Oxone®-Mediated TEMPO-Oxidized Cellulose Nanomaterials form I and form II
Abstract
:1. Introduction
2. Results & Discussion
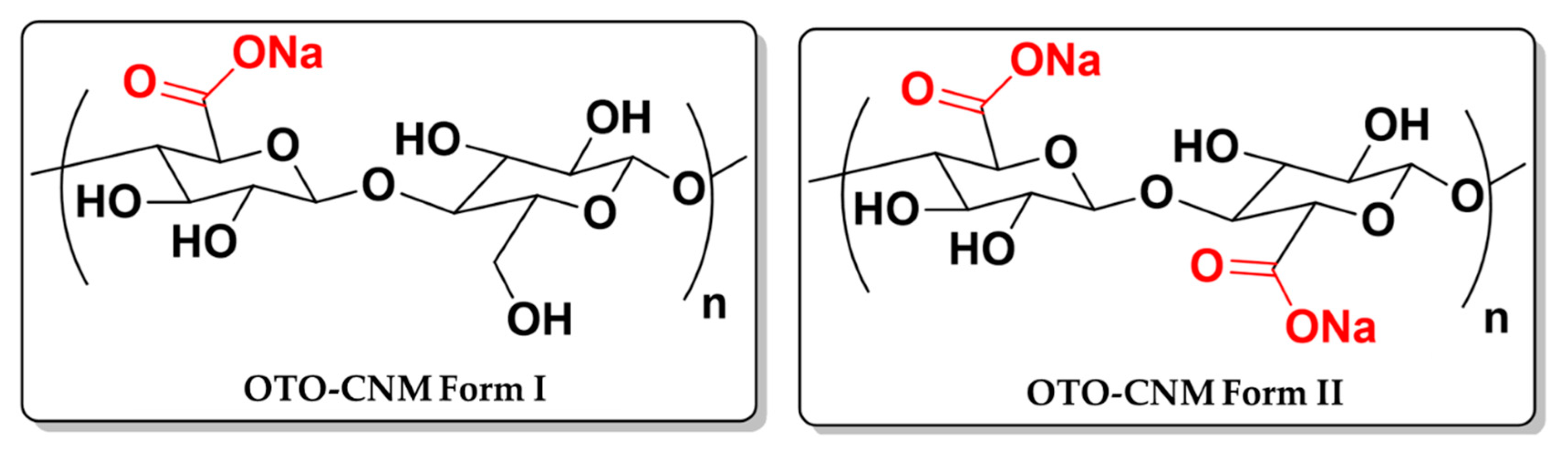
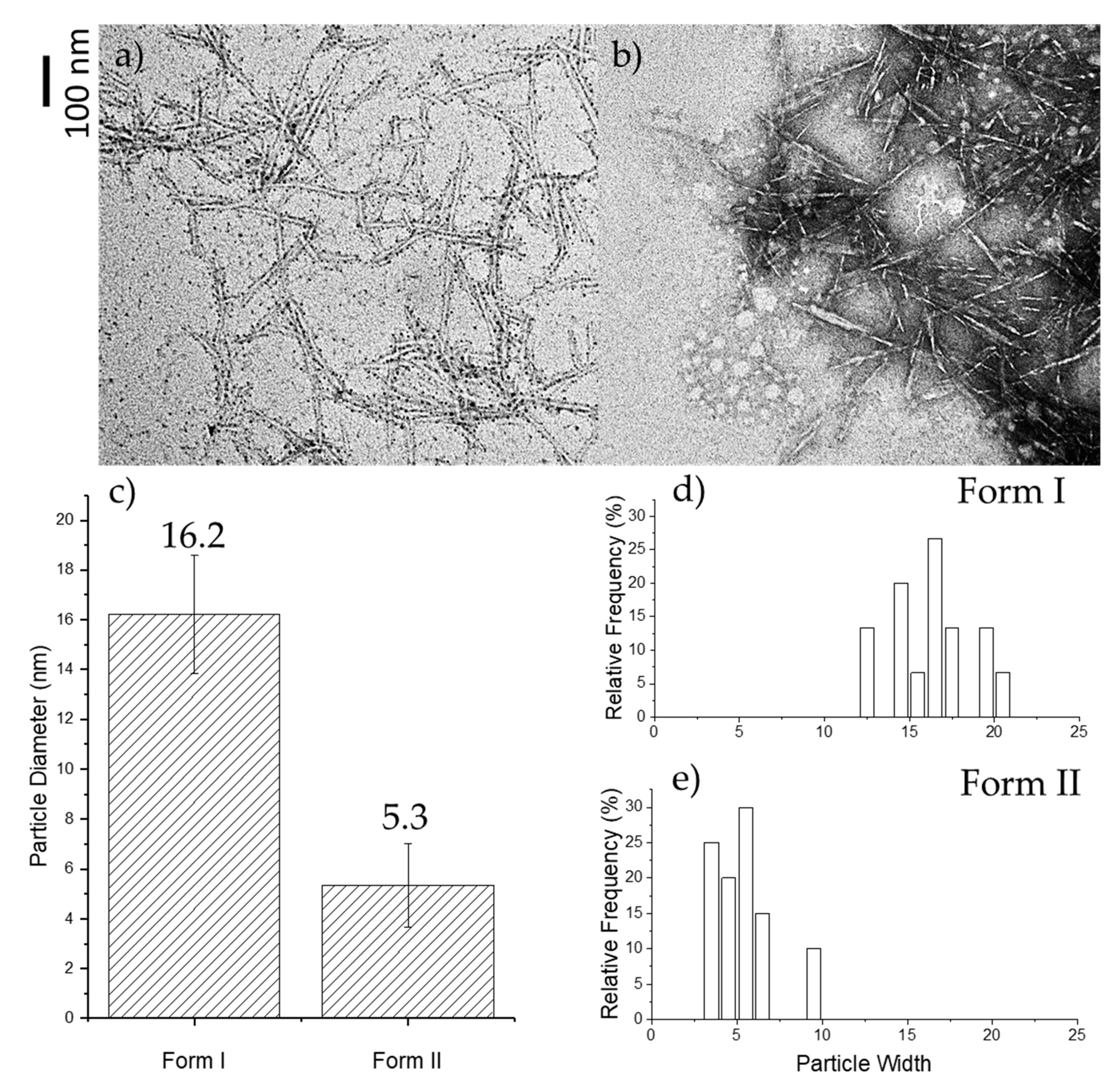
2.1. TEM Microscopy of Oxone®-Mediated TEMPO-Oxidized Cellulose
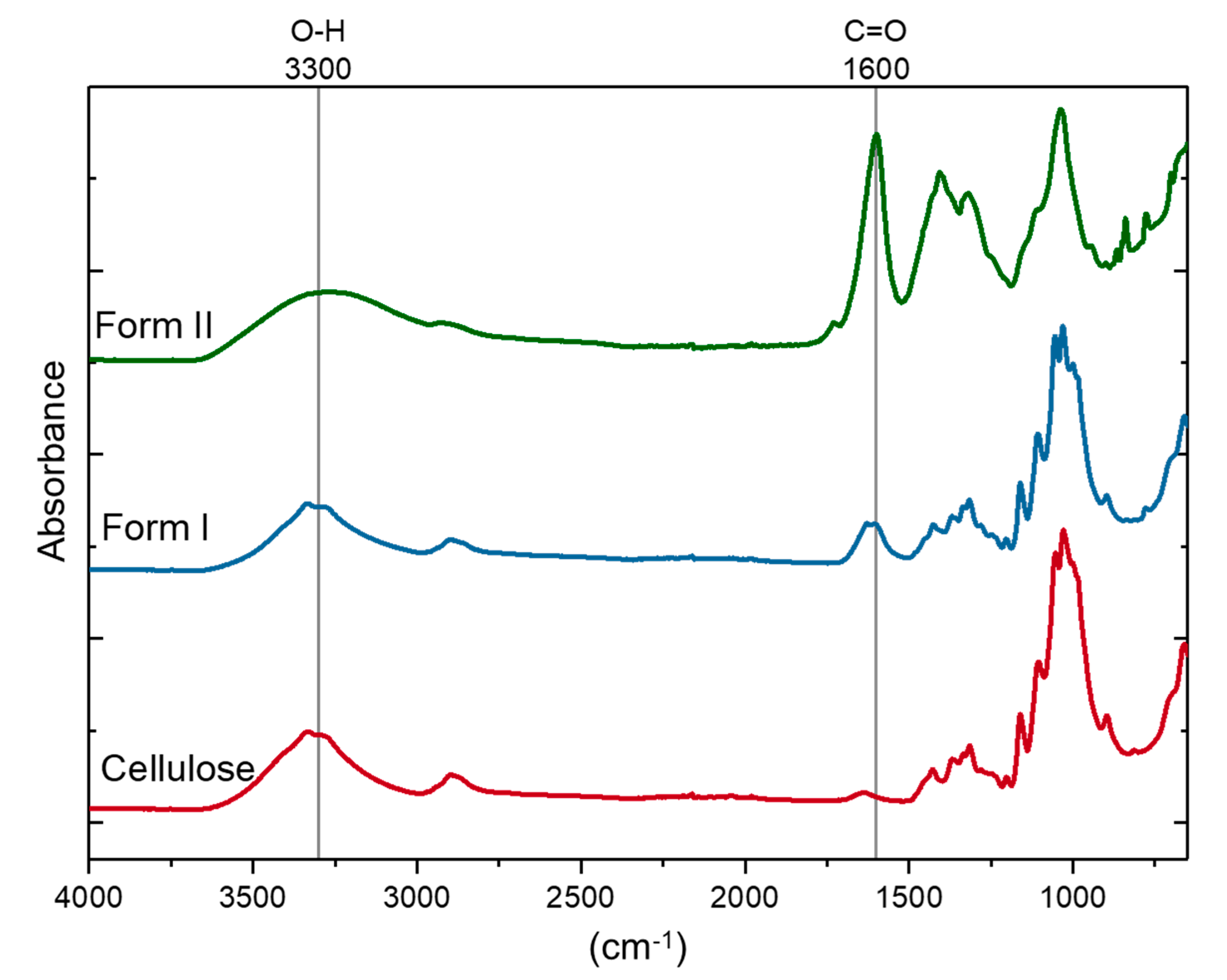
2.2. FT-IR Spectroscopy of TEMPO-Oxidized Nanocellulose
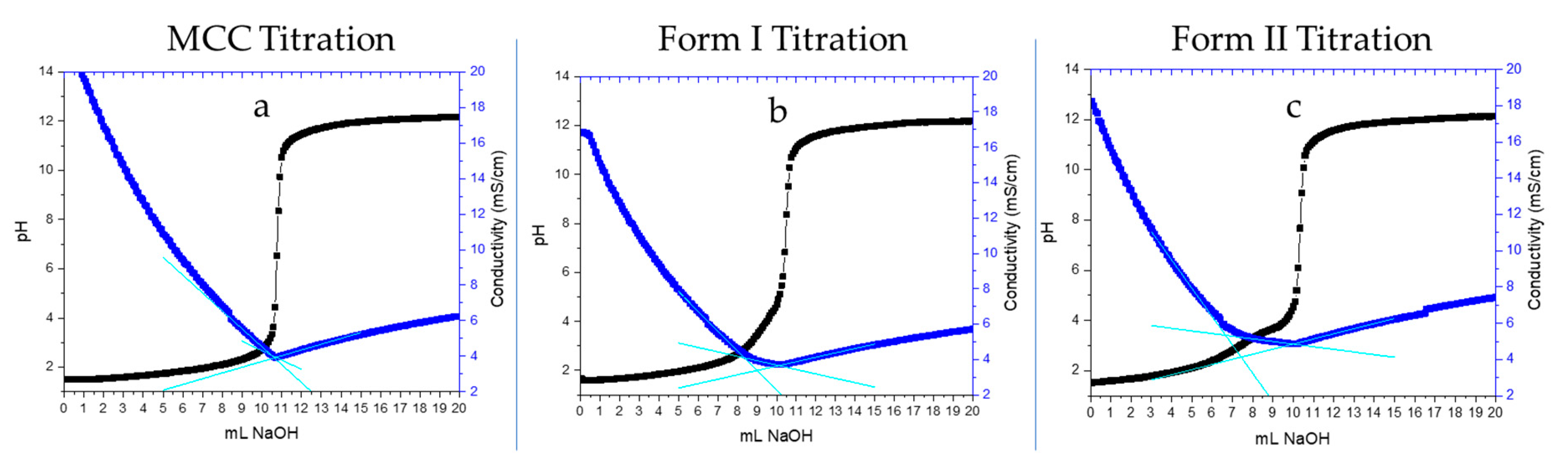
2.3. Charge Density Characterization by Conductometric Titration
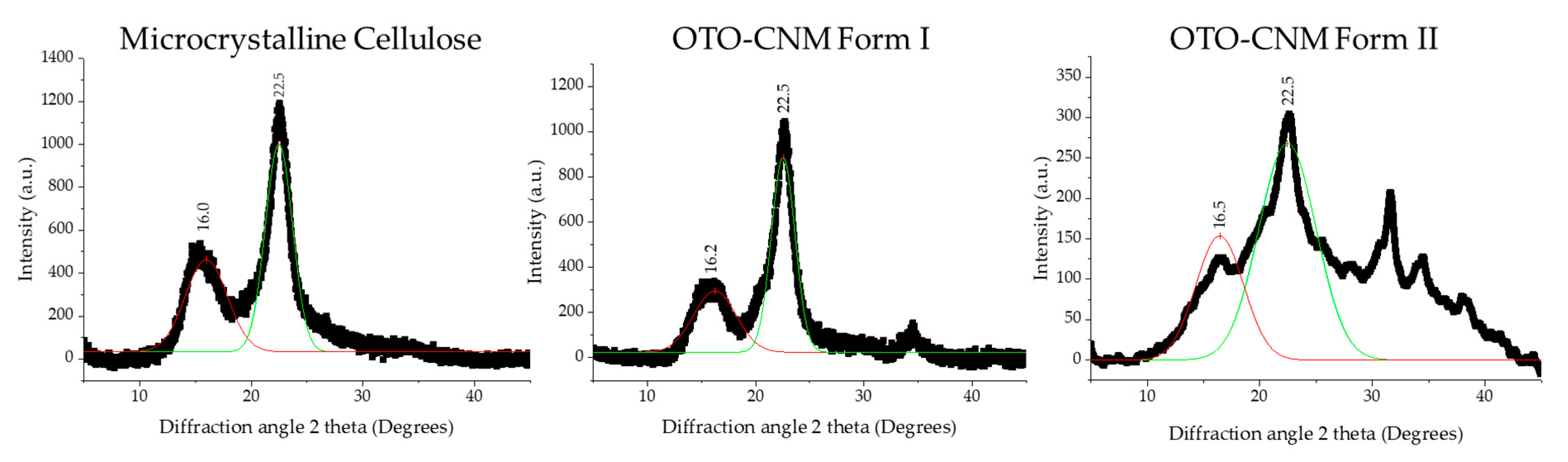
2.4. XRD Analysis of Oxone®-Mediated TEMPO-Oxidized Cellulose
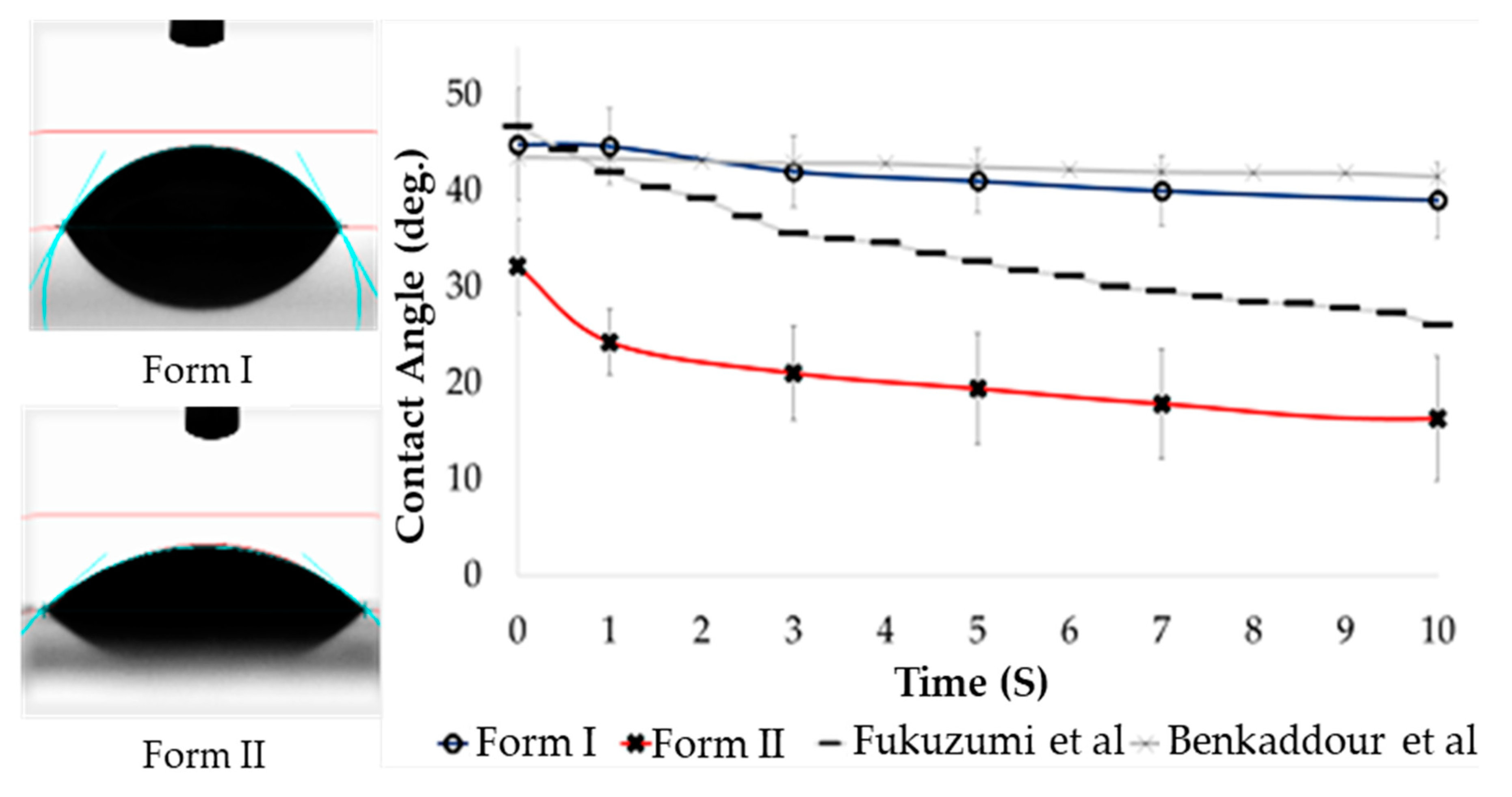
2.5. Hydrophilicity (Contact Angle) of Oxone®-Mediated TEMPO-Oxidized Cellulose
2.6. Tensile Testing Characterization
3. Materials and Methods
3.1. General Procedure for the Oxidation of Cellulose Using TEMPO, NaOCl, and Oxone®
3.2. Solution Preparation of Oxone®-Mediated TEMPO-Oxidized Cellulose
3.3. Preparation of Oxone®-Mediated TEMPO-Oxidized Cellulose Thin Films
3.4. Microscopy
3.5. Spectroscopy
3.6. Charge Density
3.7. X-Ray Diffraction (XRD)
3.8. Contact Angle
3.9. Preparation of Polymeric Nanocomposite Film
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dachavaram, S.S.; Moore, J.P.; Bommagani, S.; Penthala, N.R.; Calahan, J.L.; Delaney, S.P.; Munson, E.J.; Batta-Mpouma, J.; Kim, J.; Hestekin, J.A. A Facile Microwave Assisted TEMPO/NaOCl/Oxone (KHSO5) Mediated Micron Cellulose Oxidation Procedure: Preparation of Two Nano TEMPO-Cellulose Forms. Starch-Stärke 2020, 72, 1900213. [Google Scholar] [CrossRef]
- Krassig. Cellulose; CRC Press: Boca Raton, FL, USA, 1993; p. 293. [Google Scholar]
- Herrick, F.W.; Casebier, R.L.; Hamilton, J.K.; Sandberg, K.R. Microfibrillated Cellulose: Morphology and Accessibility. J. Appl. Polym. Sci. Appl. Polym. Symp. 1982, 37, 797–813. [Google Scholar]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated Cellulose, a New Cellulose Product: Properties, Uses, and Commercial Potential. J. Appl. Polym. Sci. Appl. Polym. Symp. 1982, 37, 291–294. [Google Scholar]
- Dachavaram, S.S.; Penthala, N.R.; Calahan, J.L.; Munson, E.J.; Crooks, P.A. Highly Sulphated Cellulose: A Versatile, Reusable and Selective Desilylating Agent for Deprotection of Alcoholic TBDMS Ethers. Org. Biomol. Chem. 2018, 16, 6057–6062. [Google Scholar] [CrossRef] [PubMed]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.; Cranston, E.D.; Eichhorn, S.J. Current Characterization Methods for Cellulose Nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Nooy, A.E.; Besemer, A.C.; van Bekkum, H. On the use of Stable Organic Nitroxyl Radicals for the Oxidation of Primary and Secondary Alcohols. Synthesis 1996, 1996, 1153–1176. [Google Scholar] [CrossRef]
- Chang, P.S.; Robyt, J.F. Oxidation of Primary Alcohol Groups of Naturally Occurring Polysaccharides with 2,2,6,6-Tetramethyl-1-Piperidine Oxoammonium Ion. J. Carbohydr. Chem. 1996, 15, 819–830. [Google Scholar] [CrossRef]
- Isogai, A.; Kato, Y. Preparation of Polyuronic Acid from Cellulose by TEMPO-Mediated Oxidation. Cellulose 1998, 5, 153–164. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-Oxidized Cellulose Nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Saito, T.; Nishiyama, Y.; Putaux, J.; Vignon, M.; Isogai, A. Homogeneous Suspensions of Individualized Microfibrils from TEMPO-Catalyzed Oxidation of Native Cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef]
- Liimatainen, H.; Visanko, M.; Sirvioö, J.A.; Hormi, O.E.; Niinimaki, J. Enhancement of the Nanofibrillation of Wood Cellulose through Sequential Periodate–chlorite Oxidation. Biomacromolecules 2012, 13, 1592–1597. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuga, S. Steric Stabilization of a Cellulose Microcrystal Suspension by Poly (Ethylene Glycol) Grafting. Langmuir 2001, 17, 21–27. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Isogai, A. TEMPO-Mediated Oxidation of Native Cellulose. The Effect of Oxidation Conditions on Chemical and Crystal Structures of the Water-Insoluble Fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- Marett, J.; Aning, A.; Foster, E.J. The Isolation of Cellulose Nanocrystals from Pistachio Shells Via Acid Hydrolysis. Ind. Crops Prod. 2017, 109, 869–874. [Google Scholar] [CrossRef]
- Mueller, S.; Weder, C.; Foster, E.J. Isolation of Cellulose Nanocrystals from Pseudostems of Banana Plants. RSC Adv. 2014, 4, 907–915. [Google Scholar] [CrossRef]
- Ruan, C.; Gustafsson, S.; Strømme, M.; Mihranyan, A.; Lindh, J. Cellulose Nanofibers Prepared Via Pretreatment Based on Oxone® Oxidation. Molecules 2017, 22, 2177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakabayashi, M.; Fujisawa, S.; Saito, T.; Isogai, A. Nanocellulose Film Properties Tunable by Controlling Degree of Fibrillation of TEMPO-Oxidized Cellulose. Front. Chem. 2020, 8, 37. [Google Scholar] [CrossRef]
- Petroudy, S.R.D.; Ranjbar, J.; Garmaroody, E.R. Eco-Friendly Superabsorbent Polymers Based on Carboxymethyl Cellulose Strengthened by TEMPO-Mediated Oxidation Wheat Straw Cellulose Nanofiber. Carbohydr. Polym. 2018, 197, 565–575. [Google Scholar] [CrossRef]
- Zhai, L.; Kim, H.C.; Kim, J.W.; Choi, E.S.; Kim, J. Cellulose Nanofibers Isolated by TEMPO-Oxidation and Aqueous Counter Collision Methods. Carbohydr. Polym. 2018, 191, 65–70. [Google Scholar]
- Liu, S.; Liang, H.; Sun, T.; Yang, D.; Cao, M. A Recoverable Dendritic Polyamidoamine Immobilized TEMPO for Efficient Catalytic Oxidation of Cellulose. Carbohydr. Polym. 2018, 202, 563–570. [Google Scholar] [CrossRef]
- Kondo, T. The Assignment of IR Absorption Bands due to Free Hydroxyl Groups in Cellulose. Cellulose 1997, 4, 281. [Google Scholar] [CrossRef]
- Hastuti, N.; Darmayanti, R.F.; Hardiningtyas, S.D.; Kanomata, K.; Sonomoto, K.; Goto, M.; Kitaoka, T. Carboxylated-Cellulose Nanofibers from Oil Palm Empty Fruit Bunches Enhanced Extractive Fermentation in Microbial Biobutanol Production. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: West Java, Indonesia, 2020. [Google Scholar]
- Camarero Espinosa, S.; Kuhnt, T.; Foster, E.J.; Weder, C. Isolation of Thermally Stable Cellulose Nanocrystals by Phosphoric Acid Hydrolysis. Biomacromolecules 2013, 14, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, T.; Kloser, E.; Gray, D.G. Estimation of the Surface Sulfur Content of Cellulose Nanocrystals Prepared by Sulfuric Acid Hydrolysis. Cellulose 2013, 20, 785–794. [Google Scholar] [CrossRef]
- Thygesen, A.; Oddershede, J.; Lilholt, H.; Thomsen, A.B.; Ståhl, K. On the Determination of Crystallinity and Cellulose Content in Plant Fibres. Cellulose 2005, 12, 563. [Google Scholar] [CrossRef]
- Mishra, S.K.; Roy, H.; Lohar, A.K.; Samanta, S.K.; Tiwari, S.; Dutta, K. A Comparative Assessment of Crystallite Size and Lattice Strain in Differently Cast A356 Aluminium Alloy. In IOP Conference Series Xi’an, The 7th Global Conference Materials Science and Engineering; IOP Publishing: Xi’an, China, 2018; p. 012001. [Google Scholar]
- Zak, A.K.; Majid, W.A.; Abrishami, M.E.; Yousefi, R. X-Ray Analysis of ZnO Nanoparticles by Williamson–Hall and Size–strain Plot Methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar]
- Benkaddour, A.; Jradi, K.; Robert, S.; Daneault, C. Study of the Effect of Grafting Method on Surface Polarity of Tempo-Oxidized Nanocellulose using Polycaprolactone as the Modifying Compound: Esterification Versus Click-Chemistry. Nanomaterials 2013, 3, 638–654. [Google Scholar] [CrossRef] [Green Version]
- Fukuzumi, H.; Saito, T.; Iwata, T.; Kumamoto, Y.; Isogai, A. Transparent and High Gas Barrier Films of Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation. Biomacromolecules 2008, 10, 162–165. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Isogai, A. Influence of TEMPO-Oxidized Cellulose Nanofibril Length on Film Properties. Carbohydr. Polym. 2013, 93, 172–177. [Google Scholar] [CrossRef]
- Di Giorgio, L.; Martín, L.; Salgado, P.R.; Mauri, A.N. Synthesis and Conservation of Cellulose Nanocrystals. Carbohydr. Polym. 2020, 238, 116187. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-Ray Line Broadening from Filed Aluminium and Wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A. Scherrer After Sixty Years: A Survey and some New Results in the Determination of Crystallite Size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung Der Grosse Und Der Inneren Struktur Von Kolloidteilchen Mittels Rontgenstrahlen. (1918); In X-ray Diffraction Methods in Polymer Science; Alexander, L.E., Ed.; IOP Publishing Ltd.: West Java, Indonesia, 1969. [Google Scholar]
- Mahmoudi, N.; Reed, L.; Moix, A.; Alshammari, N.; Hestekin, J.; Servoss, S.L. PEG-Mimetic Peptoid Reduces Protein Fouling of Polysulfone Hollow Fibers. Colloid Surf. B 2017, 149, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhang, D.; Shao, Z.; Han, B.; Lv, Y.; Gao, K.; Peng, X. Superior Effect of TEMPO-Oxidized Cellulose Nanofibrils (TOCNs) on the Performance of Cellulose Triacetate (CTA) Ultrafiltration Membrane. Desalination 2014, 332, 117–125. [Google Scholar] [CrossRef]
- Prihatiningtyas, I.; Volodin, A.; Van der Bruggen, B. 110th Anniversary: Cellulose Nanocrystals as Organic Nanofillers for Cellulose Triacetate Membranes used for Desalination by Pervaporation. Ind. Eng. Chem. Res. 2019, 58, 14340–14349. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Sample | Mmols Functional Group/kg |
|---|---|
| MCC OTO-CNM Form I | 31 ± 10 841 ± 92 |
| OTO-CNM Form II | 1620 ± 42 |
| Sample | Crystallite Size (nm) | Crystallinity (%) |
|---|---|---|
| MCC | 2.95 | 59.4 |
| Form I | 3.29 | 63.8 |
| Form II | 1.41 | 67.7 |
| Sample | Tensile Strain (%) | Tensile Stress (GPa) | Tensile Modulus (GPa) | Maximum Load (kN) |
|---|---|---|---|---|
| CTA | 5.89 ± 2.12 | 0.44 ± 1.14 | 0.77 ± 0.43 | 2.64 ± 0.19 |
| Form I | 4.28 ± 1.05 | 0.84 ± 2.00 | 2.39 ± 1.15 | 5.07 ± 0.33 |
| Form II | 4.78 ± 1.64 | 1.21 ± 1.21 | 2.60 ± 0.58 | 6.75 ± 0.22 |
| Membrane | Form I (wt%) | Form II (wt%) | CTA (wt%) | NMP (wt%) |
|---|---|---|---|---|
| Control | 0.0 | 0.0 | 10.0 | 90.0 |
| Form I | 1.0 | 0.0 | 9.0 | 90.0 |
| Form II | 0.0 | 1.0 | 9.0 | 90.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore II, J.P.; Dachavaram, S.S.; Bommagani, S.; Penthala, N.R.; Venkatraman, P.; Foster, E.J.; Crooks, P.A.; A. Hestekin, J. Oxone®-Mediated TEMPO-Oxidized Cellulose Nanomaterials form I and form II. Molecules 2020, 25, 1847. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25081847
Moore II JP, Dachavaram SS, Bommagani S, Penthala NR, Venkatraman P, Foster EJ, Crooks PA, A. Hestekin J. Oxone®-Mediated TEMPO-Oxidized Cellulose Nanomaterials form I and form II. Molecules. 2020; 25(8):1847. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25081847
Chicago/Turabian StyleMoore II, John P, Soma Shekar Dachavaram, Shobanbabu Bommagani, Narsimha Reddy Penthala, Priya Venkatraman, E. Johan Foster, Peter A. Crooks, and Jamie A. Hestekin. 2020. "Oxone®-Mediated TEMPO-Oxidized Cellulose Nanomaterials form I and form II" Molecules 25, no. 8: 1847. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25081847








