Encapsulated ASNase release (7 and 14 days): 56 % and 60% | ||||
| BSA/ASN/Pol407 NPs | (Changzhou Qianhong BioPharma Co. Ltd.) | PdI § > 0.23 | [90] | |
| SS: 4 months 4 °C (systems with 15 %, 20 % and 25 % of ASNase)– ≥ 100 % * | ||||
| ZET protocol §: in vivo safety | ||||
| SA-ASNase-CNT | E. coli (Sigma-Aldrich) | SS: 30 days–85% * (monolayer) | [91] | |
| Ca-ALG/MWCNT-COOH | E. coli (Pro-Spec) | ηi: 97% | [92] | |
| TS: 65 °C–11.13 % * | ||||
| SS: 4 weeks 30 °C–81.2 % *; OS: 14 cycles–36.4% * | ||||
| Km: 0.33 mM |
| Applications | Support | ASNase Confinement Type | Ref. |
|---|---|---|---|
| Therapeutic/Pharmaceutical (Chemotherapeutic Agent) | |||
| Stable drug support | MWCNTs | Covalent confinement | [72] |
| Novel effective drug against lung cancer | AuNPs | [76] | |
| Potential anti-lung-cancer drug | Cerium selenium nanobiocomposite | [84] | |
| Potential therapeutic agent for cervical and brain cancer | β-cyclodextrin-gelatin-ASNase nanobiocomposite | [86] | |
| Potential therapeutic agent for prostate cancer and lymphoma | β-cyclodextrin-ASNase nanobiocomposite | [85] | |
| New therapeutic system for drug delivery and anticancer therapy | AONP | [77] | |
| Potential anti-lung-, anti-liver- and anti-prostate-cancer drug | Agar cake beads, agarose pieces and gelatin blocks | Entrapment | [87] |
| Food Industry (Acrylamide Mitigation) | |||
| Efficient biocatalyst for the reduction of acrylamide in S-A food model system | APTES-modified magnetic NPs | Covalent confinement | [83] |
| Effective in asparagine cleaving for acrylamide mitigation without significant changes in reducing sugar content during frying of potato slices | Nanomagnetic particles | [144] | |
| Acrylamide formation mitigating during commercial processing of starchy foods, namely blanched potato chips | AlOPs | [78] | |
| Biosensor | |||
| Sensitive units for asparagine detection in optical devices | SA-ASNase-CNT | Entrapment | [91] |
| ASNase Confinement Support | ASNase Source | Cell Lines | Confined ASNase Cytotoxicity (%) ([ASNase] (µg mL−1) ) | NPs Cytotoxicity (%) ([ASNase] (µg mL−1)) | Free ASNase Cytotoxicity (%) ([ASNase] (µg mL−1)) | Ref. |
|---|---|---|---|---|---|---|
| AuNPs | A. terreus | A549 | IVtC *: 84.51 % (1000 µg mL−1) | IVtC: 73.68% | IVtC: 74.88% | [76] |
| A2780 | IC50 **: 18.51% (100 µg mL−1) | — | — | |||
| Cerium selenium nanobiocomposite | A. terreus | A549 | IVtC: 70.84% (1000 µg mL−1) | IC50 (24 h): 35.92% (100 µg mL−1) | — | [84,161] |
| IC50: 48.78% (125 µg mL−1) | ||||||
| β-cyclodextrin-gelatin-ASNase nanobiocomposite | A. terreus | HeLa | IVtC: 78.23% (1000 µg mL−1) | IC50: 51.4% (62.5 µg mL−1) | — | [86] |
| U87 | IVtC: 82.74% (1000 µg mL−1) | IVtC: 2.84% (7.8 µg mL−1); 57.87% (500 µg mL−1) | — | |||
| β-cyclodextrin-ASNase nanobiocomposite | A. terreus | PC3 | IVtC: 64.79% (1000 µg mL−1) | IC50: (250 µg mL−1) | IVtC: 62.15% (1000 µg mL−1) | [85] |
| IVtC: 63.04% (1000 µg mL−1) | ||||||
| U937 | IVtC: 56.42% (1000 µg mL−1) | IC50: (1000 µg mL−1) | IVtC: 45.47% (1000 µg mL−1) | |||
| IVtC: 50.5% (1000 µg mL−1) | ||||||
| AONP | E. coli | MOLT-4 | IVtC: 61% (10 µg mL−1) | IVtC: 20% (10 μg mL−1) | — | [77] |
| TONP | E. coli | MOLT-4 | IVtC: 40% (10 µg mL−1) | IVtC: 17% (5 μg mL−1) | — | [77] |
| Natural polymers: agar cake beads | Spirulina maxima | A549 | IC50: (22.54 µg mL−1) | — | — | [87] |
| Hep-G2 | IC50: (24.65 µg mL−1) | — | — | |||
| PC3 | IC50: (56.61 µg mL−1) | — | — |
| Food Product | Acrylamide Level (µg/kg) | Average Acrylamide Intake (µg/kg bw/day) |
|---|---|---|
| Cereals | <10–1354 | 0.050 |
| French Fries and other Potato Foods | <10–1999 | 0.047 |
| Potato Chips | 140–8440 | 0.038 |
| Cookies and Granola Bars | <10–1796 | 0.030 |
| Crackers | <10–2110 | 0.022 |
| Snack Foods | <10–3060 | 0.019 |
| Coffee | 70–1080 | 0.018 |
| Breads and Bakery Products | <10–102 | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, J.C.F.; Cristóvão, R.O.; Freire, M.G.; Santos-Ebinuma, V.C.; Faria, J.L.; Silva, C.G.; Tavares, A.P.M. Recent Strategies and Applications for l-Asparaginase Confinement. Molecules 2020, 25, 5827. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25245827
Nunes JCF, Cristóvão RO, Freire MG, Santos-Ebinuma VC, Faria JL, Silva CG, Tavares APM. Recent Strategies and Applications for l-Asparaginase Confinement. Molecules. 2020; 25(24):5827. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25245827
Chicago/Turabian StyleNunes, João C. F., Raquel O. Cristóvão, Mara G. Freire, Valéria C. Santos-Ebinuma, Joaquim L. Faria, Cláudia G. Silva, and Ana P. M. Tavares. 2020. "Recent Strategies and Applications for l-Asparaginase Confinement" Molecules 25, no. 24: 5827. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25245827