Modified Clerodanes from the Essential Oil of Dodonea viscosa Leaves
Abstract
:1. Introduction
2. Results and Discussion
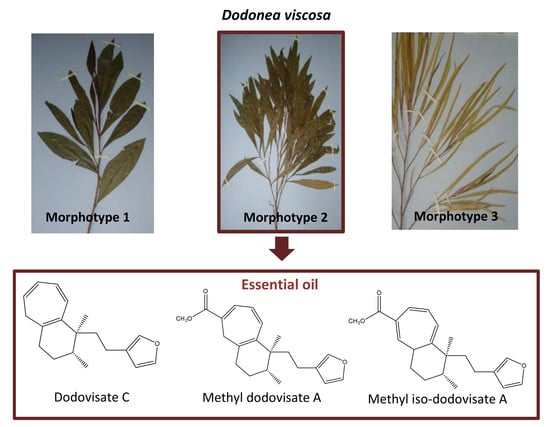
2.1. Essential Oil Composition
2.2. Terpenoids Isolation and Identification
2.3. Discussion
3. Materials and Methods
3.1. General Experimental Procedure
3.2. Plant Material
3.3. Essential oil Extraction
3.4. Isolation and Purification of Dodovisate C (1), methyl dodovisate A (2) and methyl iso-dodovisate A (3)
3.5. Identification and Quantification
3.6. Computational Details
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shanmugavasan, A.; Ramachandran, T. Investigation of the extraction process and phytochemical composition of preparations of Dodonea viscosa (L.) Jacq. J. Ethnopharmacol. 2011, 137, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.M.; Khan, M.A.; Ahmad, M.; Zafar, M.; Jahan, S.; Sultana, S. Ethnopharmacological application of medicinal plants to cure skin diseases and in folk cosmetics among the tribal communities of north-west frontier povince, Pakistan. J. Ethnopharmacol. 2010, 128, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Desta, B. Ethiopian traditional herbal drugs. Part I: Studies on the toxicity and therapeutic activity of local taenicidal medications. J. Ethnopharmacol. 1995, 45, 27–33. [Google Scholar] [PubMed]
- Giday, M.; Teklehaymanot, T.; Animut, A.; Mekonnen, Y. Medicinal plants of the Shinasha, Age-awi and Amhara peoples in northwest Ethiopia. J. Ethnopharmacol. 2007, 110, 516–525. [Google Scholar] [CrossRef]
- Chhabra, S.C.; Mahunnah, R.L.; Mshiu, E.N. Plants used in traditional medicine in eastern Tanzania. V. Angiosperms (Passifloraceae to Sapindaceae). J. Ethnopharmacol. 1991, 33, 143–157. [Google Scholar] [CrossRef]
- De Beer, J.J.J.; Van Wyk, B.E. An ethnobotanical survey of the Agter–Hantam, Northern Cape Province, South Africa. South Afr. J. Bot. 2011, 77, 741–754. [Google Scholar] [CrossRef] [Green Version]
- McGaw, L.J.; Lall, N.; Meyer, J.J.M.; Eloff, J.N. The potential of South African plants against Mycobacterium infections. J. Ethnopharmacol. 2008, 119, 482–500. [Google Scholar] [CrossRef]
- Thring, T.S.A.; Weitz, F.M. Medicinal plant use in the Bredasdorp/Elim region of the Southern Overberg in the Western Cape Province of South Africa. J. Ethnopharmacol. 2006, 103, 261–275. [Google Scholar] [CrossRef]
- van Wyk, B.E. A review of Khoi-San and Cape Dutch medical ethnobotany. J. Ethnopharmacol. 2008, 119, 331–341. [Google Scholar] [CrossRef]
- van Wyk, B.E. The potential of South African plants in the development of new medicinal products. South Afr. J. Bot. 2011, 77, 812–829. [Google Scholar] [CrossRef] [Green Version]
- Lavergne, R. Fleurs de Bourbon; Cazal Publishing: Sainte-Clotilde, France, 1984; p. 199. [Google Scholar]
- Tene, V.; Malagón, O.; Finzi, P.V.; Vidari, G.; Armijos, C.; Zaragoza, T. An ethnobotanical survey of medicinal plants used in Loja and Zamora-Chinchipe, Ecuador. J. Ethnopharmacol. 2007, 111, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Colodel, E.M.; Traverso, S.D.; Seitz, A.L.; Correa, A.; Oliveira, F.N.; Driemeier, D.; Gava, A. Spontaneous poisoning by Dodonea viscosa (Sapindaceae) in cattle. Vet. Hum. Toxicol. 2003, 45, 147–148. [Google Scholar] [PubMed]
- Mata, R.; Contreras, J.L.; Crisanto, D.; Pereda-Miranda, R.; Castañeda, P.; Del Rio, F. Chemical studies on mexican plants used in traditional medicine. XVIII. New secondary metabolites from Dodonea viscosa. J. Nat. Prod. 1991, 54, 913–917. [Google Scholar]
- Rojas, A.; Cruz, S.; Ponce-Monter, H.; Mata, R. Smooth muscle relaxing compounds from Dodonea viscosa. Planta Med. 1996, 62, 154–159. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Petitjean, A.; Ratsimamanga-Urverg, S.; Rakoto-Ratsimamanga, A. Medicinal plants used to treat malaria in Madagascar. J. Ethnopharmacol. 1992, 37, 117–127. [Google Scholar] [CrossRef]
- Gurib-Fakim, A.; Gueho, J. Plantes Médicinales de Maurice; Océan Indien Publishing: Rose-Hill, Maurice, 1997; Volume 3, p. 253. [Google Scholar]
- Gurib-Fakim, A. Plantes médicinales de Maurice et d’ailleurs; Graphic Press Ltd. Publishing: Baie du Tombeau, Maurice, 2007; p. 68. [Google Scholar]
- Gurib-Fakim, A.; Gueho, J. Plantes Médicinales de l’île Rodrigues; Océan Indien Publishing: Rose-Hill, Maurice, 1994; p. 447. [Google Scholar]
- de Cordemoy, E.J. Flore de l’Ile de la Réunion (1895); Kessinger Publishing: Paris, France, 2010; p. 381. [Google Scholar]
- Lavergne, R. Tisaneurs et plantes médicinales indigènes; Orphie Publishing: Paris, France, 1990; p. 258. [Google Scholar]
- Lavergne, R. Les plantes médicinales du Père Raimbault; Azalées Publishing: La Réunion, France, 2000; p. 81. [Google Scholar]
- Lucas, R. Cent plantes endémiques et indigènes de La Réunion; Azalées Publishing: La Réunion, France, 2007; p. 69. [Google Scholar]
- Père Nantas. Le père Raimbault et les plantes médicinales de La Réunion; Nouvelle imprimerie Dionysienne Publishing: La Réunion, France, 1984; p. 35. [Google Scholar]
- Rivière, M. Les plantes médicinales à l’île de La Réunion, leurs amis et leurs faux amis; Azalées Publishing: La Réunion, France, 2007; p. 18. [Google Scholar]
- Giraud-Técher, S.; Amédée, J.; Girard-Valenciennes, E.; Thomas, H.; Brillant, S.; Grondin, I.; Marodon, C.; Smadja, J. Plantes médicinales de La Réunion inscrites à la Pharmacopée française. Ethnopharmacologia 2016, 56, 7–33. [Google Scholar]
- Smadja, J.; Marodon, C.; Amedee, J.; Brillant, S.; Hermann, T. Plantes médicinales de l’île de La Réunion inscrites à la Pharmacopée Française; Orphie Publishing: La Réunion, France, 2016; p. 49. [Google Scholar]
- Rojas, A.; Hernandez, L.; Pereda-Miranda, R.; Mata, R. Screening for antimicrobial activity of crude drug extracts and pure natural products from Mexican medicinal plants. J. Ethnopharmacol. 1992, 35, 275–283. [Google Scholar] [CrossRef]
- Adsersen, A.; Adsersen, H. Plants from Réunion island with alleged antihypertensive and diuretic effects—An experimental and ethnobotanical evaluation. J. Ethnopharmacol. 1997, 58, 189–206. [Google Scholar] [CrossRef]
- Motsei, M.L.; Lindsey, K.L.; van Staden, J.; Jäger, A.K. Screening of traditionally used south african plants for antifungal activity against Candida albicans. J. Ethnopharmacol. 2003, 86, 235–241. [Google Scholar] [CrossRef]
- Getie, M.; Gebre-Mariam, T.; Rietz, R.; Höhne, C.; Huschka, C.; Schmidtke, M.; Abate, A.; Neubert, R.H.H. Evaluation of the anti-microbial and anti-inflammatory activities of the medicinal plants Dodonea viscosa, Rumex nervosus and Rumex abyssinicus. Fitoterapia 2003, 74, 139–143. [Google Scholar] [CrossRef]
- Clarkson, C.; Maharaj, V.J.; Crouch, N.R.; Grace, O.M.; Pillay, P.; Matsabisa, M.G.; Bhagwandin, N.; Smith, P.J.; Folb, P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 2004, 92, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Poullain, C.; Girard-Valenciennes, E.; Smadja, J. Plants from Reunion island: Evaluation of their free radical scavenging and antioxidant activities. J. Ethnopharmacol. 2004, 95, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.M.; Speretto, J.S.; Manfron, M.P. Antiinflammatory activity and acute toxicity of Dodonea viscosa. Fitoterapia 2006, 77, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Desrivot, J.; Waikedre, J.; Cabalion, P.; Herrenknecht, C.; Bories, C.; Hocquemiller, R.; Fournet, A. Antiparasitic activity of some New Caledonian medicinal plants. J. Ethnopharmacol. 2007, 112, 7–12. [Google Scholar] [CrossRef]
- Patel, M.; Coogan, M.M. Antifungal activity of the plant Dodonea viscosa var. angustifolia on Candida albicans from HIV-infected patients. J. Ethnopharmacol. 2008, 118, 173. [Google Scholar]
- Pengelly, A. Medicinal activity of Dodonea viscosa—A preliminary study. In Rural Industries Research and Development Corporation; Australian Government publishing: Canberra, Australia, 2008. [Google Scholar]
- Venkatesh, S.; Reddy, Y.S.R.; Ramesh, M.; Swamy, M.M.; Mahadevan, N.; Suresh, B. Pharmacognosy studies on Dodonea viscosa leaves. Afr. Pharm. Pharmacol. 2008, 24, 83–88. [Google Scholar]
- Arun, M.; Asha, V.V. Gastroprotective effect of Dodonea viscosa on various experimental ulcer models. J. Ethnopharmacol. 2008, 118, 460–465. [Google Scholar] [CrossRef]
- Cao, S.; Brodie, P.; Callmander, M.; Randrianaivo, R.; Razafitsalama, J.; Rakotobe, E.; Rasamison, V.E.; TenDyke, K.; Shen, Y.; Suh, E.M.; et al. Antiproliferative triterpenoid saponins of Dodonea viscosa from the Madagascar dry forest. J. Nat. Prod. 2009, 72, 1705–1707. [Google Scholar] [CrossRef] [Green Version]
- Teffo, L.S.; Aderogba, M.A.; Eloff, J.N. Antibacterial and antioxidant activities of four kaempferol methyl ethers isolated from Dodonea viscosa Jacq. var angustifolia leaf extracts. South Afr. J. Bot. 2010, 76, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Jangra, M.; Sharma, S.; Kumar, M. Evaluation of antihyperglycemic activity of Dodonea viscosa leaves in normal and STZ-diabetic rats. Int. J. Pharm. Pharm. Sci. 2011, 3, 69–74. [Google Scholar]
- Ahmad, M.; Mahmood, Q.; Gulzar, K.; Aktar, M.S.; Saleem, M.; Qadir, M.I. Antihyperlipidemic and hepatoprotective activity of Dodonaea viscosa leaves extracts in alloxan-induced diabetic rabbits (Oryctolagus cuniculus). Pak. Vet. J. 2011, 32, 50–54. [Google Scholar]
- Muhammad, A.; Tel-Çayan, G.; Öztürk, M.; Duru, M.E.; Nadeem, S.; Anis, I.; Weng Ng, S.; Shah, M.R. Phytochemicals from Dodonaea viscosa and their antioxidant and anticholinesterase activities with structure–activity relationships. Pharm. Biol. 2016, 54, 1649–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, K.V. Chemical examination of the leaves of Dodonea viscosa Linn. J. Indian Chem. Soc. 1962, 39, 561–562. [Google Scholar]
- Paris, R.; Nothis, A. Plantes de Nouvelle-Calédonie. II. Etude particulière de plantes à dérivés polyphénoliques. In Plantes Médicinales et Phytothérapie; Jouve Publishing: Paris, France, 1970; pp. 63–74. [Google Scholar]
- Hsü, H.Y.; Chen, Y.P.; Kakisawa, H. Structure of hautriwaic acid. Phytochemistry 1971, 10, 2813–2814. [Google Scholar] [CrossRef]
- Ramachandran Nair, A.G.; Sankara Subramanian, S. Isorhamnetin & quercetin glycosides from Dodonea viscosa & Sapindus emarginatus. Indian J. Chem. 1975, 13, 639–640. [Google Scholar]
- Dreyer, D.L. Kaempferol methyl ethers from flowers of Dodonea viscosa. Rev. Latinoam. Quimica 1978, 9, 97–98. [Google Scholar]
- Dominguez, X.A.; Franco, R.; Cano, C.G.; Chavez, C.N. Aislamiento de 3,6,4′-trimethoxi-5,7-dioxiflavona en el chapuliztle (Dodonea viscosa var. angustifolia Jacq) (Sapindaceae). Rev. Latinoamer. Quim. 1980, 11, 150–151. [Google Scholar]
- Sachdev, K.; Kulshreshtha, D.K. Aliarin, a new flavonoid from Dodonea viscosa Linn. Indian J. Chem. 1982, 21, 798–799. [Google Scholar]
- Sachdev, K.; Kulshreshtha, D.K. Flavonoids from Dodonea viscosa. Phytochemistry 1983, 22, 1253–1256. [Google Scholar] [CrossRef]
- Sachdev, K.; Kulshreshtha, D.K. Dodonic acid, a new diterpenoid from Dodonea viscosa. Planta Med. 1984, 50, 448–449. [Google Scholar] [CrossRef]
- Dimbi, M.Z.; Kapundu, M.; Darimont, E.; Warin, R.; Delaude, C.; Huls, R. Triterpénoïdes de Dodonea viscosa. Bull. Soc. Chim. Belg. 1985, 94, 141–148. [Google Scholar] [CrossRef]
- Sachdev, K.; Kulshreshtha, D.K. Viscosol, a C-3′ prenylated flavonoid from Dodonea viscosa. Phytochemistry 1986, 25, 1967–1969. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Fatima, I.; Fatima, A. The sapogenins from Dodonea viscosa. Fitoterapia 1987, LVIII, 361–366. [Google Scholar]
- Wagner, H.; Ludwig, C.; Grotjahn, L.; Khan, M.S.Y. Biologically active saponins from Dodonea viscosa. Phytochemistry 1987, 26, 697–701. [Google Scholar] [CrossRef]
- Khan, M.S.Y.; Javed, K.; Hasnain Khan, M. Constituents of the flowers of Dodonea viscosa. Fitoterapia 1992, 63, 83–84. [Google Scholar]
- Ortega, A.; Garcı́a, P.E.; Cárdenas, J.; Mancera, C.; Marquina, S.; del Carmen Garduño, M.L.; Maldonado, E. Methyl dodonates, a new type of diterpenes with a modified clerodane skeleton from Dodonea viscosa. Tetrahedron 2001, 57, 2981–2989. [Google Scholar] [CrossRef]
- Niu, H.M.; Zeng, D.Q.; Long, C.L.; Peng, Y.H.; Wang, Y.H.; Luo, J.F.; Wang, H.S.; Shi, Y.N.; Tang, G.H.; Zhao, F.W. Clerodane diterpenoids and prenylated flavonoids from Dodonaea viscosa. J. Asian. Nat. Prod. Res. 2010, 12, 7–14. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, Y.-D.; Hai, P.; Wang, F.; Liu, J.-K. Isoprenylated flavonoids and clerodane diterpenoids from Dodonea viscosa. Nat. Prod. Bioprospect. 2013, 3, 250–255. [Google Scholar] [CrossRef] [Green Version]
- Al-Aamri, K.K.; Hossain, M.A. New prenylated flavonoids from the leaves of Dodonea viscosa native to sultanate of Oman. Pac. Sci. Rev. A Nat. Sci. Eng. 2016, 18, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.-B.; Liao, H.-B.; Zhu, H.-Y.; Yu, M.-H.; Lei, C. Antiviral clerodane diterpenoids from Dodonea viscosa. Tetrahedron 2016, 72, 8036–8041. [Google Scholar] [CrossRef]
- Mekkawi, A.G.; Mossa, J.S. Essential oil of Dodonaea viscosa Jacq. Pharmazie 1981, 36, 517. [Google Scholar]
- Bohlmann, F.; Grenz, M.; Wegner, P.; Jakupovic, J. Clerodan-derivate und neuartige diterpene aus Conyza scabrida DC. Liebigs Ann. Chem. 1983, 11, 2008–2020. [Google Scholar] [CrossRef]
- Ohsaki, A.; Shibata, K.; Tokoroyama, T.; Kubota, T. Structure of Pilosanones A and B: Novel diterpenoids with a bicyclo[5.4.0]undecane skeleton from Portulaca pilosa L. J. Chem. Soc. Chem. Commun. 1987, 3, 151–153. [Google Scholar] [CrossRef]
- Ohsaki, A.; Kasetani, Y.; Asaka, Y.; Shibata, K.; Tokoroyama, T.; Kubota, T. Clerodane diterpenoids from the roots of Portulaca pilosa. Phytochemistry 1991, 30, 4075–4077. [Google Scholar] [CrossRef]
- Ohsaki, A.; Ohno, N.; Shibata, K.; Tokoroyama, T.; Kubota, T.; Hirotsu, K.; Higuchi, T. Minor diterpenoids from Portulaca cv Jewel. Phytochemistry 1988, 27, 2171–2173. [Google Scholar] [CrossRef]
- He, K.; Montenegro, G.; Hoffmann, J.J.; Timmermann, B.N. Diterpenoids from Baccharis linearis. Phytochemistry 1996, 41, 1123–1127. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oils Components by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured: Carol Stream, IL, USA, 2001. [Google Scholar]
- Joulain, D.; Konig, W.A. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons; EB-Verlag: Hamburg, Germany, 1998; Available online: https://0-pubs-acs-org.brum.beds.ac.uk/doi/abs/10.1021/np990755n (accessed on 27 December 2019).
- Kondjoyan, N.; Berdagué, J.L. A Compilation of Relative Retention Indices for the Analysis of Aromatic Compounds, 1st ed.; Laboratoire Flaveur: Theix, France, 1996. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D. 01; Gaussian, Inc.: Oxford, UK, 2013. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Cheeseman, J.R.; Trucks, G.W.; Keith, T.A.; Frisch, M.J. A comparison of models for calculating nuclear magnetic resonance shielding tensors. J. Chem. Phys. 1996, 104, 5497–5509. [Google Scholar] [CrossRef]
- Stephens, P.J.; Harada, N. ECD cotton effect approximated by the Gaussian curve and other methods. Chirality 2010, 22, 229–233. [Google Scholar] [CrossRef]
- Schaftenaar, G. Noordik Molden: A pre- and post-processing program for molecular and electronic structures. J. Comput. Aided Mol. Des. 2000, 14, 123–134. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of Dodonea viscosa essential oil and the isolated compounds are available from the authors. |







| Compounds a | RI b | % c | |
| Hydrocarbons | |||
| n-heneicosane | 2097 | tr | |
| n-pentacosane | 2495 | tr | |
| total | tr | ||
| Alcohols | |||
| hex-3-enol (Z/E configuration n.i.) | 853 | 0.8 | |
| hex-2-enol (Z/E configuration n.i.) | 861 | tr | |
| n-hexanol | 864 | 0.8 | |
| oct-1-en-3-ol | 978 | tr | |
| n-octanol | 1069 | m (1.3) | |
| n-decanol | 1271 | tr | |
| total | 2.9 | ||
| Ketones | |||
| isophorone | 1125 | tr | |
| 1-phenylbut-2-enone | 1312 | 0.3 | |
| (Z)-jasmone | 1401 | tr | |
| geranylacetone | 1452 | 0.2 | |
| (E)-β-ionone | 1490 | 0.2 | |
| 6,10,14-trimethylpentadecan-2-one | 1843 | 1.1 | |
| total | 1.8 | ||
| Carboxylic acids | |||
| isovaleric acid | 826 | tr | |
| 2-methylbutanoic acid | 837 | tr | |
| hexanoic acid | 973 | 0.3 | |
| benzoic acid | 1159 | tr | |
| octanoic acid | 1167 | tr | |
| dodecanoic acid | 1559 | 0.2 | |
| tetradecanoic acid | 1759 | 0.7 | |
| hexadecanoic acid | 1963 | 2.4 | |
| total | 3.6 | ||
| Esters | |||
| isopentyl isovalerate | 1104 | 1.5 | |
| n-hexyl butanoate | 1190 | tr | |
| n-hexyl 2-methylbutanoate | 1236 | 0.4 | |
| n-hexyl 3-methylbutanoate | 1239 | 2.3 | |
| total | 4.2 | ||
| Oxygenated monoterpenes | |||
| trans-linalool oxide (furanoid) | 1074 | 0.1 | |
| cis-linalool oxide (furanoid) | 1091 | tr | |
| linalool | 1100 | 1.0 | |
| terpinen-4-ol | 1182 | 0.4 | |
| α-terpineol | 1195 | 0.3 | |
| nerol | 1230 | 0.2 | |
| geraniol | 1255 | tr | |
| total | 2.0 | ||
| Sesquiterpene hydrocarbons | |||
| aromadendrene | 1449 | 0.2 | |
| (E,E)-α-farnesene | 1508 | tr | |
| δ-cadinene | 1530 | tr | |
| α-calacorene | 1551 | tr | |
| total | 0.2 | ||
| Oxygenated sesquiterpenes | |||
| (E)-nerolidol | 1565 | 0.2 | |
| (Z)-dihydro-apofarnesol | 1579 | tr | |
| spathulenol | 1588 | 0.5 | |
| globulol | 1595 | 0.5 | |
| viridiflorol | 1604 | 0.2 | |
| epi-α-muurolol | 1658 | tr | |
| α-cadinol | 1664 | 0.2 | |
| (2E,6Z)-farnesol | 1744 | tr | |
| total | 1.6 | ||
| Oxygenated diterpenes | |||
| isophytol | 1947 | tr | |
| (1) | 2036 | 35.0 | |
| phytol | 2111 | 0.5 | |
| (2) + (3) | 2420 | m (16.0) | |
| hardwickiic acid, methyl ester | 2431 | m (16.0) | |
| total | 51.5 | ||
| Aromatic compounds | |||
| benzaldehyde | 963 | 0.1 | |
| benzyl alcohol | 1035 | 1.8 | |
| benzene acetaldehyde | 1045 | 0.1 | |
| o-cresol | 1054 | 0.7 | |
| acetophenone | 1068 | m (1.3) | |
| phenylethyl alcohol | 1116 | tr | |
| methyl salicylate | 1199 | 0.5 | |
| 4-vinyl phenol | 1218 | 0.1 | |
| chavicol | 1254 | tr | |
| p-anisaldehyde dimethyl acetal | 1256 | tr | |
| p-ethylacetophenone | 1285 | tr | |
| 1-phenylbut-2-en-1-one | 1312 | 0.3 | |
| 2-methoxy-4-vinylphenol | 1317 | 0.5 | |
| benzyl butanoate | 1346 | tr | |
| eugenol | 1360 | 0.9 | |
| methyl p-anisate | 1378 | tr | |
| benzyl isovalerate | 1395 | 2.1 | |
| vanillin | 1401 | tr | |
| methyl eugenol | 1403 | 0.4 | |
| 2-methylbutyl benzoate | 1440 | 0.2 | |
| ethyl vanillin | 1461 | tr | |
| phenylethyl 3-methylbutanoate | 1493 | 0.5 | |
| asaricin | 1500 | tr | |
| isopentyl salicylate | 1539 | 0.1 | |
| (3Z)-hexenyl benzoate | 1574 | 0.8 | |
| n-hexyl benzoate | 1580 | 1.0 | |
| (3Z)-hexenyl salicylate | 1673 | 0.2 | |
| n-hexyl salicylate | 1681 | 0.2 | |
| benzyl benzoate | 1771 | 0.6 | |
| benzyl salicylate | 1877 | 0.5 | |
| total | 12.9 | ||
| Others | |||
| 7-methyl-1,6-dioxaspiro [4,5] decane (stereoisomer n.i.) | 1058 | 1.1 | |
| vitispirane | 1286 | tr | |
| total | 1.1 | ||
| Total identified | 80.5 | ||
| Position | Dodovisate C (1) | Methyl Dodovisate A (2) | Methyl Iso-Dodovisate A (3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| δ 13C (150 MHz) | δ 1H (600 MHz) | HMBC (H→C) | δ 13C (500 MHz) | δ 1H (125 MHz) | HMBC (H→C) | δ 13C (500 MHz) | δ 1H (125 MHz) | HMBC (H→C) | |
| 1 | 130.9 s | 135.5 s | 38.3 d | 1.52 m a | 2, 4, 6, 7, 10, 11 | ||||
| 2 | 33.8 t | 2.28 dd (12.3, 7.0) 2.07 dd (12.3, 6.8) | 1, 3, 4, 7, 11 | 33.3 t | 2.30 m 2.71 bd (11.5) | 1, 3, 4, 7 | 135.0 d | 6.31 d (3.7) | 1, 4, 5w, 11 |
| 3 | 123.2 d | 5.49 ddd (8.9, 7.0, 6.8) | 1, 2, 5 | 123.7 s | 126.8 s | ||||
| 4 | 124.7 d | 5.98 dd (8.9, 5.1) | 2, 6 | 132.0 d | 7.10 d (5.6) | 2, 3, 5, 6 | 125.9 d | 7.06 d (11.1) | 2, 6 |
| 5 | 128.0 d | 6.40 dd (11.4, 5.1) | 3, 4, 7 | 127.6 d | 6.58 dd (11.6, 5.6) | 1, 3, 4 | 131.5 d | 6.75 dd (11.1, 5.7) | 2 w, 4, 6, 7 |
| 6 | 131.1 d | 6.64 d (11.4) | 1, 4, 5, 7, 8 | 136.6 d | 6.97 d (11.6) | 3, 4, 5, 7, 8 | 119.5 d | 6.13 d (5.7) | 4 |
| 7 | 134.0 s | 134.5 s | 143.5 s | ||||||
| 8 | 39.1 s | 40.4 s | 42.3 s | ||||||
| 9 | 32.3 d | 1.69 m | 7, 8, 19 | 33.2 d | 1.78 m | 19 | 34.9 d | 1.89 m a | 19 |
| 10 | 26.2 t | 1.40 m | 1, 8, 9 | 26.9 t | 1.45 m 1.55 m | 1, 8, 9 | 26.3 t | 1.54 m a 1.73 m (dquin like) | 1 w, 8, 11 |
| 11 | 31.4 t | 2.16 m 2.34 m | 1, 7, 10 | 31.8 t | 2.28 m 2.43 m | 1, 7, 10 | 28.4 t | 1.78 m2.02 m | 1 w, 9, 10 |
| 12 | 166.9 s | 167.6 s | |||||||
| 13 | 37.5 t | 1.69 m | 7, 8, 9, 14, 15 | 38.3 t | 1.80 m | 8, 9, 14 | 37.0 t | 1.83 m a, 1.89 ma | |
| 14 | 18.4 t | 1.88 m 2.16 m | 13, 15, 16, 18 | 19.5 t | 1.97 m 2.23 m | 13, 15, 16, 18 | 19.6 t | 2.53 dbrt (3.8, 12.2) 2.43 dbrt (5.2, 12.2) | 9 w, 13, 15, 16, 18 |
| 15 | 124.9 s | 125.6 s | 125.6 s | ||||||
| 16 | 110.0 d | 6.18 s | 15, 17, 18 | 110.9 d | 6.25 s | 15, 17, 18 | 110.9 d | 6.34 brs | 15 w, 17, 18 |
| 17 | 141.5 d | 7.20 s | 15, 16, 18 | 142.6 d | 7.34 s | 15, 16, 18 | 142.8 d | 7.34 brs | 15, 16 |
| 18 | 137.3 d | 7.10 s | 15, 16, 17 | 138.4 d | 7.18 s | 15, 16, 17 | 138.4 d | 7.25 brs | 15, 16, 17 |
| 19 | 22.0 q | 0.85 s | 7, 8, 9, 13 | 23.3 q | 0.88 s | 8, 13 | 30.0 q | 0.72 s | 7, 8, 9, 13, 14 w |
| 20 | 15.1 q | 0.82 d (3.6) | 8, 9, 10 | 15.9 q | 0.83 d (6.8) | 8, 9, 10 | 16.3 q | 0.81 d (7) | 8, 9, 10 |
| 21 | 51.9 q | 3.78 s | 12 | 51.8 q | 3.75 s | 12 | |||
| Conformer | a | b | c | d | e | f |
| Figure |  |  |  |  |  |  |
| E (ua) | −813.474252 | −813.473483 | −813.474156 | −813.473265 | −813.472512 | −813.473392 |
| Dipole moment | 1.83 | 1.77 | 1.47 | 1.85 | 1.81 | 1.57 |
| Dihedral angle | −111.4° | 1.2° | 110.3° | −110.7° | 1.3° | 110.2° |
| ΔE (kJ/mol) | 0.00 | 2.02 | 0.25 | 2.59 | 4.57 | 2.26 |
| Population (%) | 30.7 | 13.6 | 27.7 | 10.8 | 4.9 | 12.3 |
| Conformer | g | h | i | j | k | l |
| Figure |  |  |  |  |  |  |
| E (ua) | −813.467509 | −813.466609 | −813.467563 | −813.468819 | −813.467850 | −813.469001 |
| Dipole moment | 1.65 | 1.66 | 1.34 | 1.71 | 1.72 | 1.43 |
| Dihedral angle | −111.0° | 5.4 | 109.1 | −109.8 | 4.3 | 110.0 |
| ΔE (kJ/mol) | 17.70 | 20.07 | 17.56 | 14.26 | 16.81 | 13.79 |
| Population (%) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Conformer | a’ | b’ | c’ | d’ | e’ | f’ |
| Figure |  |  |  |  |  |  |
| E (ua) | −813.463138 | −813.462352 | −813.463090 | −813.464746 | −813.463918 | −813.464838 |
| Dipole moment | 1.76 | 1.74 | 1.46 | 1.82 | 1.81 | 1.56 |
| Dihedral angle | −111.4° | 4.8° | 109.4° | −110.9° | 3.5° | 110.3° |
| ΔE (kJ/mol) | 4.46 | 6.53 | 4.59 | 0.24 | 2.42 | 0.00 |
| Population (%) | 6.1 | 2.7 | 5.8 | 33.6 | 14.0 | 37.1 |
| Conformer | g’ | h’ | i’ | j’ | k’ | l’ |
| Figure |  |  |  |  |  |  |
| E (ua) | −813.460304 | −813.459717 | −813.460261 | −813.458557 | −813.457959 | −813.4585678 |
| Dipole moment | 1.95 | 1.78 | 1.60 | 1.97 | 1.79 | 1.64 |
| Dihedral angle | −112.7° | 1.8° | 113.4° | −111.6° | 0.9° | 112.7 |
| ΔE (kJ/mol) | 11.91 | 13.45 | 12.02 | 16.49 | 18.06 | 16.46 |
| Population (%) | 0.3 | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 |
| Conformer | 5 | 10 | 4 | 11 | 12 | 8 |
| Figure |  |  |  |  |  |  |
| E (ua) | −1041.381622 | −1041.380520 | −1041.381631 | −1041.380467 | −1041.379800 | −1041.380902 |
| Dipole moment | 1.08 | 3.62 | 0.87 | 3.12 | 3.12 | 0.35 |
| Dihedral angle | −111.6° | −112.5° | 110.5° | 109.7° | 3.6° | 2.4° |
| ΔE (kJ/mol) | 3.15 | 6.04 | 3.12 | 6.18 | 7.93 | 5.04 |
| Population (%) | 7.2 | 2.2 | 7.2 | 2.1 | 1.1 | 3.3 |
| Conformer | 6 | 1 | 7 | 2 | 3 | 9 |
| Figure |  |  |  |  |  |  |
| E (ua) | −1041.381577 | −1041.382821 | −1041.381499 | −1041.382724 | −1041.382110 | −1041.380875 |
| Dipole moment | 2.92 | 1.61 | 2.05 | 1.73 | 0.98 | 2.70 |
| Dihedral angle | −111.8° | −111.8 | 110.4 | 110.4 | 1.3 | 1.2 |
| ΔE (kJ/mol) | 3.27 | 0.00 | 3.47 | 0.25 | 1.87 | 5.11 |
| Population (%) | 6.8 | 25.5 | 6.3 | 23.0 | 12.0 | 3.3 |
| Conformer | 4 | 2 | 5 | 1 | 3 | 8 |
| Figure |  |  |  |  |  |  |
| E (ua) | −1041.379759 | −1041.380966 | −1041.379717 | −1041.381006 | −1041.380144 | −1041.378913 |
| Dipole moment | 3.65 | 1.13 | 3.12 | 0.98 | 0.28 | 3.20 |
| dihedral angle | −112.4° | −111.7 | 109.5° | 110.6° | 3.7° | 6.0° |
| ΔE (kJ/mol) | 3.27 | 0.11 | 3.39 | 0.00 | 2.26 | 5.49 |
| Population (%) | 7.8 | 28.0 | 7.5 | 29.2 | 11.7 | 3.2 |
| Conformer | 11 | 7 | 10 | 6 | 9 | 12 |
| Figure |  |  |  |  |  |  |
| E (ua) | −1041.378027 | −1041.379098 | −1041.378051 | −1041.379135 | −1041.378310 | −1041.377274 |
| Dipole moment | 2.73 | 1.96 | 1.93 | 1.86 | 1.22 | 2.61 |
| dihedral angle | −111.8° | −111.7° | 109.6° | 109.5° | 4.9° | 4.9° |
| ΔE (kJ/mol) | 7.82 | 5.01 | 7.76 | 4.91 | 7.08 | 9.80 |
| Population (%) | 1.2 | 3.9 | 1.3 | 4.0 | 1.7 | 0.5 |
| Compound | Isomers | Slope | Intercept | Coefficient Correlation | Fischer-F Statistic | s in ppm |
|---|---|---|---|---|---|---|
| Dodovisate C (1) | 8R,9S | 1.027(7) | 4.5(6) | 1.000(6) | 26661 | 1.4 |
| 8R,9R | 1.006(10) | 8.1(1.0) | 0.999(10) | 10440 | 2.2 | |
| Methyl dodovisate A (2) | 8R,9S | 0.9685(7) | 4.7(7) | 0.999(7) | 18342 | 1.7 |
| 8R,9R | 0.9453(9) | 6.2(9) | 0.999(9) | 11412 | 2.1 | |
| Methyl iso-dodovisate A (3) | 1S,8R,9R | 0.9405(7) | 7.0(8) | 0.999(8) | 15132 | 1.8 |
| 1S,8R,9S | 0.9610(10) | 4.3(1.0) | 0.999(10) | 10007 | 2.3 | |
| 1S,8S,9R | 0.9479(10) | 6.2(1.9) | 0.996(20) | 2593 | 4.4 | |
| 1S,8S,9S | 0.9613(13) | 4.5(1.3) | 0.998(13) | 5556 | 3.1 |
| Conformer | 7 | 10 | 9 | 12 | 11 | 5 |
| Figure |  |  |  |  |  |  |
| E (ua) | −1041.365849 | −1041.365746 | −1041.365780 | −1041.364999 | −1041.365036 | −1041.366448 |
| Dipole moment | 2.90 | 1.61 | 3.20 | 1.11 | 3.72 | 1.01 |
| dihedral angle | −114.6° | 112.0° | 112.2° | 1.0° | 1.0° | 2.7° |
| ΔE (kJ/mol) | 3.44 | 3.71 | 3.62 | 5.68 | 5.58 | 1.87 |
| Population (%) | 4.2 | 3.7 | 3.9 | 1.7 | 1.7 | 7.9 |
| Conformer | 6 | 8 | 2 | 1 | 4 | 3 |
| Figure |  |  |  |  |  |  |
| E (ua) | −1041.366428 | −1041.365784 | −1041.367137 | −1041.367160 | −1041.367124 | −1041.367129 |
| Dipole moment | 3,11 | 0,82 | 0,75 | 3,58 | 0,70 | 2,74 |
| dihedral angle | 2.8° | −114.4° | −112.9° | −112.9° | 110.8° | 110.7° |
| ΔE (kJ/mol) | 1.92 | 3.61 | 0.06 | 0.00 | 0.10 | 0.08 |
| Population (%) | 7.7 | 3.9 | 16.3 | 16.7 | 16.1 | 16.2 |
| Conformer | 3 | 1 | 4 | 2 | 5 | 6 |
| Figure |  |  |  |  |  |  |
| E (ua) | −1041.365913 | −1041.365955 | −1041.365867 | −1041.365937 | −1041.365388 | −1041.365382 |
| Dipole moment | 1.21 | 3.73 | 0.97 | 2.88 | 1.73 | 3.31 |
| dihedral angle | −116.4° | −116.1° | 112.4 | 112.2° | 1.7° | 2.0° |
| ΔE (kJ/mol) | 0.11 | 0.00 | 0.3 | 0.05 | 1.49 | 1.50 |
| Population (%) | 17.9 | 18.7 | 17.0 | 18.3 | 10.2 | 10.2 |
| Conformer | 8 | 9 | 7 | |||
| Figure |  |  |  | |||
| E (ua) | −1041.364092 | −1041.364040 | −1041.364102 | |||
| Dipole moment | 0.77 | 0.76 | 2.71 | |||
| dihedral angle | −112.5° | 110.4 | 110.4° | |||
| ΔE (kJ/mol) | 4.89 | 5.03 | 4.86 | |||
| Population (%) | 2.6 | 2.5 | 2.6 |
| Conformer | 1 | 2 | 5 | 10 | 3 | 4 |
| Figure |  |  |  |  |  |  |
| E (ua) | −1041.369364 | −1041.368947 | −1041.367744 | −1041.367199 | −1041.368890 | −1041.368444 |
| Dipole moment | 2.51 | 3.23 | 2.36 | 2.49 | 2.51 | 2.60 |
| dihedral angle | −105.1° | −105.8° | 2.8° | 3.9° | 92.7° | 97.5 |
| ΔE (kJ/mol) | 0.00 | 1.09 | 4.25 | 5.68 | 1.24 | 2.42 |
| Population (%) | 28.7 | 18. | 5.2 | 2.9 | 17.4 | 10.8 |
| Conformer | 9 | 8 | 12 | 7 | 6 | 11 |
| Figure |  |  |  |  |  |  |
| E (ua) | −1041.367202 | −1041.367402 | −1041.366173 | −1041.367417 | −1041.367530 | −1041.366354 |
| Dipole moment | 2.40 | 3.50 | 2.50 | 2.92 | 2.12 | 2.69 |
| dihedral angle | −107.4° | −109.8° | −2.1° | 109.9° | 111.0° | 0.6° |
| ΔE (kJ/mol) | 5.68 | 5.15 | 8.38 | 5.11 | 4.82 | 7.90 |
| Population (%) | 2.9 | 3.6 | 1.0 | 3.7 | 4.1 | 1.2 |
| Conformer | 1 | 3 | 5 | 6 | 2 | 4 |
| Figure |  |  |  |  |  |  |
| E (ua) | −1041.368995 | −1041.368611 | −1041.367491 | −1041.366883 | −1041.368632 | −1041.368189 |
| Dipole moment | 2,58 | 3.24 | 2.38 | 2.56 | 2.57 | 2.66 |
| dihedral angle | −104.0° | −105.3° | 2.4° | 3.7° | 94.4° | 98.6° |
| ΔE (kJ/mol) | 0.00 | 1.01 | 3.95 | 5.55 | 0.95 | 2.12 |
| Population (%) | 32.4 | 21.6 | 6.6 | 3.5 | 22 | 13.8 |
| Conformer | 7 | |||||
| Figure |  | |||||
| E (ua) | −1041.362938 | |||||
| Dipole moment | 1.89 | |||||
| dihedral angle | 110.9° | |||||
| ΔE (kJ/mol) | 15.90 | |||||
| Population (%) | 0.1 |
| Voucher Number | Collection Places (Altitude) | Geographical Coordinates (GPS System) | Date | Oil Yield (%) |
|---|---|---|---|---|
| REU08438 | Vincendo (67 m) | 21°22′859 S 55°40′108 E | February 2009 | 0.10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marvilliers, A.; Illien, B.; Gros, E.; Sorres, J.; Kashman, Y.; Thomas, H.; Smadja, J.; Gauvin-Bialecki, A. Modified Clerodanes from the Essential Oil of Dodonea viscosa Leaves. Molecules 2020, 25, 850. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25040850
Marvilliers A, Illien B, Gros E, Sorres J, Kashman Y, Thomas H, Smadja J, Gauvin-Bialecki A. Modified Clerodanes from the Essential Oil of Dodonea viscosa Leaves. Molecules. 2020; 25(4):850. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25040850
Chicago/Turabian StyleMarvilliers, Arnaud, Bertrand Illien, Emmanuelle Gros, Jonathan Sorres, Yoel Kashman, Hermann Thomas, Jacqueline Smadja, and Anne Gauvin-Bialecki. 2020. "Modified Clerodanes from the Essential Oil of Dodonea viscosa Leaves" Molecules 25, no. 4: 850. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25040850