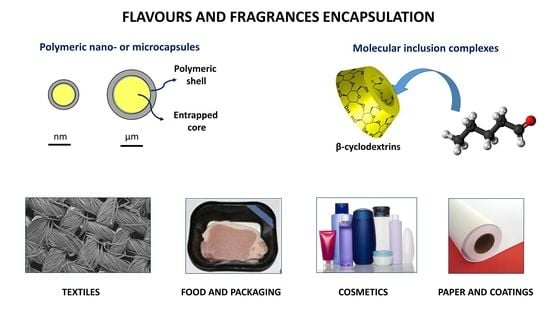
Encapsulation of Flavours and Fragrances into Polymeric Capsules and Cyclodextrins Inclusion Complexes: An Update
Abstract
:1. Introduction
2. Methods of Preparation for Micro/Nanoencapsulation of Flavours/Fragrances in Polymeric Capsules and Molecular Inclusion Complexes
2.1. Polymeric Capsules
2.2. Molecular Inclusion Complexes with CDs
3. Applications of Micro-/Nanoencapsulated Fragrances and Flavours
3.1. Textile Applications
3.2. Food Applications
3.3. Cosmetic Applications
3.4. Paper Applications
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guentert, M. The flavour and fragrance industry-past, present, and future. In Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Springer: Berlin/Heidelberg, Germnay, 2007; pp. 1–14. ISBN 9783540493389. [Google Scholar]
- Bauer, K.; Garbe, D.; Surburg, H. Common Fragrance and Flavor Materials: Preparation, Properties and Uses; Wiley-VCH: Hoboken, NJ, USA, 1997; ISBN 3-527-28850-3. [Google Scholar]
- Rowe, D.J. Chemistry and Technology of Flavors and Fragrances; Rowe, D.J., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2004; ISBN 9781444305517. [Google Scholar]
- Nagegowda, D.A.; Dudareva, N. Plant Biochemistry and Biotechnology of Flavor Compounds and Essential Oils. In Medicinal Plant Biotechnology: From Basic Research to Industrial Applications; Kayser, O., Quax, W.J., Eds.; Wiley-VCH: Weinheim, Germany, 2007; ISBN 9783527314430. [Google Scholar]
- Can Başer, K.H.; Buchbauer, G. Handbook of Essential Oils: SCIENCE, Technology, and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781466590472. [Google Scholar]
- Nieto, G. Biological Activities of Three Essential Oils of the Lamiaceae Family. Medicines 2017, 4, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, A. The Chemistry and Biology of Volatiles; Herrmann, A., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2010; ISBN 9780470669532. [Google Scholar]
- Weerawatanakorn, M.; Wu, J.C.; Pan, M.H.; Ho, C.T. Reactivity and stability of selected flavor compounds. J. Food Drug Anal. 2015, 23, 176–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branta, D.; Speranza, L.P.; Macedo, G.A. A new approach for flavor and aroma encapsulation. In Novel Approaches of Nanotechnology in Food Nanotechnology in the Agri-Food Industry Volume 1 Nanotechnology in the Agri-Food Industry; Academic Press Inc.: Cambridge, MA, USA, 2016; pp. 623–661. [Google Scholar]
- Van Soest, J.J.G. Encapsulation of fragrances and flavours: A way to control odour and aroma in consumer products. In Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Springer: Berlin/Heidelberg, Germany, 2007; pp. 439–455. ISBN 9783540493389. [Google Scholar]
- Estevinho, B.N.; Roch, F. A Key for the Future of the Flavors in Food Industry: Nanoencapsulation and Microencapsulation. In Nanotechnology Applications in Food Flavor, Stability, Nutrition and Safety; Oprea, A.E., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–19. ISBN 978-0-12-811942-6. [Google Scholar]
- Chakraborty, S. Carrageenan for encapsulation and immobilization of flavor, fragrance, probiotics, and enzymes: A review. J. Carbohydr. Chem. 2017, 36, 1–19. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pagliaro, M. Sol-gel microencapsulation of odorants and flavors: Opening the route to sustainable fragrances and aromas. Chem. Soc. Rev. 2013, 42, 9243–9250. [Google Scholar] [CrossRef]
- Sadovoy, A.V.; Lomova, M.V.; Antipina, M.N.; Braun, N.A.; Sukhorukov, G.B.; Kiryukhin, M.V. Layer-by-layer assembled multilayer shells for encapsulation and release of fragrance. ACS Appl. Mater. Interfaces 2013, 5, 8948–8954. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kukkar, D.; Bhardwaj, S.K.; Kim, K.H.; Deep, A. Potential use of polymers and their complexes as media for storage and delivery of fragrances. J. Control. Release 2018, 285, 81–95. [Google Scholar] [CrossRef]
- He, L.; Hu, J.; Deng, W. Preparation and application of flavor and fragrance capsules. Polym. Chem. 2018, 9, 4926–4946. [Google Scholar] [CrossRef]
- Dardelle, G.; Jacquemond, M.; Erni, P. Delivery Systems for Low Molecular Weight Payloads: Core/Shell Capsules with Composite Coacervate/Polyurea Membranes. Adv. Mater. 2017, 29, 1606099. [Google Scholar] [CrossRef]
- Yow, H.N.; Routh, A.F. Formation of liquid core-polymer shell microcapsules. Soft Matter 2006, 2, 940–949. [Google Scholar] [CrossRef]
- Esser-Kahn, A.P.; Odom, S.A.; Sottos, N.R.; White, S.R.; Moore, J.S. Triggered release from polymer capsules. Macromolecules 2011, 44, 5539–5553. [Google Scholar] [CrossRef]
- Paret, N.; Trachsel, A.; Berthier, D.L.; Herrmann, A. Developing Multi Stimuli-Responsive Core/Shell Microcapsules to Control the Release of Volatile Compounds. Macromol. Mater. Eng. 2019, 304, 1800599. [Google Scholar] [CrossRef]
- Lee, H.; Choi, C.-H.; Abbaspourrad, A.; Wesner, C.; Caggioni, M.; Zhu, T.; Weitz, D.A. Encapsulation and Enhanced Retention of Fragrance in Polymer Microcapsules. ACS Appl. Mater. Interfaces 2016, 8, 4007–4013. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, A.; Landy, D.; Fourmentin, S. Complexation efficiency of cyclodextrins for volatile flavor compounds. Food Res. Int. 2013, 53, 110–114. [Google Scholar] [CrossRef]
- Cabral-Marques, H. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Wadhwa, G.; Kumar, S.; Chhabra, L.; Mahant, S.; Rao, R. Essential oil–cyclodextrin complexes: An updated review. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 39–58. [Google Scholar] [CrossRef]
- Saifullah; Shishir, M.R.I.; Ferdowsi, R.; Rahman, R.T.; Vuong, Q. Micro and nano encapsulation, retention and controlled release of flavor and aroma compounds: A critical review. Trends Food Sci. Technol. 2019, 86, 230–251. [Google Scholar] [CrossRef]
- Wei, M.; Pan, X.; Rong, L.; Dong, A.; He, Y.; Song, X.; Li, J. Polymer carriers for controlled fragrance release. Mater. Res. Express 2020, 7, 082001. [Google Scholar] [CrossRef]
- Stasse, M.; Ribaut, T.; Héroguez, V.; Schmitt, V. Elaboration of double emulsion-based polymeric capsules for fragrance. Colloid Polym. Sci. 2020, 1–13. [Google Scholar] [CrossRef]
- Ji, Z.; Tang, Y.; Wang, C.; Yang, J. Preparation of submicron capsules containing fragrance and their application as emulsifier. Polym. Bull. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Manfredini, N.; Ilare, J.; Invernizzi, M.; Polvara, E.; Mejia, D.C.; Sironi, S.; Moscatelli, D.; Sponchioni, M. Polymer Nanoparticles for the Release of Fragrances: How the Physicochemical Properties Influence the Adsorption on Textile and the Delivery of Limonene. Ind. Eng. Chem. Res. 2020, 59, 12766–12773. [Google Scholar] [CrossRef]
- Stasse, M.; Laurichesse, E.; Vandroux, M.; Ribaut, T.; Héroguez, V.; Schmitt, V. Cross-linking of double oil-in-water-in-oil emulsions: A new way for fragrance encapsulation with tunable sustained release. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125448. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Aloys, H.; Korma, S.A.; Alice Tuyishime, M.; Ali, A.H.; Marie Alice, T.; Chantal, N.; Abed, S.M.; Ildephonse, H. Microencapsulation by Complex Coacervation: Methods, Techniques, Benefits, and Applications-A Review. Am. J. Food Sci. Nutr. Res. 2016, 3, 188–192. [Google Scholar]
- Martins, I.M.; Barreiro, M.F.; Coelho, M.A.; Rodrigues, A.E. Microencapsulation of essential oils with biodegradable polymeric carriers for cosmetic applications. Chem. Eng. J. 2014, 245, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2015, 15, 143–182. [Google Scholar] [CrossRef]
- Niu, Y.; Liu, W.; Zhu, G.; Zhou, R.; Niu, Y. A review of the preparation and application of flavour and essential oils microcapsules based on complex coacervation technology. J. Sci. Food Agric. 2013, 94, 1482–1494. [Google Scholar] [CrossRef]
- Muhoza, B.; Xia, S.; Wang, X.; Zhang, X.; Li, Y.; Zhang, S. Microencapsulation of essential oils by complex coacervation method: Preparation, thermal stability, release properties and applications. Crit. Rev. Food Sci. Nutr. 2020, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, J.; Xu, Q. Polyelectrolyte complex from cationized casein and sodium alginate for fragrance controlled release. Colloids Surf. B Biointerfaces 2019, 178, 439–444. [Google Scholar] [CrossRef]
- Hernández-Nava, R.; López-Malo, A.; Palou, E.; Ramírez-Corona, N.; Jiménez-Munguía, M.T. Encapsulation of oregano essential oil (Origanum vulgare) by complex coacervation between gelatin and chia mucilage and its properties after spray drying. Food Hydrocoll. 2020, 109, 106077. [Google Scholar] [CrossRef]
- Ammendola, M.; Gomez, R.R.; Garcia-Valls, R. Perfume Encapsulation via Vapor Induced Phase Separation. Processes 2019, 7, 865. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Khan, S.; Muzafar, M.; Kushwaha, M.; Yadav, A.K.; Gupta, A.P. Encapsulation: Entrapping essential oil/flavors/aromas in food. Encapsulations 2016, 229–268. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C.; Ishwarya, S.P. Spray Drying Techniques for Food Ingredient Encapsulation; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industry applications–A review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Reineccius, G.A.; Yan, C. Factors controlling the deterioration of spray dried flavourings and unsaturated lipids. Flavour Fragr. J. 2015, 31, 5–21. [Google Scholar] [CrossRef]
- Veiga, R.D.S.D.; Da Silva-Buzanello, R.A.; Corso, M.P.; Canan, C. Essential oils microencapsulated obtained by spray drying: A review. J. Essent. Oil Res. 2019, 31, 457–473. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Tan, C.P.; Manap, M.Y.A.; Muhialdin, B.J.; Hussin, A.S.M. Spray Drying for the Encapsulation of Oils—A Review. Molecules 2020, 25, 3873. [Google Scholar] [CrossRef] [PubMed]
- Assadpour, E.; Jafari, S.M. Advances in Spray-Drying Encapsulation of Food Bioactive Ingredients: From Microcapsules to Nanocapsules. Annu. Rev. Food Sci. Technol. 2019, 10, 103–131. [Google Scholar] [CrossRef]
- Hu, Q.; Li, X.; Chen, F.; Wan, R.; Yu, C.; Li, J.; McClements, D.J.; Deng, Z.-Y. Microencapsulation of an essential oil (cinnamon oil) by spray drying: Effects of wall materials and storage conditions on microcapsule properties. J. Food Process. Preserv. 2020, 44. [Google Scholar] [CrossRef]
- Ramos, F.D.M.; Júnior, V.S.; Prata, A.S. Assessing the Vacuum Spray Drying Effects on the Properties of Orange Essential Oil Microparticles. Food Bioprocess Technol. 2019, 12, 1917–1927. [Google Scholar] [CrossRef]
- Procopio, F.R.; Oriani, V.; Paulino, B.N.; Prado-Silva, L.D.; Pastore, G.M.; Sant’Ana, A.S.; Hubinger, M.D. Solid lipid microparticles loaded with cinnamon oleoresin: Characterization, stability and antimicrobial activity. Food Res. Int. 2018, 113, 351–361. [Google Scholar] [CrossRef]
- Sillick, M.; Gregson, C.M. Spray chill encapsulation of flavors within anhydrous erythritol crystals. LWT 2012, 48, 107–113. [Google Scholar] [CrossRef]
- Đorđević, V.; Balanc, B.D.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in Encapsulation Technologies for Delivery of Food Bioactive Compounds. Food Eng. Rev. 2015, 7, 452–490. [Google Scholar] [CrossRef]
- Coelho, S.C.; Estevinho, B.N.; Rocha, F. Encapsulation in food industry with emerging electrohydrodynamic techniques: Electrospinning and electrospraying–A review. Food Chem. 2020, 339, 127850. [Google Scholar] [CrossRef]
- Haider, S.; Haider, A.; Alghyamah, A.A.; Khan, R.; Almasry, W.A.; Khan, N. Electrohydrodynamic Processes and Their Affecting Parameters. In Electrospinning and Electrospraying-Techniques and Applications; IntechOpen: London, UK, 2019. [Google Scholar]
- Wen, P.; Wen, Y.; Zong, M.-H.; Linhardt, R.J.; Wu, H. Encapsulation of Bioactive Compound in Electrospun Fibers and Its Potential Application. J. Agric. Food Chem. 2017, 65, 9161–9179. [Google Scholar] [CrossRef] [PubMed]
- Ataei, S.; Azari, P.; Hassan, A.; Pingguan-Murphy, B.; Yahya, R.; Muhamad, F. Essential Oils-Loaded Electrospun Biopolymers: A Future Perspective for Active Food Packaging. Adv. Polym. Technol. 2020, 2020, 1–21. [Google Scholar] [CrossRef]
- Solaberrieta, I.; Jiménez, A.; Cacciotti, I.; Garrigós, M. Encapsulation of Bioactive Compounds from Aloe Vera Agrowastes in Electrospun Poly (Ethylene Oxide) Nanofibers. Polymer 2020, 12, 1323. [Google Scholar] [CrossRef]
- Mele, E. Electrospinning of Essential Oils. Polymer 2020, 12, 908. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Saito, Y.; Ullah, S.; Haider, K.; Nawaz, H.; Duy-Nam, P.; Kharaghani, D.; Kim, I.-S. Bioactive Sambong oil-loaded electrospun cellulose acetate nanofibers: Preparation, characterization, and in-vitro biocompatibility. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef]
- Venturelli, R.; Immich, A.P.; De Souza, S.; De Souza, A.A. Recycled polyester nanofiber as a reservoir for essential oil release. J. Appl. Polym. Sci. 2020, 11, 50258. [Google Scholar] [CrossRef]
- Kamrudi, N.; Akbari, S.; Kish, M.H. Enhanced control release of thyme essential oils from electrospun nanofiber/polyamidoamine dendritic polymer for antibacterial platforms. Polym. Adv. Technol. 2020, 31, 1719–1731. [Google Scholar] [CrossRef]
- Dehghani, S.; Noshad, M.; Rastegarzadeh, S.; Hojjati, M.; Fazlara, A. Electrospun chia seed mucilage/PVA encapsulated with green cardamonmum essential oils: Antioxidant and antibacterial property. Int. J. Biol. Macromol. 2020, 161, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rezaeinia, H.; Emadzadeh, B.; Ghorani, B. Electrospun balangu (Lallemantia royleana) hydrocolloid nanofiber mat as a fast-dissolving carrier for bergamot essential oil. Food Hydrocoll. 2020, 100, 105312. [Google Scholar] [CrossRef]
- Buzgo, M.; Mickova, A.; Rampichova, M.; Doupnik, M. Blend electrospinning, coaxial electrospinning, and emulsion electrospinning techniques. In Core-Shell Nanostructures for Drug Delivery and Theranostics; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 325–347. [Google Scholar]
- Yao, Z.-C.; Chen, S.; Ahmad, Z.; Huang, J.; Chang, M.-W.; Li, J. Essential Oil Bioactive Fibrous Membranes Prepared via Coaxial Electrospinning. J. Food Sci. 2017, 82, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Camerlo, A.; Vebert-Nardin, C.; Rossi, R.M.; Popa, A.-M. Fragrance encapsulation in polymeric matrices by emulsion electrospinning. Eur. Polym. J. 2013, 49, 3806–3813. [Google Scholar] [CrossRef]
- Dehcheshmeh, M.A.; Fathi, M. Production of core-shell nanofibers from zein and tragacanth for encapsulation of saffron extract. Int. J. Biol. Macromol. 2019, 122, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Yang, H.; Lee, I.Y.; Yong, T.-S.; Lee, S. Core/Sheath-Structured Composite Nanofibers Containing Cinnamon Oil: Their Antibacterial and Antifungal Properties and Acaricidal Effect against House Dust Mites. Polymer 2020, 12, 243. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Dong, R.-H.; Yan, X.; Yu, G.-F.; You, M.-H.; Ning, X.; Long, Y.-Z. Recent Advances in Needleless Electrospinning of Ultrathin Fibers: From Academia to Industrial Production. Macromol. Mater. Eng. 2017, 302, 1700002. [Google Scholar] [CrossRef]
- Karim, M.; Fathi, M.; Soleimanian-Zad, S. Nanoencapsulation of cinnamic aldehyde using zein nanofibers by novel needle-less electrospinning: Production, characterization and their application to reduce nitrite in sausages. J. Food Eng. 2020, 288, 110140. [Google Scholar] [CrossRef]
- Vafania, B.; Fathi, M.; Soleimanian-Zad, S. Nanoencapsulation of thyme essential oil in chitosan-gelatin nanofibers by nozzle-less electrospinning and their application to reduce nitrite in sausages. Food Bioprod. Process. 2019, 116, 240–248. [Google Scholar] [CrossRef]
- Castro, N.; Durrieu, V.; Raynaud, C.; Rouilly, A.; Rigal, L.; Quellet, C. Melt Extrusion Encapsulation of Flavors: A Review. Polym. Rev. 2016, 56, 137–186. [Google Scholar] [CrossRef] [Green Version]
- Hoyos-Leyva, J.D.; Bello-Pérez, L.A.; Alvarez-Ramirez, J.; Garcia, H.S. Microencapsulation using starch as wall material: A review. Food Rev. Int. 2018, 34, 148–161. [Google Scholar] [CrossRef]
- Zhang, J.; Normand, V. Gelatinization of octenyl succinate starch affects oil encapsulation in melt extrusion. LWT 2020, 133, 109920. [Google Scholar] [CrossRef]
- Fahim, T.; Zaidul, I.; Abu Bakar, M.R.; Salim, U.; Awang, M.; Sahena, F.; Jalal, K.; Sharif, K.; Sohrab, M. Particle formation and micronization using non-conventional techniques- review. Chem. Eng. Process. Process. Intensif. 2014, 86, 47–52. [Google Scholar] [CrossRef]
- Soh, S.H.; Lee, L.Y. Microencapsulation and Nanoencapsulation Using Supercritical Fluid (SCF) Techniques. Pharmaceutics 2019, 11, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akolade, J.; Nasir-Naeem, K.O.; Swanepoel, A.; Yusuf, A.A.; Balogun, M.; Labuschagne, P. CO2-assisted production of polyethylene glycol/lauric acid microparticles for extended release of Citrus aurantifolia essential oil. J. CO2 Util. 2020, 38, 375–384. [Google Scholar] [CrossRef]
- Akolade, J.; Balogun, M.; Swanepoel, A.; Ibrahim, R.B.; A Yusuf, A.; Labuschagne, P. Microencapsulation of eucalyptol in polyethylene glycol and polycaprolactone using particles from gas-saturated solutions. RSC Adv. 2019, 9, 34039–34049. [Google Scholar] [CrossRef] [Green Version]
- Reis, P.M.C.L.; Mezzomo, N.; Aguiar, G.P.S.; Senna, E.M.T.L.; Hense, H.; Ferreira, S.R.S. Ultrasound-assisted emulsion of laurel leaves essential oil (Laurus nobilis L.) encapsulated by SFEE. J. Supercrit. Fluids 2019, 147, 284–292. [Google Scholar] [CrossRef]
- Tian, B.; Xiao, D.; Hei, T.; Ping, R.; Hua, S.; Liu, J. The application and prospects of cyclodextrin inclusion complexes and polymers in the food industry: A review. Polym. Int. 2020, 69, 597–603. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of cyclodextrins in food science. A review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Haji, A. Functional Finishing of Textiles with β-Cyclodextrin. In Frontiers of Textile Materials; Wiley: Hoboken, NJ, USA, 2020; pp. 87–116. [Google Scholar]
- Bezerra, F.M.; Lis, M.; Firmino, H.B.; Da Silva, J.G.D.; Valle, R.D.C.S.C.; Valle, J.A.B.; Scacchetti, F.A.P.; Tessaro, A.L. The Role of β-Cyclodextrin in the Textile Industry—Review. Molecules 2020, 25, 3624. [Google Scholar] [CrossRef]
- Behr, A.; Seidensticker, T. Cyclic Carbohydrates-Cyclodextrins. In Chemistry of Renewables; Springer: Berlin/Heidelberg, Germnay, 2020; pp. 191–198. [Google Scholar]
- Rodríguez-López, M.I.; Mercader-Ros, M.T.; Lucas-Abellán, C.; Pellicer, J.A.; Pérez-Garrido, A.; Pérez-Sánchez, H.; Yáñez-Gascón, M.J.; Gabaldón, J.; Núñez-Delicado, E. Comprehensive Characterization of Linalool-HP-β-Cyclodextrin Inclusion Complexes. Molecules 2020, 25, 5069. [Google Scholar] [CrossRef]
- Niu, Y.; Deng, J.; Xiao, Z.; Kou, X.; Zhu, G.; Liu, M.; Liu, S. Preparation and slow release kinetics of apple fragrance/β-cyclodextrin inclusion complex. J. Therm. Anal. Calorim. 2020, 1–7. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, G.; Xiao, Z. Study of formation constant, thermodynamics and β-ionone release characteristic of β-ionone-hydroxypropyl-β-cyclodextrin inclusion complex. Polym. Bull. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Christoforides, E.; Fourtaka, K.; Andreou, A.; Bethanis, K. X-ray crystallography and molecular dynamics studies of the inclusion complexes of geraniol in β-cyclodextrin, heptakis (2,6-di-O-methyl)-β-cyclodextrin and heptakis (2,3,6-tri-O-methyl)-β-cyclodextrin. J. Mol. Struct. 2020, 1202, 127350. [Google Scholar] [CrossRef]
- Kfoury, M.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Encapsulation in cyclodextrins to widen the applications of essential oils. Environ. Chem. Lett. 2019, 17, 129–143. [Google Scholar] [CrossRef]
- Saffarionpour, S. Nanoencapsulation of Hydrophobic Food Flavor Ingredients and Their Cyclodextrin Inclusion Complexes. Food Bioprocess Technol. 2019, 12, 1157–1173. [Google Scholar] [CrossRef]
- Yildiz, Z.I.; Celebioglu, A.; Kilic, M.E.; Durgun, E.; Uyar, T. Fast-dissolving carvacrol/cyclodextrin inclusion complex electrospun fibers with enhanced thermal stability, water solubility, and antioxidant activity. J. Mater. Sci. 2018, 53, 15837–15849. [Google Scholar] [CrossRef] [Green Version]
- Celebioglu, A.; Yildiz, Z.I.; Uyar, T. Thymol/cyclodextrin inclusion complex nanofibrous webs: Enhanced water solubility, high thermal stability and antioxidant property of thymol. Food Res. Int. 2018, 106, 280–290. [Google Scholar] [CrossRef] [Green Version]
- Celebioglu, A.; Aytac, Z.; Kilic, M.E.; Durgun, E.; Uyar, T. Encapsulation of camphor in cyclodextrin inclusion complex nanofibers via polymer-free electrospinning: Enhanced water solubility, high temperature stability, and slow release of camphor. J. Mater. Sci. 2017, 53, 5436–5449. [Google Scholar] [CrossRef] [Green Version]
- Yildiz, Z.I.; Celebioglu, A.; Kilic, M.E.; Durgun, E.; Uyar, T. Menthol/cyclodextrin inclusion complex nanofibers: Enhanced water-solubility and high-temperature stability of menthol. J. Food Eng. 2018, 224, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Aytac, Z.; Yildiz, Z.I.; Kayaci-Senirmak, F.; Keskin, N.O.S.; Kusku, S.I.; Durgun, E.; Tekinay, T.; Uyar, T. Fast-Dissolving, Prolonged Release, and Antibacterial Cyclodextrin/Limonene-Inclusion Complex Nanofibrous Webs via Polymer-Free Electrospinning. J. Agric. Food Chem. 2016, 64, 7325–7334. [Google Scholar] [CrossRef]
- Aytac, Z.; Celebioglu, A.; Yildiz, Z.I.; Uyar, T. Efficient Encapsulation of Citral in Fast-Dissolving Polymer-Free Electrospun Nanofibers of Cyclodextrin Inclusion Complexes: High Thermal Stability, Longer Shelf-Life, and Enhanced Water Solubility of Citral. Nanomaterials 2018, 8, 793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celebioglu, A.; Yildiz, Z.I.; Uyar, T. Electrospun nanofibers from cyclodextrin inclusion complexes with cineole and p -cymene: Enhanced water solubility and thermal stability. Int. J. Food Sci. Technol. 2017, 53, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Celebioglu, A.; Yildiz, Z.I.; Uyar, T. Fabrication of Electrospun Eugenol/Cyclodextrin Inclusion Complex Nanofibrous Webs for Enhanced Antioxidant Property, Water Solubility, and High Temperature Stability. J. Agric. Food Chem. 2018, 66, 457–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aytac, Z.; Ipek, S.; Durgun, E.; Tekinay, T.; Uyar, T. Antibacterial electrospun zein nanofibrous web encapsulating thymol/cyclodextrin-inclusion complex for food packaging. Food Chem. 2017, 233, 117–124. [Google Scholar] [CrossRef]
- Wang, Z.; Zou, W.; Liu, L.; Wang, M.; Li, F.; Shen, W. Characterization and bacteriostatic effects of β-cyclodextrin/quercetin inclusion compound nanofilms prepared by electrospinning. Food Chem. 2020, 338, 127980. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrohydrodynamic encapsulation of eugenol-cyclodextrin complexes in pullulan nanofibers. Food Hydrocoll. 2021, 111, 106264. [Google Scholar] [CrossRef]
- Chen, Y.; Mensah, A.; Wang, Q.; Li, D.; Qiu, Y.; Weic, Q. Hierarchical porous nanofibers containing thymol/beta-cyclodextrin: Physico-chemical characterization and potential biomedical applications. Mater. Sci. Eng. C 2020, 115, 111155. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Enescu, D.; Torres-Giner, S.; Cabedo, L.; Cerqueira, M.; Pastrana, L.; Fuciños, P.; Lagaron, J.M. Development of electrospun active films of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by the incorporation of cyclodextrin inclusion complexes containing oregano essential oil. Food Hydrocoll. 2020, 108, 106013. [Google Scholar] [CrossRef]
- Ghayempour, S.; Montazer, M. Micro/nanoencapsulation of essential oils and fragrances: Focus on perfumed, antimicrobial, mosquito-repellent and medical textiles. J. Microencapsul. 2016, 33, 497–510. [Google Scholar] [CrossRef]
- Teixeira, C.S.N.R.; Martins, I.M.D.; Mata, V.L.G.; Barreiro, M.F.F.; Rodrigues, A.E. Characterization and evaluation of commercial fragrance microcapsules for textile application. J. Text. Inst. 2011, 103, 1–13. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Rodríguez, O.; Rodrigues, S.; Martins, I.; Rodrigues, A.E. A case study of product engineering: Performance of microencapsulated perfumes on textile applications. AIChE J. 2011, 58, 1939–1950. [Google Scholar] [CrossRef]
- Wang, C.X.; Chen, S.L. Fragrance-release Property of β-Cyclodextrin Inclusion Compounds and their Application in Aromatherapy. J. Ind. Text. 2005, 34, 157–166. [Google Scholar] [CrossRef]
- Schindler, W.D.; Hauser, P.J. Chemical Finishing of Textiles; Woodhead Publishing: Cambridge, UK, 2004; ISBN 978-1-85573-905-5. [Google Scholar]
- Bezerra, F.M.; Lis, M.; Óscar, C.G.; Carmona, C.G.; Moisés, M.P.; Zanin, G.M.; Moraes, F.F. Assessment of the delivery of citronella oil from microcapsules supported on wool fabrics. Powder Technol. 2019, 343, 775–782. [Google Scholar] [CrossRef]
- Zhao, D.; Jiao, X.; Zhang, M.; Ye, K.; Shi, X.; Lu, X.; Qiu, G.; Shea, K.J. Preparation of high encapsulation efficiency fragrance microcapsules and their application in textiles. RSC Adv. 2016, 6, 80924–80933. [Google Scholar] [CrossRef]
- Bruyninckx, K.; Dusselier, M. Sustainable Chemistry Considerations for the Encapsulation of Volatile Compounds in Laundry-Type Applications. ACS Sustain. Chem. Eng. 2019, 7, 8041–8054. [Google Scholar] [CrossRef]
- Cerempei, A. Aromatherapeutic Textiles. In Active Ingredients from Aromatic and Medicinal Plants; IntechOpen: London, UK, 2017. [Google Scholar]
- Rodrigues, S.; Martins, I.M.; Fernandes, I.P.; Gomes, P.; Mata, V.; Barreiro, M.F.; Rodrigues, A.E. Scentfashion®: Microencapsulated perfumes for textile application. Chem. Eng. J. 2009, 149, 463–472. [Google Scholar] [CrossRef]
- Ye, L.; Li, Z.; Niu, R.; Zhou, Z.; Shen, Y.; Jiang, L. All-Aqueous Direct Deposition of Fragrance-Loaded Nanoparticles onto Fabric Surfaces by Electrospraying. ACS Appl. Polym. Mater. 2019, 1, 2590–2596. [Google Scholar] [CrossRef]
- Liu, X.; Huang, L.; Chen, H.; Qian, M.C.; Ji, H. Pore size matching up: A novel insight into cotton textile aromatic finishing. Flavour Fragr. J. 2019, 35, 149–156. [Google Scholar] [CrossRef]
- Romagnoli, M.J.; Gonzalez, J.S.; Martinez, M.A.; Alvarez, V. Micro- and Nanotechnology Applied on Eco-friendly Smart Textiles. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2020; pp. 1–19. [Google Scholar]
- Sharkawy, A.; Fernandes, I.P.; Barreiro, M.F.; Rodrigues, A.E.; Shoeib, T. Aroma-Loaded Microcapsules with Antibacterial Activity for Eco-Friendly Textile Application: Synthesis, Characterization, Release, and Green Grafting. Ind. Eng. Chem. Res. 2017, 56, 5516–5526. [Google Scholar] [CrossRef]
- Ghayempour, S.; Mortazavi, S.M. Microwave curing for applying polymeric nanocapsules containing essential oils on cotton fabric to produce antimicrobial and fragrant textiles. Cellulose 2015, 22, 4065–4075. [Google Scholar] [CrossRef]
- Specos, M.M.; García, J.; Tornesello, J.; Marino, P.; Della Vecchia, M.; Tesoriero, M.D.; Hermida, L.G. Microencapsulated citronella oil for mosquito repellent finishing of cotton textiles. Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 653–658. [Google Scholar] [CrossRef]
- Eyupoglu, S.; Kut, D.; Girisgin, A.O.; Eyupoglu, C.; Ozuicli, M.; Dayioglu, H.; Civan, M.; Aydin, L. Investigation of the bee-repellent properties of cotton fabrics treated with microencapsulated essential oils. Text. Res. J. 2019, 89, 1417–1435. [Google Scholar] [CrossRef]
- Chen, K.; Xu, C.; Zhou, J.; Zhao, R.; Gao, Q.; Wang, C. Multifunctional fabric coatings with slow-releasing fragrance and UV resistant properties from ethyl cellulose/silica hybrid microcapsules. Carbohydr. Polym. 2020, 232, 115821. [Google Scholar] [CrossRef]
- Ma, J.; Xu, W.; Kou, X.; Niu, Y.; Xia, Y.; Wang, Y.; Tian, G.; Zhao, Y.; Ke, Q. Green fabrication of control-released, washable and non-adhesives aromatic-nanocapsules/cotton fabrics via electrostatic-adsorption/in-situ immobilization. ACS Sustain. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Persico, P.; Carfagna, C. Cosmeto-Textiles: State of the Art and Future Perspectives. Adv. Sci. Technol. 2012, 80, 39–46. [Google Scholar] [CrossRef]
- Mamta, M.; Saini, H.K.; Kaur, M. Cosmetotextiles: A novel technique of developing wearable skin care. Asian J. HOME Sci. 2017, 12, 12. [Google Scholar] [CrossRef]
- Cheng, S.; Yuen, C.; Kan, C.; Cheuk, K. Development of Cosmetic Textiles Using Microencapsulation Technology. Res. J. Text. Appar. 2008, 12, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Jamal, Z.; Rani, S.; Zeba, C.; Hd, J.P.; Scholars, R. Cosmetotextiles: A wearable skin care. Int. J. Home Sci. 2018, 4, 31–35. [Google Scholar]
- Gonçalves, F.; Ribeiro, A.; Silva, C.; Cavaco-Paulo, A. Release of Fragrances from Cotton Functionalized with Carbohydrate-Binding Module Proteins. ACS Appl. Mater. Interfaces 2019, 11, 28499–28506. [Google Scholar] [CrossRef]
- Yingngam, B.; Chiangsom, A.; Pharikarn, P.; Vonganakasame, K.; Kanoknitthiran, V.; Rungseevijitprapa, W.; Prasitpuriprecha, C. Optimization of menthol-loaded nanocapsules for skin application using the response surface methodology. J. Drug Deliv. Sci. Technol. 2019, 53, 101138. [Google Scholar] [CrossRef]
- Yingngam, B.; Kacha, W.; Rungseevijitprapa, W.; Sudta, P.; Prasitpuriprecha, C.; Brantner, A.H. Response surface optimization of spray-dried citronella oil microcapsules with reduced volatility and irritation for cosmetic textile uses. Powder Technol. 2019, 355, 372–385. [Google Scholar] [CrossRef]
- Wang, L.; Li, T.; Xin, B.; Liu, Y.; Zhang, F. Preparation and characterization of wormwood-oil-contained microcapsules. J. Microencapsul. 2020, 37, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.; Coimbra, P.; Carreira, A.S.; Figueiredo, M.M.; Gil, M.H.; Simões, P.N. Eugenol-loaded microspheres incorporated into textile substrates. Cellulose 2020, 27, 4109–4121. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Luo, Y.; Wang, M.; Chen, F.; Liu, J.; Meng, K.; Zhao, H. Double-Layered Microcapsules Significantly Improve the Long-Term Effectiveness of Essential Oil. Polymer 2020, 12, 1651. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, C.; Wang, M.; Zhao, T.; Li, W. Bifunctional Microcapsules with n-Octadecane/Thyme Oil Core and Polyurea Shell for High-Efficiency Thermal Energy Storage and Antibiosis. Polymer 2020, 12, 2226. [Google Scholar] [CrossRef]
- Kudligi, S.J.; Malligawad, L.H.; Naikwadi, S.; Jamadar, D. Antimicrobial and aroma finishing of organic cotton knits using natural colourants and Palmarosa oil microcapsules. Flavour Fragr. J. 2020, 35, 59–69. [Google Scholar] [CrossRef]
- Stan, M.S.; Chirila, L.; Popescu, A.; Radulescu, D.M.; Radulescu, D.E.; Dinischiotu, A. Essential Oil Microcapsules Immobilized on Textiles and Certain Induced Effects. Materials 2019, 12, 2029. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, L.; Singh, S.S.J. Comparative analysis of lemongrass oil application on textile substrate through microencapsulation and exhaust method. Int. J. Adv. Res. Sci. Eng. 2018, 7, 313–320. [Google Scholar]
- Fiedler, J.O.; Óscar, C.G.; Carmona, C.G.; Lis, M.J.; Plath, A.M.S.; Samulewski, R.B.; Bezerra, F.M. Application of Aloe vera microcapsules in cotton nonwovens to obtain biofunctional textiles. J. Text. Inst. 2020, 111, 68–74. [Google Scholar] [CrossRef]
- Karagönlü, S.; Başal, G.; Özyıldız, F.; Uzel, A. Preparation of Thyme Oil Loaded Microcapsules for Textile Applications. Int. J. New Technol. Res. 2018, 4, 1–8. [Google Scholar]
- Sannapapamma, K.J.; Lokanath, H.M.; Naikwadi, S. Antimicrobial and Aroma Finishing of Organic Cotton Knits Using Vetiver Oil Microcapsules for Health Care Textiles. Int. J. Mater. Text. Eng. 2018, 12. [Google Scholar]
- Beşen, B.S. Production of Disposable Antibacterial Textiles Via Application of Tea Tree Oil Encapsulated into Different Wall Materials. Fibers Polym. 2019, 20, 2587–2593. [Google Scholar] [CrossRef]
- Ben Abdelkader, M.; Azizi, N.; Baffoun, A.; Chevalier, Y.; Majdoub, M. Fragrant Microcapsules Based On β-Cyclodextrin for Cosmetotextile Application. J. Renew. Mater. 2019, 7, 1347–1362. [Google Scholar] [CrossRef] [Green Version]
- Mamusa, M.; Sofroniou, C.; Resta, C.; Murgia, S.; Fratini, E.; Smets, J.; Baglioni, P. Tuning the Encapsulation of Simple Fragrances with an Amphiphilic Graft Copolymer. ACS Appl. Mater. Interfaces 2020, 12, 28808–28818. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liang, B.; Wang, Y.; Li, Y.; Shi, G. Core-shell nanocapsules containing essential oil for textile application. J. Appl. Polym. Sci. 2018, 135, 45695. [Google Scholar] [CrossRef]
- Đorđević, V.; Paraskevopoulou, A.; Mantzouridou, F.; Lalou, S.; Pantic, M.; Bugarski, B.; Nedović, V. Encapsulation Technologies for Food Industry. In Food Engineering Series; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2015; pp. 329–382. [Google Scholar]
- Kayaci, F.; Uyar, T. Encapsulation of vanillin/cyclodextrin inclusion complex in electrospun polyvinyl alcohol (PVA) nanowebs: Prolonged shelf-life and high temperature stability of vanillin. Food Chem. 2012, 133, 641–649. [Google Scholar] [CrossRef]
- Maswal, M.; Dar, A.A. Formulation challenges in encapsulation and delivery of citral for improved food quality. Food Hydrocoll. 2014, 37, 182–195. [Google Scholar] [CrossRef]
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K.; Rather, I.A. Application of Nanotechnology in Food Science: Perception and Overview. Front. Microbiol. 2017, 8, 1501. [Google Scholar] [CrossRef] [Green Version]
- Sanguansri, L.; Augustin, M.A. Microencapsulation in Functional Food Product Development. In Functional Food Product Development; Wiley: Hoboken, NJ, USA, 2010; pp. 1–23. [Google Scholar]
- Rajabi, H.; Ghorbani, M.; Jafari, S.M.; Mahoonak, A.S.; Rajabzadeh, G. Retention of saffron bioactive components by spray drying encapsulation using maltodextrin, gum Arabic and gelatin as wall materials. Food Hydrocoll. 2015, 51, 327–337. [Google Scholar] [CrossRef]
- Zanin, R.C.; Smrke, S.; Kurozawa, L.E.; Yamashita, F.; Yeretzian, C. Modulation of aroma release of instant coffees through microparticles of roasted coffee oil. Food Chem. 2020, 341, 128193. [Google Scholar] [CrossRef]
- Froiio, F.; Mosaddik, A.; Morshed, M.T.; Paolino, D.; Fessi, H.; Elaissari, A. Edible Polymers for Essential Oils Encapsulation: Application in Food Preservation. Ind. Eng. Chem. Res. 2019, 58, 20932–20945. [Google Scholar] [CrossRef]
- Yang, W.; Wang, L.; Ban, Z.; Yan, J.; Lu, H.; Zhang, X.; Wu, Q.; Aghdam, M.S.; Luo, Z.; Li, L. Efficient microencapsulation of Syringa essential oil; the valuable potential on quality maintenance and storage behavior of peach. Food Hydrocoll. 2019, 95, 177–185. [Google Scholar] [CrossRef]
- Ban, Z.; Zhang, J.; Li, L.; Luo, Z.; Wang, Y.; Yuan, Q.; Zhou, B.; Liu, H. Ginger essential oil-based microencapsulation as an efficient delivery system for the improvement of Jujube (Ziziphus jujuba Mill.) fruit quality. Food Chem. 2020, 306, 125628. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.P.M.; De Oliveira, J.; Biazotto, A.D.M.; Parisi, M.M.; Da Gloria, E.M.; Spoto, M.H.F. Essential oils from Eucalyptus staigeriana F. Muell. ex Bailey and Eucalyptus urograndis W. Hill ex Maiden associated to carboxymethylcellulose coating for the control of Botrytis cinerea Pers. Fr. and Rhizopus stolonifer (Ehrenb.:Fr.) Vuill. in strawberries. Ind. Crop. Prod. 2020, 156, 112884. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [Green Version]
- Wyrwa, J.; Barska, A. Innovations in the food packaging market: Active packaging. Eur. Food Res. Technol. 2017, 243, 1681–1692. [Google Scholar] [CrossRef]
- De Abreu, D.A.P.; Cruz, J.M.; Losada, P.P. Active and Intelligent Packaging for the Food Industry. Food Rev. Int. 2012, 28, 146–187. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Murphy, T.V.; Morris, M.J.; Cummins, E.; Kerry, J.P. Antimicrobial activity of chitosan, organic acids and nano-sized solubilisates for potential use in smart antimicrobially-active packaging for potential food applications. Food Control. 2013, 34, 393–397. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2020, 128403. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun Functional Materials toward Food Packaging Applications: A Review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Ai, F.; Shao, P.; Chen, H.; Gao, H. Development of polyvinyl alcohol/β-cyclodextrin antimicrobial nanofibers for fresh mushroom packaging. Food Chem. 2019, 300, 125249. [Google Scholar] [CrossRef] [PubMed]
- Simionato, I.; Domingues, F.; Nerin, C.; Silva, F. Encapsulation of cinnamon oil in cyclodextrin nanosponges and their potential use for antimicrobial food packaging. Food Chem. Toxicol. 2019, 132, 110647. [Google Scholar] [CrossRef] [PubMed]
- Adel, A.M.; Ibrahim, A.A.; El-Shafei, A.M.; Al-Shemy, M. Inclusion complex of clove oil with chitosan/β-cyclodextrin citrate/oxidized nanocellulose biocomposite for active food packaging. Food Packag. Shelf Life 2019, 20, 100307. [Google Scholar] [CrossRef]
- Alehosseini, A.; Gómez-Mascaraque, L.G.; Ghorani, B.; Lopez-Rubio, A. Stabilization of a saffron extract through its encapsulation within electrospun/electrosprayed zein structures. LWT 2019, 113, 108280. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Caleb, O.J.; Van Reenen, A.J.; Opara, U.L. Physical and antifungal properties of β-cyclodextrin microcapsules and nanofibre films containing cinnamon and oregano essential oils. LWT 2018, 87, 413–422. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Y.; Tian, J.; Chu, Z. Effect of a new shell material—Jackfruit seed starch on novel flavor microcapsules containing vanilla oil. Ind. Crop. Prod. 2018, 112, 47–52. [Google Scholar] [CrossRef]
- Jedlińska, A.; Samborska, K.; Janiszewska-Turak, E.; Witrowa-Rajchert, D.; Seuvre, A.-M.; Voilley, A. Physicochemical properties of vanilla and raspberry aromas microencapsulated in the industrial conditions by spray drying. J. Food Process. Eng. 2018, 41, e12872. [Google Scholar] [CrossRef]
- Yuan, C.; Thomas, D.S.; Hook, J.M.; Qin, G.; Qi, K.; Zhao, J. Molecular Encapsulation of Eucalyptus staigeriana Essential Oil by Forming Inclusion Complexes with Hydroxypropyl-β-Cyclodextrin. Food Bioprocess Technol. 2019, 12, 1264–1272. [Google Scholar] [CrossRef]
- Chen, B.; Su, M.; Pan, Q.; Zhang, Z.; Chen, S.; Huang, Z.; Cai, Z.; Li, Z.; Qian, X.; Hu, X.; et al. Fully Printed Geranium-Inspired Encapsulated Arrays for Quantitative Odor Releasing. ACS Omega 2019, 4, 19977–19982. [Google Scholar] [CrossRef] [Green Version]
- Samakradhamrongthai, R.S.; Angeli, P.T.; Kopermsub, P.; Utama-Ang, N. Optimization of gelatin and gum arabic capsule infused with pandan flavor for multi-core flavor powder encapsulation. Carbohydr. Polym. 2019, 226, 115262. [Google Scholar] [CrossRef]
- Mehran, M.; Masoum, S.; Memarzadeh, M. Microencapsulation of Mentha spicata essential oil by spray drying: Optimization, characterization, release kinetics of essential oil from microcapsules in food models. Ind. Crop. Prod. 2020, 154, 112694. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Amraie, M.; Salehi, M.; Mohseni, M.; Aloui, H. Effect of chitosan-based coatings enriched with savory and/or tarragon essential oils on postharvest maintenance of kumquat (Fortunella sp.) fruit. Food Sci. Nutr. 2019, 7, 155–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, K.; Ortiz, M.; Albis, A.; Castañeda, C.G.G.; Zapata, M.E.V.; Tovar, C.D.G. The Effect of Edible Chitosan Coatings Incorporated with Thymus capitatus Essential Oil on the Shelf-Life of Strawberry (Fragaria x ananassa) during Cold Storage. Biomol. 2018, 8, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chávez-Magdaleno, M.E.; González-Estrada, R.; Ramos-Guerrero, A.; Plascencia-Jatomea, M.; Gutierrez-Martinez, P. Effect of pepper tree (Schinus molle) essential oil-loaded chitosan bio-nanocomposites on postharvest control of Colletotrichum gloeosporioides and quality evaluations in avocado (Persea americana) cv. Hass. Food Sci. Biotechnol. 2018, 27, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Buendía-Moreno, L.; Ros-Chumillas, M.; Navarro-Segura, L.; Sánchez-Martínez, M.J.; Soto-Jover, S.; Antolinos, V.; Martínez-Hernández, G.B.; López–Gómez, A. Effects of an Active Cardboard Box Using Encapsulated Essential Oils on the Tomato Shelf Life. Food Bioprocess Technol. 2019, 12, 1548–1558. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Langroodi, A.M. Chitosan coatings incorporated with propolis extract and Zataria multiflora Boiss oil for active packaging of chicken breast meat. Int. J. Biol. Macromol. 2019, 141, 401–409. [Google Scholar] [CrossRef]
- Paglione, I.D.S.; Galindo, M.V.; De Medeiros, J.A.S.; Yamashita, F.; Alvim, I.D.; Grosso, C.R.F.; Sakanaka, L.S.; Shirai, M.A. Comparative study of the properties of soy protein concentrate films containing free and encapsulated oregano essential oil. Food Packag. Shelf Life 2019, 22, 100419. [Google Scholar] [CrossRef]
- Chen, H.; Li, L.; Ma, Y.; McDonald, T.P.; Wang, Y. Development of active packaging film containing bioactive components encapsulated in β-cyclodextrin and its application. Food Hydrocoll. 2019, 90, 360–366. [Google Scholar] [CrossRef]
- Xiao, Z.; Hou, W.; Kang, Y.; Niu, Y.; Kou, X. Encapsulation and sustained release properties of watermelon flavor and its characteristic aroma compounds from γ-cyclodextrin inclusion complexes. Food Hydrocoll. 2019, 97, 105202. [Google Scholar] [CrossRef]
- Hernández-Fernández, M.Á.; García-Pinilla, S.; Ocampo-Salinas, O.I.; Gutiérrez-López, G.F.; Hernández-Sánchez, H.; Cornejo-Mazón, M.; Perea-Flores, M.D.J.; Dávila-Ortiz, G. Microencapsulation of Vanilla Oleoresin (V. planifolia Andrews) by Complex Coacervation and Spray Drying: Physicochemical and Microstructural Characterization. Foods 2020, 9, 1375. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Lin, C.T.; Chen, J.M.; Lei, D.M. The study of size and stability of n-butylcyanoacrylate nanocapsule suspensions encapsulating green grass fragrance. IOP Conf. Series: Mater. Sci. Eng. 2018, 292, 012094. [Google Scholar] [CrossRef]
- Cui, G.; Wang, J.; Wang, X.; Li, W.; Zhang, X. Preparation and Properties of Narrowly Dispersed Polyurethane Nanocapsules Containing Essential Oil via Phase Inversion Emulsification. J. Agric. Food Chem. 2018, 66, 10799–10807. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Singh, V.K.; Dwivedy, A.K.; Chaudhari, A.K.; Upadhyay, N.; Singh, P.; Sharma, S.; Dubey, N.K. Encapsulation in chitosan-based nanomatrix as an efficient green technology to boost the antimicrobial, antioxidant and in situ efficacy of Coriandrum sativum essential oil. Int. J. Biol. Macromol. 2019, 133, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M.; Mazon, L.R.; De Meneses, A.C.; Silva, L.L.; De Araújo, P.H.H.; Fiori, M.A.; De Oliveira, D. Encapsulation of geranyl cinnamate in polycaprolactone nanoparticles. Mater. Sci. Eng. C 2019, 97, 198–207. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Kang, H. Chitosan coatings incorporated with free or nano-encapsulated Paulownia Tomentosa essential oil to improve shelf-life of ready-to-cook pork chops. LWT 2019, 116, 108580. [Google Scholar] [CrossRef]
- Min, T.; Sun, X.; Yuan, Z.; Zhou, L.; Jiao, X.; Zha, J.; Zhu, Z.; Wen, Y. Novel antimicrobial packaging film based on porous poly(lactic acid) nanofiber and polymeric coating for humidity-controlled release of thyme essential oil. LWT 2021, 135, 110034. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Lan, X.; Huang, D.; Luo, T.; Ji, J.; Mafang, Z.; Miao, X.; Wang, H.; Wang, W. Electrospun Gelatin Nanofibers Encapsulated with Peppermint and Chamomile Essential Oils as Potential Edible Packaging. J. Agric. Food Chem. 2019, 67, 2227–2234. [Google Scholar] [CrossRef]
- European Parliament & Council of the European Union. Regulation (EC) NO 1223/2009 of the European Parliament and of the Council on Cosmetic Products. Off. J. Eur. Union 2009, 342, 59–209. [Google Scholar]
- Global Beauty & Personal Care Market | Trends, Growth, Size, Share. Available online: https://www.inkwoodresearch.com/reports/beauty-and-personal-care-market/ (accessed on 6 November 2020).
- Beauty Becomes a $532 billion Industry Thanks to These trends-Business Insider. Available online: https://www.businessinsider.com/beauty-multibillion-industry-trends-future-2019-7?IR=T (accessed on 6 November 2020).
- Carvalho, I.T.; Estevinho, B.N.; Santos, L.R.D.A.D. Application of microencapsulated essential oils in cosmetic and personal healthcare products - a review. Int. J. Cosmet. Sci. 2016, 38, 109–119. [Google Scholar] [CrossRef]
- Corrêa-Filho, L.C.; Moldão-Martins, M.; Alves Vitor, D. Advances in the Application of Microcapsules as Carriers of Functional Compounds for Food Products. Appl. Sci. 2019, 9, 571. [Google Scholar] [CrossRef] [Green Version]
- Casanova, F.; Santos, L. Encapsulation of cosmetic active ingredients for topical application—a review. J. Microencapsul. 2016, 33, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.M.S.; Srebernich, S.M.; Vercelino, B.G.; Zampieri, B.M. Influence of the presence and type of fragrance on the sensory perception of cosmetic formulations. Braz. Arch. Biol. Technol. 2013, 56, 203–212. [Google Scholar] [CrossRef]
- Tumová, J.; Šauer, P.; Golovko, O.; Koba Ucun, O.; Grabic, R.; Máchová, J.; Kocour Kroupová, H. Effect of polycyclic musk compounds on aquatic organisms: A critical literature review supplemented by own data. Sci. Total Environ. 2019, 651, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
- Ammala, A. Biodegradable polymers as encapsulation materials for cosmetics and personal care markets. Int. J. Cosmet. Sci. 2012, 35, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Civera, C.; Heras, A. Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives. Polym. 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohsenabadi, N.; Rajaei, A.; Tabatabaei, M.; Mohsenifar, A. Physical and antimicrobial properties of starch-carboxy methyl cellulose film containing rosemary essential oils encapsulated in chitosan nanogel. Int. J. Biol. Macromol. 2018, 112, 148–155. [Google Scholar] [CrossRef]
- Devi, N.; Sarmah, M.; Khatun, B.; Maji, T.K. Encapsulation of active ingredients in polysaccharide–protein complex coacervates. Adv. Colloid Interface Sci. 2017, 239, 136–145. [Google Scholar] [CrossRef]
- Van Tran, V.; Nguyen, T.L.; Moon, J.-Y.; Lee, Y.-C. Core-shell materials, lipid particles and nanoemulsions, for delivery of active anti-oxidants in cosmetics applications: Challenges and development strategies. Chem. Eng. J. 2019, 368, 88–114. [Google Scholar] [CrossRef]
- Kesente, M.; Kavetsou, E.; Roussaki, M.; Blidi, S.; Loupassaki, S.; Chanioti, S.; Siamandoura, P.; Stamatogianni, C.; Philippou, E.; Papaspyrides, C.; et al. Encapsulation of Olive Leaves Extracts in Biodegradable PLA Nanoparticles for Use in Cosmetic Formulation. Bioengineering 2017, 4, 75. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, C.; Álvaro, V.; Fronza, N.; Dos Santos, J.H.Z. Structural, textural and morphological characteristics of tannins from Acacia mearnsii encapsulated using sol-gel methods: Applications as antimicrobial agents. Colloids Surf. B Biointerfaces 2017, 151, 26–33. [Google Scholar] [CrossRef]
- Sansukcharearnpon, A.; Wanichwecharungruang, S.; Leepipatpaiboon, N.; Kerdcharoen, T.; Arayachukeat, S. High loading fragrance encapsulation based on a polymer-blend: Preparation and release behavior. Int. J. Pharm. 2010, 391, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Triyono, K.; Suhartatik, N.; Wulandari, Y.W. Nanoencapsulating of Kaffir Lime Oil with Coacervation Method using Arabic Gum and Maltodextrin as Encapsulant. Int. J. Food Nutr. Sci. 2018, 3, 43–48. [Google Scholar]
- Ji, W.; Zhang, T.; Lu, Z.; Shen, J.; Xiao, Z.; Zhang, X. Synthesis and characterization of novel biocompatible nanocapsules encapsulated lily fragrance. Chin. Chem. Lett. 2019, 30, 739–742. [Google Scholar] [CrossRef]
- Hu, J.; Xiao, Z.; Zhou, R.; Li, Z.; Wang, M.; Ma, S. Synthesis and characterization of polybutylcyanoacrylate-encapsulated rose fragrance nanocapsule. Flavour Fragr. J. 2011, 26, 162–173. [Google Scholar] [CrossRef]
- Xiao, Z.; Tian, T.; Hu, J.; Wang, M.; Zhou, R. Preparation and characterization of chitosan nanoparticles as the delivery system for tuberose fragrance. Flavour Fragr. J. 2013, 29, 22–34. [Google Scholar] [CrossRef]
- Song, J.; Chen, H. Preparation of aroma microcapsules with sodium alginate and tetradecylallyldimethylammonium bromide (TADAB) and its potential applications in cosmetics. Flavour Fragr. J. 2018, 33, 160–165. [Google Scholar] [CrossRef]
- Saito, N.; Taguchi, Y.; Tanaka, M. Preparation of Microcapsules Containing Camellia Oil with Heterocoagulation between Chitosan and Oleic Acid. J. Cosmet. Dermatol. Sci. Appl. 2018, 8, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Tinoco, A.; Gonçalves, F.; Costa, A.F.; Freitas, D.S.; Cavaco-Paulo, A.; Ribeiro, A. Keratin:Zein particles as vehicles for fragrance release on hair. Ind. Crop. Prod. 2020, 159, 113067. [Google Scholar] [CrossRef]
- Yang, S.; Liu, L.; Han, J.; Tang, Y. Encapsulating plant ingredients for dermocosmetic application: An updated review of delivery systems and characterization techniques. Int. J. Cosmet. Sci. 2020, 42, 16–28. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, X.; Xiao, Z.; Chen, H.; Ji, H. Preparation and release behaviour of the inclusion complexes of phenylethanol withβ-cyclodextrin. Flavour Fragr. J. 2015, 31, 206–216. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, Y.; Zhu, G.; Zhou, R.; Niu, Y. Preparation and sustained-releasing characterization of aromatic wallpaper. Prog. Org. Coat. 2017, 104, 50–57. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, Y.; Zhu, G.; Niu, Y.; Xu, Z.; Zhu, J. Preparation of micro-encapsulated strawberry fragrance and its application in the aromatic wallpaper. Pol. J. Chem. Technol. 2017, 19, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Kandirmaz, E.A.; Birtane, H.; Çiğil, A.B.; Ozcan, A. pH-controlled lavender oil capsulation with ABA-type block copolymer and usage in paper coating. Flavour Fragr. J. 2020, 35, 174–181. [Google Scholar] [CrossRef]
- Rungwasantisuk, A.; Raibhu, S. Application of encapsulating lavender essential oil in gelatin/gum-arabic complex coacervate and varnish screen-printing in making fragrant gift-wrapping paper. Prog. Org. Coat. 2020, 149, 105924. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, Z.; Yang, J.; Wang, J.; Shen, J.; Wang, X.; Xiao, Z.; Niu, Y.; Chen, L.; Zhang, X. Chitosan-based nanofragrance with antibacterial function applied to wallpaper. Eng. Life Sci. 2020, 20, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Šumiga; Ravnjak, D.; Bojana, B.P. Antimicrobial Paper Coatings Containing Microencapsulated Cymbopogon citratus Oil. Coatings 2019, 9, 470. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Wan, S.; Niu, Y.; Kou, X. Effect of Preparation Parameters on Microparticles with High Loading Capacity and Adsorption Property Adsorbed on Functional Paper. Coatings 2019, 9, 704. [Google Scholar] [CrossRef] [Green Version]



| Encapsulate System | Carrier Agent | Encapsulated Substance | Encapsulation Method | Applications | Ref. |
|---|---|---|---|---|---|
| Microcapsule | Gelatin/Arabic gum | Citronella oil | Complex coacervation | Textiles | [108] |
| Microcapsule | Gelatin/Arabic gum | Wormwood oil | Complex coacervation | Potential application in health care textiles | [129] |
| Microcapsule | Chitosan | Citronella oil | Coacervation | Textiles | [114] |
| Microcapsule | Ethyl cellulose/silica | Lavender fragrance oil | One-step emulsion solvent diffusion | Fragrant and UV resistant textiles | [120] |
| Microsphere | Cellulose derivatives/Polyvinyl alcohol (PVA) | Eugenol | Oil-in-water emulsion solvent evaporation | Textiles | [130] |
| Microcapsule | Crosslinked polymers | Model fragrances | Double-emulsion | Potential application in textiles | [27] |
| Double-Layered Microcapsule | β-CD (inner layer)/chitosan and sodium alginate (outer layer) | Lavender EO | Inclusion encapsulation method | Potential application in textiles | [131] |
| Microcapsule | Polyurea Shell | Thyme oil | Interfacial polymerisation method | Potential application in textiles | [132] |
| Microcapsule | Methyltrimethoxysilane/Tetraethyl orthosilicate | Palmarosa oil | Interfacial co-hydrolysis and co-condensation | Health care textiles | [133] |
| Microcapsule | Melamine | Sage and rose EO | Purchased | Potential application in cosmetotextile | [134] |
| Microcapsules | Gelatin/Gum acacia | Lemongrass oil | Coacervation | Potential application as antibacterial textile | [135] |
| Microcapsule | Starch/Glutaraldehyde | Aloe vera EO | Coacervation | Functional textiles | [136] |
| Microcapsule | Gelatin/Gum Arabic | Thyme Oil | Complex coacervation | Potential application as antibacterial textile | [137] |
| Microcapsule | Silane/orthosilicates/surfactant | Vetiver EO | Interfacial polymerisation technique | Potential application in Health care textiles | [138] |
| Microcapsule | PVA and Arabic gum and β-CD Inclusion Complex | Tea tree oil | Simple coacervation and inclusion encapsulation | Potential application as antibacterial textiles | [139] |
| Microcapsule | Acacia gum | Citronella oil | Two-step approach: oil-in-water emulsification and spray-drying | Potential application as cosmetic textiles | [128] |
| Microcapsule | Polyurethane/β-CD | Neroline | Interfacial polycondensation | Fragrant cosmetotextile | [140] |
| Encapsulate System | Carrier Agent | Encapsulated Substance | Encapsulation Method | Applications | Ref. |
|---|---|---|---|---|---|
| Polymeric micelle | poly(ethylene glycol)-graft poly(vinyl acetate) (PEG-g-PVAc) graft copolymer | 2-phenyl ethanol, L-carvone, and α-pinene | Homogenisation | Potential use in textiles | [141] |
| Nanocapsule | Chitosan | Citronella oil | Coacervation/High-speed homogenisation | Textiles | [114] |
| Nanoparticle | butyl methacrylate/ethylene glycol dimethacrylate | Limonene | Free-radicalemulsion polymerisation | Potential application in fragrance-release textiles | [29] |
| Nanocapsule | Epichlorohydrin modified CD | Lavender essence | Purchased | Potential applications as aromatic medical care textiles and household, clothing | [121] |
| Composite nanoparticle | 2-hydroxypropyl-β-CD/regenerated silk fibroin | Limonene Rose Oxide | Complex inclusion/electro-spraying | Fragrant textiles | [113] |
| Core-shell nanocapsules | Core: styrene/methyl methacrylate copolymer, shell: poly(butyl acrylate) modified by octamethylcyclotetrasiloxane | Jasmine EO | Two-stage emulsion polymerisation | Textile | [142] |
| Nanofiber | Polyamidoamine dendritic polymer | Thyme EOs | Electrospinning | Potential application as antibacterial textiles | [60] |
| Nanocapsule | Poly(ε-caprolactone) caprylic/capric triglycerides | Menthol | Nanoprecipitation | Potential application a as cosmetotextiles | [127] |
| Encapsulate System | Carrier Agent | Encapsulated Substance | Encapsulation Method | Applications | Ref. |
|---|---|---|---|---|---|
| Microcapsules | β-CD | Cinnamon and oregano EOs | Solvent evaporation/complex inclusion | Potential application for active packaging | [164] |
| Nanofibers | Hydroxypropyl-β-CD (HPβCD) and hydroxypropyl-γ-CD (HPγCD) | Cineole and p-cymene | Complex inclusion and electrospinning | Food and oral care applications | [96] |
| Microcapsule | Different shell materials including jackfruit seed starch, chitosan, and β-CD | Vanilla EO | Ultrasonic method | Potential use in food industry | [165] |
| Microcapsule | Maltodextrin and gum Arabic | Vanilla and raspberry aromas | Spray-drying | Potential use in food industry | [166] |
| Inclusion complex | β-CD | Syringa EO | Complex Inclusion | Improving storage of peaches | [151] |
| Inclusion complex | β-CD | Eucalyptus staigeriana Essential Oil | Complex Inclusion | Potential use in food | [167] |
| Core-shell array | poly(lactic-co-glycolic acid) (PLGA) | Longan milk, or vanilla spices | Ink-Jet Printing | Potential use in food security and anticounterfeiting | [168] |
| Microcapsule | Gelatin/gum arabic | Pandan flavour | Complex coacervation | Potential use in food industry | [169] |
| Microsphere | Modified food starch derived from waxy maize | Roasted coffee oil | Spray-drying | Improving quality and acceptance of instant coffee products | [149] |
| Microcapsule | Chitosan/sodium carboxymethyl cellulose | Zingiber officinale EO | Emulsion/freeze-drying | Improving Jujube (Ziziphus jujuba) fruit quality | [152] |
| Microcapsule | Inulin/gum Arabic | Mentha spicata EO | Spray-drying | Potential use in pharmaceutical and food applications | [170] |
| Microcapsule | Carboxymethyl cellulose coating | EOs from Eucalyptus staigeriana and Eucalyptus urograndis | Mixing | Microorganisms growth control in strawberries | [153] |
| Microcapsule | Chitosan coating | Savoury and/or tarragon EOs | Mixing | Postharvest maintenance of kumquat (Fortunella sp.) fruit | [171] |
| Microcapsule | Chitosan coating | Thymus capitatus EO | Mixing | Improving shelf-Life of Strawberry during Cold Storage | [172] |
| Nanocapsule | Chitosan | Pepper tree (Schinus molle) EO-loaded | Nanoprecipitation | Postharvest control of Colletotrichum gloeosporioides and quality evaluations in avocado | [173] |
| Inclusion complex | β-CD | Carvacrol, oregano and cinnamon EO | Complex inclusion | Improving the quality of fresh tomatoes during storage through packaging active materials | [174] |
| Microcapsule | Chitosan coating | Propolis extract and Zataria multiflora oil | Mixing | Active packaging of chicken breast meat | [175] |
| Microparticle | Sodium alginate | Oregano EO | Ionic gelation | Potential use as active and biodegradable packages in food conservation | [176] |
| Inclusion complex | β-CD | Citral/trans- cinnamaldehyde | Complex inclusion | antimicrobial active packaging for food | [177] |
| Inclusion complex | β-CD | Watermelon flavour | Complex inclusion | Potential application in food industry | [178] |
| Microsphere | Chitosan/gum Arabic | Vanilla Oleoresin | Complex coacervation and spray-drying | Potential application in various food matrices | [179] |
| Nanocapsule | Polybutylcyanoacrylate (PBCA) | Green grass fragrance | Emulsion polymerisation | Potential application in food industry | [180] |
| Core-shell nanofiber | Zein/tragacanth gum | Saffron extract | Coaxial electrospinning | Potential use in food industry (chewing gum and tea bag development) | [66] |
| Nanocapsules | Polyurethane | Lavender EO | Emulsion inversion point method | Potential application in food industry | [181] |
| Nanofiber | chitosan–gelatin | Thyme EO | Nozzleless electrospinning | Nitrite substitute for meat products | [70] |
| Nanocapsule | Chitosan | Coriandrum sativum EO | Emulsion formation/ionic gelation | Prolong shelf life and control the fungal and aflatoxin contamination of stored rice | [182] |
| Nanoparticle | Polycaprolactone | Geranyl cinnamate | Mini-emulsification/solvent evaporation technique | Potential use antimicrobial packaging | [183] |
| Nanoparticle | Chitosan | Paulownia Tomentosa EO | Ionic gelation method | Improve shelf-life of ready-to-cook pork chops | [184] |
| Fast-dissolving fibre mats | Balangu seed gum | Bergamot EO | Electrospinning method | Potential strategy for enhancing the flavour in the food systems | [62] |
| Nanofiber | PVA/β-CD | Cinnamon EO | Electrospinning method | Antimicrobial packaging for fresh mushroom | [160] |
| Nanosponge | CD | Cinnamon EO | Synthesis via a crosslinking agent | Antimicrobial food packaging | [161] |
| Nanobiocomposite | Chitosan/β-CD citrate/oxidised nanocellulose | Clove oil | Impregnation of biocomposite | Active food packaging | [162] |
| Microparticle and nanofiber | Zein | Saffron extract | Electrohydrodynamic processing | Potential use in for active packaging applications or in food formulations. | [163] |
| Nanofiber in a film | Polylactic acid (PLA) | Thyme EO | Electrospinning | Antimicrobial and humidity sensitive food packaging system | [185] |
| Nanofiber | Gelatin | Peppermint and chamomile EOs | Electrospinning | Potential application as edible food packaging | [186] |
| Nanofibrous film | Chitosan/PVA/β-CD | Cinnamon and oregano EOs | Complex inclusion and electrospinning | Potential application in active packaging | [164] |
| Nanofiber | Zein | Cinnamic aldehyde | Needleless electrospinning | Food additive to reduce nitrites in sausages | [69] |
| Encapsulate System | Carrier Agent | Encapsulated Substance | Encapsulation Method | Applications | Ref. |
|---|---|---|---|---|---|
| Microcapsule | Gelatin/gum Arabic | Lavender EO | Complex coacervation | Making fragrant gift-wrapping paper | [215] |
| Nanocapsule | Chitosan/PLGA | Vanillin | Emulsion method | Antibacterial function applied to wallpaper | [216] |
| Nanocapsule | Polyethylene oxide-polypropylene glycol-polyethylene oxide (PEO-b-PPG-b-PEO) | Lavender EO | Micellisation of interactions between the hydrophilic–lipophilic–hydrophilic polymer and oil | Paper coating applications | [214] |
| Microcapsule | Gelatin/Carboxymethylcellulose or gum arabic | Citronella Oil | Coacervation | Antimicrobial Paper Coatings | [217] |
| Microcapsule | PLGA or PLGA/Chitosan | Orange EO | Emulsion solvent evaporation | Biodegradable functional packaging paper | [218] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perinelli, D.R.; Palmieri, G.F.; Cespi, M.; Bonacucina, G. Encapsulation of Flavours and Fragrances into Polymeric Capsules and Cyclodextrins Inclusion Complexes: An Update. Molecules 2020, 25, 5878. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25245878
Perinelli DR, Palmieri GF, Cespi M, Bonacucina G. Encapsulation of Flavours and Fragrances into Polymeric Capsules and Cyclodextrins Inclusion Complexes: An Update. Molecules. 2020; 25(24):5878. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25245878
Chicago/Turabian StylePerinelli, Diego Romano, Giovanni Filippo Palmieri, Marco Cespi, and Giulia Bonacucina. 2020. "Encapsulation of Flavours and Fragrances into Polymeric Capsules and Cyclodextrins Inclusion Complexes: An Update" Molecules 25, no. 24: 5878. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25245878