Synthesis and Structure–Activity Relationship of Palmatine Derivatives as a Novel Class of Antibacterial Agents against Helicobacter pylori
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Pharmacological Evaluation
2.3. Acute Toxicity Assay of Compound 1c
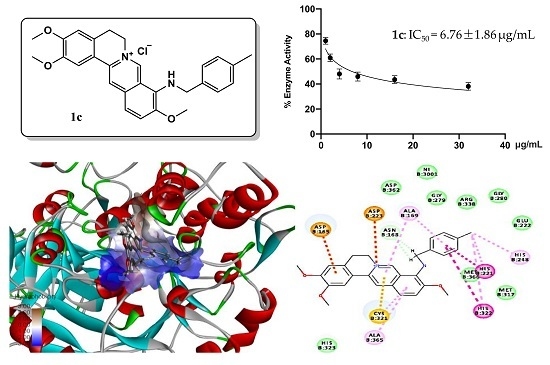
2.4. Molecular Docking Study of Key Compound 1c
2.5. The Inhibitory Effects on Urease of Compound 1c
3. Experimental Section
3.1. Apparatus, Materials, and Analysis Reagents
3.2. General Synthesis Procedures for Compounds 1a–i and 2
3.3. Synthesis of 3
3.4. General Procedure for the Synthesis of Compounds 4a–i
3.5. Biology Assay
3.5.1. Antimicrobial Assay
3.5.2. Acute Toxicity
3.5.3. Molecular Docking Assay
3.5.4. Enzyme Inhibition Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kishikawa, H.; Ojiro, K.; Nakamura, K.; Katayama, T.; Arahata, K.; Takarabe, S.; Miura, S.; Kanai, T.; Nishida, J. Previous Helicobacter pylori infection-induced atrophic gastritis: A distinct disease entity in an understudied population without a history of eradication. Helicobacter 2019, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Chen, C.; Hu, J.; Su, R.; Zhang, J.; Han, Z.; Chen, H.; Li, Y. Molecular mechanism of Helicobacter pylori-induced autophagy in gastric cancer. Oncol. Lett. 2019, 18, 6221–6227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pichon, M.; Broutin, L.; Touroult-Jupin, P.; Cremniter, J.; Plouzeau, C.; Faure, J.P.; Olivier, R.; Burucoa, C. First detection in Helicobacter suis of a mutation conferring resistance to clarithromycin in Helicobacter pylori: Case report and review of the literature. Microb. Drug Resist. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Metz, D.C.; Ellenberg, S.; Kaplan, D.E.; Goldberg, D.S. Risk factors and incidence of gastric cancer after detection of Helicobacter pylori infection: A large cohort study. Gastroenterology 2019. [Google Scholar] [CrossRef] [Green Version]
- Kato, M.; Ota, H.; Okuda, M.; Kikuchi, S.; Satoh, K.; Shimoyama, T.; Suzuki, H.; Handa, O.; Furuta, T.; Mabe, K.; et al. Guidelines for the management of Helicobacter pylori infection in Japan. Helicobacter 2019, 24. [Google Scholar] [CrossRef]
- Gisbert, J.P.; McNicholl, A.G. Optimization strategies aimed to increase the efficacy of H. pylori eradication therapies. Helicobacter 2017, 22. [Google Scholar] [CrossRef]
- Liu, Y.X.; Xiao, C.L.; Wang, Y.X.; Li, Y.H.; Yang, Y.H.; Li, Y.B.; Bi, C.W.; Gao, L.M.; Jiang, J.D.; Song, D.Q. Synthesis, structure-activity relationship and in vitro anti-mycobacterial evaluation of 13-n-octylberberine derivatives. Eur. J. Med. Chem. 2012, 52, 151−158. [Google Scholar] [CrossRef]
- Fan, T.Y.; Wang, Y.X.; Tang, S.; Hu, X.X.; Zeng, Q.X.; Pang, J.; Yang, Y.S.; You, X.F.; Song, D.Q. Synthesis and antibacterial evaluation of 13-substituted cycloberberine derivatives as a novel class of anti-MRSA agents. Eur. J. Med. Chem. 2018, 157, 877–886. [Google Scholar] [CrossRef]
- Fan, T.Y.; Hu, X.X.; Tang, S.; Liu, X.J.; Wang, Y.X.; Deng, H.B.; You, X.F.; Jiang, J.D.; Li, Y.H.; Song, D.Q. Discovery and development of 8-substituted cycloberberine derivatives as novel antibacterial agents against MRSA. ACS Med. Chem. Lett. 2018, 9, 484–489. [Google Scholar] [CrossRef]
- Yang, Y.S.; Wei, W.; Hu, X.X.; Tang, S.; Pang, J.; You, X.F.; Fan, T.Y.; Wang, Y.X.; Song, D.Q. Evolution and antibacterial evaluation of 8-hydroxy-cycloberberine derivatives as a novel family of antibacterial agents against MRSA. Molecules 2019, 24, 984. [Google Scholar] [CrossRef] [Green Version]
- Tarabasz, D.; Kukula-Koch, W. Palmatine: A review of pharmacological properties and pharmacokinetics. Phytother. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.L.; Usami, S.; Hsieh, M.T.; Jiang, M.J. Effects of palmatine on isometric force and intracellular calcium levels of arterial smooth muscle. Life Sci. 1999, 64, 597–606. [Google Scholar] [CrossRef]
- Vennerstrom, J.L.; Klayman, D.L. Protoberberine Alkaloids as Antimalarials. J. Med. Chem. 1988, 31, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.C.; Kong, X.B.; Mai, W.P.; Sun, G.C.; Zhao, S.Z. Synthesis and antimicrobial activity of 9-o-substituted palmatine derivatives. Indian J. Pharm. Sci. 2015, 77, 196–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, T.W.; Ye, X.L.; Li, X.G.; Zhang, B.S.; Jiang, X.F.; Chen, Z. Synthesis and antimicrobial activity of 8-alkylpalmatine derivatives. Lett. Drug Des. Discov. 2011, 8, 464–468. [Google Scholar] [CrossRef]
- Song, L.; Zhang, H.J.; Deng, A.J.; Li, J.; Li, X.; Li, Z.H.; Zhang, Z.H.; Wu, L.Q.; Wang, S.Q.; Qin, H.L. Syntheses and structure-activity relationships on antibacterial and anti-ulcerative colitis properties of quaternary 13-substituted palmatines and 8-oxo-13-substituted dihydropalmatines. Bioorg. Med. Chem. 2018, 26, 2586–2598. [Google Scholar] [CrossRef]
- Menteşe, E.; Akyuz, G.; Emirik, M.; Baltaş, N. Synthesis, in vitro urease inhibition and molecular docking studies of some novel quinazolin-4 (3H)-one derivatives containing triazole, thiadiazole and thiosemicarbazide functionalities. Bioorg. Chem. 2019, 83, 289–296. [Google Scholar] [CrossRef]
- Alomari, M.; Taha, M.; Imran, S.; Jamil, W.; Selvaraj, M.; Uddin, N.; Rahim, F. Design, synthesis, in vitro evaluation, molecular docking and ADME properties studies of hybrid bis-coumarin with thiadiazole as a new inhibitor of Urease. Bioorg. Chem. 2019, 92. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, W.K.; Ren, S.Z.; Ni, W.W.; Li, W.Y.; Chen, H.M.; Liu, P.; Yuan, J.; He, X.S.; Liu, J.J.; et al. Arylamino containing hydroxamic acids as potent urease inhibitors for the treatment of Helicobacter pylori infection. Eur. J. Med. Chem. 2018, 156, 126–136. [Google Scholar] [CrossRef]
- Li, W.Y.; Ni, W.W.; Ye, Y.X.; Fang, H.L.; Pan, X.M.; He, J.L.; Zhou, T.L.; Yi, J.; Liu, S.S.; Zhou, M.; et al. N-monoarylacetothioureas as potent urease inhibitors: Synthesis, SAR, and biological evaluation. J. Enzyme Inhib. Med. Chem. 2020, 35, 404–413. [Google Scholar] [CrossRef] [Green Version]
- Bailie, N.C.; Osborne, C.A.; Leininger, J.R.; Fletcher, T.F.; Johnston, S.D.; Ogburn, P.N.; Griffith, D.P. Teratogenic effect of acetohydroxamic acid in clinically normal beagles. Am. J. Vet. Res. 1986, 47, 2604–2611. [Google Scholar] [PubMed]
- Zhou, J.T.; Li, C.L.; Tan, L.H.; Xu, Y.F.; Liu, Y.H.; Mo, Z.Z.; Dou, Y.X.; Su, R.; Su, Z.R.; Huang, P.; et al. Inhibition of Helicobacter pylori and its associated urease by palmatine: Investigation on the potential mechanism. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, Q.; Xu, M.; Dong, Q.; Zhang, Y.L.; Li, Y.L.; Ye, G.; Zhao, L. In vitro and in vivo bactericidal activity of Tinospora sagittata (Oliv.) Gagnep. var. craveniana (S.Y.Hu) Lo and its main effective component, palmatine, against porcine Helicobacter pylori. BMC Complement Altern. Med. 2016, 16, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Pharmacopoeia Committee. Pharmacopoeia of Peoples Republic of China, 2015 ed.; Chinese Medical Science and Technology Press: Beijing, China, 2015; ISBN 978-7-5067-7337-9. [Google Scholar]
- Jung, J.; Choi, J.S.; Jeong, C.S. Inhibitory Activities of Palmatine from Coptis chinensis Against Helicobactor pylori and Gastric Damage. Toxicol. Res. 2014, 30, 45–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, M.F.; Drown, B.S.; Riley, A.P.; Garcia, A.; Shirai, T.; Svec, R.L.; Hergenrother, P.J. Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature 2017, 545, 299–304. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.X.; Pang, W.Q.; Zeng, Q.X.; Deng, Z.S.; Fan, T.Y.; Jiang, J.D.; Deng, H.B.; Song, D.Q. Synthesis and biological evaluation of new berberine derivatives as cancer immunotherapy agents through targeting IDO1. Eur. J. Med. Chem. 2018, 143, 1858–1868. [Google Scholar] [CrossRef]
- Naruto, S.; Mizuta, H.; Nishimura, H. A novel amination of isoquinolinium salts via nucleophilic substitution reaction. Tetrahedron Lett. 1976, 17, 1597–1600. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, version 5.0. 2015. Available online: http://www.eucast.org (accessed on 14 February 2019).
- Wang, G.Z.; Pang, J.; Hu, X.X.; Nie, T.Y.; Lu, X.; Li, X.; Wang, X.K.; Lu, Y.; Yang, X.Y.; Jiang, J.D.; et al. Daphnetin: A Novel Anti-Helicobacter pylori Agent. Int. J. Mol. Sci. 2019, 20, 850. [Google Scholar] [CrossRef] [Green Version]
- Ha, N.C.; Oh, S.T.; Sung, J.Y.; Cha, K.A.; Oh, B.H. Supramolecular assembly and acid resistance of Helicobacter pylori urease. Nat. Struct. Biol. 2001, 8, 505–509. [Google Scholar] [CrossRef]
- Balasubramanian, A.; Ponnuraj, K. Crystal Structure of the First Plant Urease From Jack Bean: 83 Years of Journey From Its First Crystal to Molecular Structure. J. Mol. Biol. 2010, 400, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Whitmire, J.M.; Merrell, D.S. Successful Culture Techniques for Helicobacter Species: General Culture Techniques for Helicobacter pylori. In Helicobacter Species; Humana Press: Totowa, NJ, USA, 2012; pp. 37–40. [Google Scholar]
- Fan, T.Y.; Hu, X.X.; Wang, Y.X.; You, X.F.; Song, D.Q. Anti-MRSA activities of cycloberberine derivatives with a novel chemical scaffold. Acta Pharm. Sin. 2019, 54, 1627–1635. [Google Scholar]
- Wu, D.W.; Yu, X.D.; Xie, J.H.; Su, Z.Q.; Su, J.Y.; Tan, L.R.; Huang, X.Q.; Chen, J.N.; Su, Z.R. Inactivation of jack bean urease by scutellarin: Elucidation of inhibitory efficacy, kinetics and mechanism. Fitoterapia 2013, 91, 60–67. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1a–i, 2, 3 and 4a–i are available from the authors. |





| Code | R | ATCC 43504 a | CCPMAP 160007 | CCPMAP 160008 | CCPMAP 160010 | CCPMAP 160011 | CCPMAP 160017 |
|---|---|---|---|---|---|---|---|
| PMT | - | 64 | 64 | 128 | 128 | 128 | 256 |
| 1a | C6H5 | 32 | 8 | 64 | 16 | 64 | 64 |
| 1b | m-CH3C6H4 | 16 | 4 | 8 | 4 | 16 | 16 |
| 1c | p-CH3C6H4 | 16 | 4 | 4 | 4 | 8 | 16 |
| 1d | p-CH3OC6H4 | 16 | 4 | 32 | 8 | 16 | 32 |
| 1e | m-CNC6H4 | >256 | 128 | >256 | 256 | >256 | >256 |
| 1f | p-FC6H4 | 128 | 64 | 128 | 8 | 128 | 128 |
| 1g | p-BrC6H4 | 64 | 8 | 16 | 4 | 64 | 64 |
| 1h | 2′-furyl | 32 | 8 | 64 | 16 | 16 | 16 |
| 1i | 2′-pyridyl | 64 | 16 | 128 | 32 | 64 | 128 |
| 2 | 2′,4′-(CH3O)2 C6H3 | 16 | 4 | 32 | 4 | 16 | 16 |
| 3 | - | >128 | >128 | >128 | >128 | >128 | >128 |
| 4a | C6H5 | >256 | 64 | >256 | >256 | >256 | >256 |
| 4b | p-(CH3)3CC6H4 | >256 | 16 | >256 | 128 | >256 | >256 |
| 4c | m-FC6H4 | >256 | 128 | >256 | 256 | >256 | >256 |
| 4d | 2′-furyl | 256 | 128 | >256 | 256 | >256 | >256 |
| 4e |  | 256 | 128 | >256 | 256 | >256 | >256 |
| 4f |  | >256 | 128 | >256 | 256 | >256 | >256 |
| 4g |  | >256 | >256 | >256 | >256 | >256 | >256 |
| 4h |  | >256 | >256 | >256 | >256 | >256 | >256 |
| 4i | 3′-pyridyl | >256 | >256 | >256 | >256 | >256 | >256 |
| MTZ b | - | 128 | 32 | 32 | 16 | 64 | 128 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, T.; Guo, X.; Zeng, Q.; Wei, W.; You, X.; Pang, J.; Wang, Y.; Song, D. Synthesis and Structure–Activity Relationship of Palmatine Derivatives as a Novel Class of Antibacterial Agents against Helicobacter pylori. Molecules 2020, 25, 1352. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25061352
Fan T, Guo X, Zeng Q, Wei W, You X, Pang J, Wang Y, Song D. Synthesis and Structure–Activity Relationship of Palmatine Derivatives as a Novel Class of Antibacterial Agents against Helicobacter pylori. Molecules. 2020; 25(6):1352. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25061352
Chicago/Turabian StyleFan, Tianyun, Xixi Guo, Qingxuan Zeng, Wei Wei, Xuefu You, Jing Pang, Yanxiang Wang, and Danqing Song. 2020. "Synthesis and Structure–Activity Relationship of Palmatine Derivatives as a Novel Class of Antibacterial Agents against Helicobacter pylori" Molecules 25, no. 6: 1352. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25061352