Valorisation of By-Products from Soybean (Glycine max (L.) Merr.) Processing
Abstract
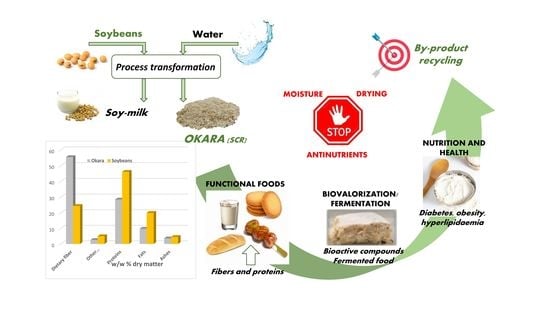
:1. Introduction
1.1. Use of By-Products in the Food Sector
1.2. Soybeans: Characteristics, Processing and Use
1.3. Okara: between Production and Consumption
2. Composition of Okara, A By-Product of Soymilk Production
2.1. Dietary Fibre
2.2. Protein Component
2.3. Lipid Fraction
2.4. Isoflavones
2.5. Nutritional and Anti-Nutritional Elements
3. Production and Use of Okara
- (1)
- SB washing to remove impurities
- (2)
- SB soaking/hydration for 12 h at 25 °C, then draining and rinsing with water
- (3)
- cooking at 98 °C for 5 min, with the aim of both sterilising and improving aroma and nutritional value via the inactivation of trypsin inhibitors
- (4)
- grinding in a blender with distilled water (1:10 ratio w/v SBs/water) for the preparation of a slurry
- (5)
4. Application of Okara in Functional Foods
4.1. Production of Food for Human Consumption
4.2. A Study on Paste Production
4.3. Fibre Recovery and Enhancement
4.4. Applications in Animal Nutrition
4.5. Application in Ecological Materials
5. Biovalorisation through Fermentation
5.1. Fungal Fermentation
5.1.1. Production of Bioactive Compounds
5.1.2. Production of Food Fermented by Fungi
5.2. Bacterial Fermentation
5.2.1. Production of Bioactive Compounds
5.2.2. Using Dried Okara as A Prebiotic
5.3. Fermentation Using Yeasts
6. Nutrition and Health
6.1. Diabetes
6.2. Hyperlipidaemia
6.3. Obesity
6.4. Antioxidant Activity
7. Limiting Factors for Okara Valorisation
7.1. Humidity and Drying
7.2. Anti-Nutrients
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References and Notes
- FAO. Global Food Losses and Food Waste. 2011 [Retrieved 01.05.2014]. Available online: http://www.fao.org/docrep/014/mb060e/mb060e00.pdf (accessed on 20 December 2019).
- FAO. Food Wastage Footprint-Impacts on Natural Resources. 2013 [Retrieved 01.05.2014]. Available online: http://www.fao.org/docrep/018/i3347e/i3347e.pdf (accessed on 20 December 2019).
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Cleaner Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Laufenberg, G.; Kunz, B.; Nystroem, M. Transformation of vegetable waste into value added products: (A) the upgrading concept; (B) practical implementations. Bioresour. Technol. 2003, 87, 167–198. [Google Scholar] [CrossRef]
- Cravotto, G.; Mariatti, F.; Gunjevic, V.; Secondo, M.; Villa, M.; Parolin, J.; Cavaglià, G. Pilot scale cavitational reactors and other enabling technologies to design the industrial recovery of polyphenols from agro-food by-products, a technical and economical overview. Foods 2018, 7, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karuga, J. 10 Countries with Largest Soybean Production. WorldAtlas. 2018. Available online: worldatlas.com/articles/world-leaders-in-soya-soybean-production-by-country.html (accessed on 18 March 2020).
- Messina, M. Soy and health update: Evaluation of the clinical and epidemiologic literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabanillas, B.; Jappe, U.; Novak, N. Allergy to peanut, soybean, and other legumes: Recent advances in allergen characterization, stability to processing and IgE cross-reactivity. Mol. Nutr. Food Res. 2018, 62, 1700446. [Google Scholar] [CrossRef]
- Dipietro, C.M.; Liener, I.E. Soybean protease inhibitors in foods. J. Food Sci. 1989, 54, 606–609. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Potential role of bioactive peptides in prevention and treatment of chronic diseases: A narrative review. Br. J. Pharmacol. 2017, 174, 1378–1394. [Google Scholar] [CrossRef]
- Chen, L.-R.; Ko, N.-Y.; Chen, K.-H. Isoflavone Supplements for Menopausal Women: A Systematic Review. Nutrients 2019, 11, 2649. [Google Scholar] [CrossRef] [Green Version]
- US Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory. USDA National Nutrient Database for Standard Reference, Release 28. Version Current: Sep 2015, Slightly Revised May 2016. Products in Table 1: 1 soybeans, mature seeds, raw, FDC ID: 174270, NDB N.: 16108; 2 soybeans, mature seeds, dry roasted, FDC ID 172441, NDB N. 16111; 3 soybeans, mature cooked, boiled, without salt, FDC ID 174271, NDB N. 16109; 4 soy flour, full-fat, raw, FDC ID 174273, NDB N. 16115; 5 soy meal, defatted, raw, FDC ID 172445, NDB N. 16119; 6 soy protein concentrate, produced by acid wash, FDC ID 174301, NDB N. 16420; 7 Soy protein isolate, FDC ID 174276, NDB N. 16122; 8 soymilk: https://www.soya.be/nutritional-values-of-soy-milk.php (accessed on 18 March 2020); 9 okara, FDC ID 172452, NDB N. 16130. Available online: https://www.ars.usda.gov/Services/docs.htm?docid=8964 (accessed on 4 March 2020). [CrossRef]
- Ma, C.-Y. Soybean—Soy Concentrates and Isolates. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Database indicated in Ref. [12]. Products in Table 2: 1 tempeh, FDC ID 174272, NDB N. 16114; 2 miso, FDC ID 172442, NDB N. 16112; 3 natto, FDC ID 172443, NDB N. 16113; 4 soy sauce made from soy (tamari), FDC ID 174278, NDB N. 16124; 5 tofu, salted and fermented (fuyu), FDC ID 174280, NDB N. 16132.
- Jayachandran, M.; Xu, B. An insight into the health benefits of fermented soy products. Food Chem. 2019, 271, 362–371. [Google Scholar] [CrossRef]
- FAO. Technology of Production of Edible Flours and Protein Products from Soybeans. 1992 [Retrieved 01.05.2014]. Available online: http://www.fao.org/docrep/t0532e/t0532e00.htm#con (accessed on 20 December 2019).
- Zenith-International. Soy Beverages Double in Four Years. 2007 [Retrieved 01.05.2014]. Available online: http://www.zenithinternational.com/articles/692/Soy+beverages+double+in+four+years (accessed on 20 December 2019).
- Wang, H.J.; Murphy, P.A. Mass balance study of isoflavones during soybean processing. J. Agric. Food Chem. 1996, 44, 2377–2383. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, R.; Yin, L.; Zhang, N. Novel blasting extrusion processing improved the physicochemical properties of soluble dietary fiber from soybean residue and in vivo evaluation. J. Food Eng. 2014, 120, 1–8. [Google Scholar] [CrossRef]
- Ahn, S.H.; Oh, S.C.; Choi, I.-G.; Han, G.-S.; Jeong, H.-S.; Kim, K.-W.; Yoon, Y.-H.; Yang, I. Environmentally friendly wood preservatives formulated with enzymatic hydrolysed okara, copper and/or boron salts. J. Hazard. Mater. 2010, 178, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Long, D.; Peng, J.; Ming, J.; Zhao, G. A novel in-situ enhanced blasting extrusion technique—Extrudate analysis and optimization of processing conditions with okara. Innovative Food Sci. Emerg. Technol. 2012, 16, 80–88. [Google Scholar] [CrossRef]
- Khare, S.K.; Jha, K.; Gandhi, A.P. Citric acid production from okara (soy residue) by solid-state fermentation. Biores. Technol. 1995, 54, 323–325. [Google Scholar] [CrossRef]
- Muroyama, K.; Mochizuki, T.; Wakamura, T. Methane fermentation of bean curd refuse. J. Biosci. Bioeng. 2001, 91, 208–212. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Redondo-Cuenca, A.; Villanueva-Suarez, M.J. Isolation and characterisation of cell wall polysaccharides from legume by-products: Okara (soymilk residue), pea pod and broad bean pod. Food Chem. 2010, 122, 339–345. [Google Scholar] [CrossRef]
- Redondo-Cuenca, A.; Villanueva-Suarez, M.J.; Mateos-Aparicio, I. Soybean seeds and its by-product okara as sources of dietary fibre. Measurement by AOAC and Englyst methods. Food Chem. 2008, 108, 1099–1105. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Mateos-Peinado, C.; Jimenez-Escrig, A.; Ruperez, P. Multifunctional antioxidant activity of polysaccharide fractions from the soybean byproduct okara. Carbohydr. Polym. 2010, 82, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Mateos-Aparicio, I.; Redondo-Cuenca, A.; Villanueva-Suarez, M.-J.; Zapata-Revilla, M.-A.; Tenorio-Sanz, M.-D. Pea pod, broad bean pod and okara, potential sources of functional compounds. LWT Food Sci. Technol. 2010, 43, 1467–1470. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Mateos-Peinado, C.; Rupérez, P. High hydrostatic pressure improves the functionality of dietary fibre in okara by-product from soybean. Innovative Food Sci. Emerg. Technol. 2010, 11, 445–450. [Google Scholar] [CrossRef]
- Rinaldi, V.E.A.; Ng, P.K.W.; Bennink, M.R. Effects of extrusion on dietary fiber and isoflavone contents of wheat extrudates enriched with wet okara. Cereal Chem. 2000, 77, 237–240. [Google Scholar] [CrossRef]
- Li, Y.O.; Komarek, A.R. Dietary fibre basics: Health, nutrition, analysis, and applications. Food Qual. Safety 2017, 1, 47–59. [Google Scholar] [CrossRef]
- Nagata, Y.; Yamsasaki, S.; Torisu, N.; Suzuki, T.; Shimamoto, S.; Tamaru, S.; Tanaka, K. Okara, a by-product of tofu manufacturing, modifies triglyceride metabolism at the intestinal and hepatic levels. J. Nutr. Sci. 2016, 62, 162–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishibori, N.; Kishibuchi, R.; Morita, K. Suppressive effect of okara on intestinal lipid digestion and absorption in mice ingesting high-fat diet. Int. J. Food Sci. Nutr. 2017, 69, 1–6. [Google Scholar] [CrossRef]
- Villanueva-Suárez, M.-J.; Pérez-Cózar, M.-L.; Mateos-Aparicio, I.; Redondo-Cuenca, A. Potential fat-lowering and prebiotic effect of enzymatically treated okara in high-cholesterol-fed Wistar rats. Int. J. Food Sci. Nutr. 2016, 67, 828–833. [Google Scholar] [CrossRef]
- Villanueva, M.-J.; Yokoyama, W.H.; Hong, Y.J.; Barttley, G.E.; Rupérez, P. Effect of high-fat diets supplemented with okara soybean by-product on lipid profiles of plasma, liver and faeces in Syrian hamsters. Food Chem. 2011, 124, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Meena, M.; Kumar, D.; Dubey, A.K.; Hassan, M.I. Structural and functional analysis of various globulin proteins from soy seed. Crit. Rev. Food Sci. Nutr. 2015, 55, 1491–1502. [Google Scholar] [CrossRef]
- Chan, W.M.; Ma, C.Y. Modification of proteins from soymilk residue (okara) by trypsin. J. Food Sci. 1999, 64, 781–786. [Google Scholar] [CrossRef]
- Ma, C.Y.; Liu, W.S.; Kwok, K.C.; Kwok, F. Isolation and characterization of proteins from soymilk residue (okara). Food Res. Int. 1996, 29, 799–805. [Google Scholar] [CrossRef]
- Jimenez-Escrig, A.; Alaiz, M.; Vioque, J.; Ruperez, P. Health-promoting activities of ultra-filtered okara protein hydrolysates released by in vitro gastrointestinal digestion: Identification of active peptide from soybean lipoxygenase. Eur. Food Res. Technol. 2010, 230, 655–663. [Google Scholar] [CrossRef]
- Stanojevic, S.P.; Barac, M.B.; Pesic, M.B.; Jankovic, V.S.; Vucelic-Radovic, B.V. Bioactive proteins and energy value of okara as a byproduct in hydrothermal processing of soy milk. J. Agric. Food Chem. 2013, 61, 9210–9219. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.M.; Ma, C.Y. Acid modification of proteins from soymilk residue (okara). Food Res. Int. 1999, 32, 119–127. [Google Scholar] [CrossRef]
- Vishwanathan, K.H.; Singh, V.; Subramanian, R. Influence of particle size on protein extractability from soybean and okara. J. Food Eng. 2011, 102, 240–246. [Google Scholar] [CrossRef]
- Yuan, S.; Chang, S.K. Selected odor compounds in soymilk as affected by chemical composition and lipoxygenases in five soybean materials. J. Agric. Food Chem. 2007, 55, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Quitain, A.T.; Oro, K.; Katoh, S.; Moriyoshi, T. Recovery of oil components of okara by ethanol-modified supercritical carbon dioxide extraction. Biores. Technol. 2006, 97, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.J.C.; Dini, J.P.; Lavandier, C.; Rupasinghe, H.P.V.; Faulkner, H.; Poysa, V.; Buzzell, D.; DeGrandis, S. Effects of processing on the content and composition of isoflavones during manufacturing of soy beverage and tofu. Process Biochem. 2002, 37, 1117–1123. [Google Scholar] [CrossRef]
- Cederroth, C.R.; Nef, S. Soy, phytoestrogens and metabolism: A review. Mol. Cell. Endocrinol. 2009, 304, 30–42. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Cassidy, A. Dietary isoflavones: Biological effects and relevance to human health. J. Nutr. 1999, 129, 758–767. [Google Scholar] [CrossRef] [Green Version]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signaling 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Villares, A.; Rostagno, M.A.; García-Lafuente, A.; Guillamón, E.; Martínez, J.A. Content and profile of isoflavones in soy-based foods as a function of the production process. Food Bioprocess Technol. 2011, 4, 27–38. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.H.; Chen, B.H. Functional components in soybean cake and their effects on antioxidant activity. J. Agric. Food Chem. 2006, 54, 7544–7555. [Google Scholar] [CrossRef] [PubMed]
- Preedy, V.R. Isoflavones: Chemistry, Analysis, Function and Effects (Food and Nutritional Components in Focus, Book 5), 1st ed.; Royal Society of Chemistry Publishing: Cambridge, UK, 2013; pp. 1–710. [Google Scholar]
- Valls, J.; Millán, S.; Martí, M.P.; Borràs, E.; Arola, L. Advanced separation methods of food anthocyanins, isoflavones and flavanols. J. Chromatogr. A 2009, 1216, 7143–7172. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Murphy, P.A.; Sala, I. Isoflavone profiles of soymilk as affected by high-pressure treatments of soymilk and soybeans. Food Chem. 2008, 111, 592–598. [Google Scholar] [CrossRef]
- Rickert, D.A.; Meyer, M.A.; Hu, J.; Murphy, P.A. Effect of extraction pH and temperature on isoflavone and saponin partitioning and profile during soy protein isolate production. J. Food Sci. 2004, 69, 623–631. [Google Scholar] [CrossRef]
- Kao, T.H.; Lu, Y.F.; Hsieh, H.C.; Chen, B.H. Stability of isoflavone glucosides during processing of soymilk and tofu. Food Res. Int. 2004, 37, 891–900. [Google Scholar] [CrossRef]
- Balisteiro, D.M.; Rombaldi, C.V.; Genovese, M.I. Protein, isoflavones, trypsin inhibitory and in vitro antioxidant capacities: Comparison among conventionally and organically grown soybeans. Food Res. Int. 2013, 51, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Coward, L.; Barnes, N.C.; Setchell, K.D.R.; Barnes, S. Genistein, daidzein, and their beta-glycoside conjugates—Antitumor isoflavones in soybean foods from American and Asian diets. J. Agric. Food Chem. 1993, 41, 1961–1967. [Google Scholar] [CrossRef]
- Izumi, T.; Piskula, M.K.; Osawa, S.; Obata, A.; Tobe, K.; Saito, M.; Kikuchi, M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J. Nutr. 2000, 130, 1695–1699. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, Y.; Mishra, S.; Bisaria, V. Microbial β-glucosidases: Cloning, properties, and applications. Crit. Rev. Biotechnol. 2002, 22, 375–407. [Google Scholar] [CrossRef]
- Ishihara, M.; Singh, H.; Chung, G.; Tam, C. Content composition and antioxidant activity of isoflavones in commercial and homemade soymilk and tofu. J. Sci. Food Agric. 2007, 87, 2844–2852. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Perera, C.O. Effect of extraction methods and UHT treatment conditions on the level of isoflavones during soymilk manufacture. Food Chem. 2006, 99, 231–237. [Google Scholar] [CrossRef]
- Surel, O.; Couplet, B. Influence of the dehydration process on active compounds of okara during its fractionation. J. Sci. Food Agric. 2005, 85, 1343–1349. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef]
- Bordenave, N.; Hamaker, B.R.; Ferruzzi, M.G. Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods. Food Funct. 2014, 5, 18–34. [Google Scholar] [CrossRef]
- Deng, J.; Xu, Z.; Xiang, C.; Liu, J.; Zhou, L.; Li, T.; Ding, C. Comparative evaluation of maceration and ultrasonic-assisted extraction of phenolic compounds from fresh olives. Ultrason. Sonochem. 2017, 37, 328–334. [Google Scholar] [CrossRef]
- Tian, S.; Xie, S.; Pan, J. Preparation and performance detection of soybean dietary fiber. China Oils Fats 2007, 32, 64–66. [Google Scholar]
- Muliterno, M.M.; Rodrigues, D.; Lima, F.S.; Ida, E.I.; Kurozawa, L.E. Conversion/degradation of isoflavones and color alterations during the drying of okara. LWT Food Sci. Technol. 2017, 75, 512–519. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Goulas, V.; Tsakona, S.; Manganaris, G.A.; Gekas, V. A knowledge base for the recovery of natural phenols with different solvents. Int. J. Food Prop. 2013, 16, 382–396. [Google Scholar] [CrossRef] [Green Version]
- Chebil, L.; Humeau, C.; Anthoni, J.; Dehez, F.; Engasser, J.-M.; Ghoul, M. Solubility of flavonoids in organic solvents. J. Chem. Eng. Data 2007, 52, 1552–1556. [Google Scholar] [CrossRef]
- Lásztity, R.; Hidvégi, M.; Bata, A. Saponins in food. Food Rev. Int. 1998, 14, 371–390. [Google Scholar] [CrossRef]
- Fenwick, D.E.; Oakenfull, D. Saponin content of food plants and some prepared foods. J. Sci. Food Agric. 1983, 34, 186–191. [Google Scholar] [CrossRef]
- Gurfinkel, D.M.; Rao, A.V. Soybeansaponins: The relationship between chemical structure and colon anticarcinogenic activity. Nutr. Cancer 2003, 47, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.L.; Wolf, W.J. Compositional changes in trypsin inhibitors, phytic acid, saponins and isoflavones related to soybean processing. J. Nutr. 1995, 125, 518–588. [Google Scholar]
- Wang, T.; Qin, G.-X.; Sun, Z.-W.; Zhao, Y. Advances of research on glycinin and b-conglycinin: A review of two major soybean allergenic proteins. Crit. Rev. Food Sci. Nutr. 2014, 54, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, S.P.; Barac, M.B.; Pesic, M.B.; Zilic, S.M.; Kresovic, M.M.; Vucelic-Radovic, B.V. Mineral elements, lipoxygenase activity, and antioxidant capacity of okara as a byproduct in hydrothermal processing of soy milk. J. Agric. Food Chem. 2014, 62, 9017–9023. [Google Scholar] [CrossRef]
- Iqbal, S.; Klammer, N.; Ekmekcioglu, C. The effect of electrolytes on blood pressure: A brief summary of meta-analyses. Nutrients 2019, 1, 1362. [Google Scholar] [CrossRef] [Green Version]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef]
- Verstovsek, S.; Harrison, C.N.; Kiladjian, J.J.; Miller, C.; Naim, A.B.; Paranagama, D.C.; Habr, D.; Vannucchi, A.M. Markers of iron deficiency in patients with polycythemia vera receiving ruxolitinib or best available therapy. Leuk Res. 2017, 56, 52–59. [Google Scholar] [CrossRef]
- De Assumpção, D.; Dias, M.R.; de Azevedo Barros, M.B.; Fisberg, R.M.; de Azevedo Barros Filho, A. Calcium intake by adolescents: A population-based health survey. J. Pediatr. 2016, 92, 251–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golbitz, P. Traditional soyfoods—Processing and products. J. Nutr. 1995, 125, 570–572. [Google Scholar]
- O’Toole, D.K. Characteristics and use of okara, the soybean residue from soy milk production—A review. J. Agric. Food Chem. 1999, 47, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Choicharoen, K.; Devahastin, S.; Soponronnarit, S. Performance and energy consumption of an impinging stream dryer for high-moisture particulate materials. Drying Technol. 2010, 28, 20–29. [Google Scholar] [CrossRef]
- Wachiraphansakul, S.; Devahastin, S. Drying kinetics and quality of okara dried in a jet spouted bed of sorbent particles. LWT Food Sci. Technol. 2007, 40, 207–219. [Google Scholar] [CrossRef]
- Itaya, Y.; Kobayashi, N.; Nakamiya, T. Okara drying by pneumatically swirling two-phase flow in entrained bed riser with enlarged zone. Drying Technol. 2010, 28, 972–980. [Google Scholar] [CrossRef]
- Katayama, M.; Wilson, L.A. Utilization of okara, a byproduct from soymilk production, through the development of soy-based snack food. J. Food Sci. 2008, 73, 152–157. [Google Scholar] [CrossRef]
- Aplevic, K.S.; Demiate, I.M. Physicochemical analyses of commercial samples of cheese bread premix and production of cheese breads with addition of okara. Ciencia Agrotec. 2007, 31, 1416–1422. [Google Scholar]
- Bowles, S.; Demiate, I.M. Caracterização físico-química de okara e aplicação em pães do tipo francês. Food Sci. Technol. 2006, 26, 652–659. [Google Scholar] [CrossRef] [Green Version]
- Nagai, T.; Li, L.T.; Ma, Y.L.; Sarkar, P.K.; Nout, R.; Park, K.Y.; Jeong, J.K.; Lee, J.E.; Lee, G.I.; Lee, C.H.; et al. Diversity of plant-based food products involving alkaline fermentation. In Handbook of Indigenous Foods Involving Alkaline Fermentation; Sarkar, P.K., Nout, M.R., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 78–87. [Google Scholar]
- Turhan, S.; Temiz, H.; Sagir, I. Utilization of wet okara in low-fat beef patties. J. Muscle Foods 2007, 18, 226–235. [Google Scholar] [CrossRef]
- Su, S.I.T.; Pedroso Yoshida, C.M.; Contreras-Castillo, C.J.; Quiñones, E.M.; Venturini, A.C. Okara, a soymilk industry by-product, as a non-meat protein source in reduced fat beef burgers. Food Sci. Technol. 2013, 33, 52–56. [Google Scholar] [CrossRef] [Green Version]
- Radocaj, O.; Dimic, E. Valorization of wet okara, a value-added functional ingredient, in a coconut-based baked snack. Cereal Chem. 2013, 90, 256–262. [Google Scholar] [CrossRef]
- Espinosa-Martos, I.; Ruperez, P. Indigestible fraction of okara from soybean: Composition, physicochemical properties and in vitro fermentability by pure cultures of Lactobacillus acidophilus and Bifidobacterium bifidum. Eur. Food Res. Technol. 2009, 228, 685–693. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Nan, H.; Liu, Y. Isolation and structural characterisation of okara polysaccharides. Molecules 2012, 17, 753–761. [Google Scholar] [CrossRef]
- Stanojevic, S.P.; Barac, M.B.; Pesic, M.B.; Vucelic-Radovic, B.V. Composition of proteins in okara as a byproduct in hydrothermal processing of soy milk. J. Agric. Food Chem. 2012, 60, 9221–9228. [Google Scholar] [CrossRef]
- Vishwanathan, K.; Govindaraju, K.; Singh, V.; Subramanian, R. Production of okara and soy protein concentrates using membrane technology. J. Food Sci. 2011, 76, 158–164. [Google Scholar] [CrossRef]
- Yokomizo, A.; Takenaka, Y.; Takenaka, T. Antioxidative activity of peptides prepared from Okara protein. Food Sci. Technol. Res. 2002, 8, 357–359. [Google Scholar] [CrossRef] [Green Version]
- Amin, I.; Mukhrizah, O. Antioxidant capacity of methanolic and water extracts prepared from food-processing by-products. J. Sci. Food Agric. 2006, 86, 778–784. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Y.; Yang, C.; Wang, Z. Enzymatic preparation and characterization of soybean oligosaccharides from okara. Procedia Eng. 2012, 37, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Wiboonsirikul, J.; Mori, M.; Khuwijitjaru, P.; Adachi, S. Properties of extract from okara by its subcritical water treatment. Int. J. Food Prop. 2013, 16, 974–982. [Google Scholar] [CrossRef] [Green Version]
- Genovese, M.I.; Lajolo, F.M. Isoflavones in soy-based foods consumed in Brazil: Levels, distribution, and estimated intake. J. Agric. Food Chem. 2002, 50, 5987–5993. [Google Scholar] [CrossRef] [PubMed]
- Alezandro, M.R.; Granato, D.; Lajolo, F.M.; Genovese, M.I. Nutritional aspects of second generation soy foods. J. Agric. Food Chem. 2011, 59, 5490–5497. [Google Scholar] [CrossRef] [PubMed]
- Wickramarathna, G.L.; Arampath, P.C. Utilization of okara in bread making. Cey. J. Sci. (Biol. Sci.) 2003, 31, 29–33. [Google Scholar]
- Suda, T.; Kido, Y.; Tsutsui, S.; Tsutsui, D.; Fujita, M.; Nakaya, Y. Nutritional evaluation of the new OKARA powder for food processing material. Foods Food Ingredients J. Jpn. 2007, 212, 320–328. [Google Scholar]
- Yao, X.; Song, W.; Zhang, Y.; Xiao, W. On the application of enzyme on preparations in bread containing soybeand. Cereal Feed Ind. 2006, 11, 22–23. [Google Scholar]
- Wang, Y.; Tang, J. Application of soybean fiber on bread. J. Zhengzhou Inst. Technol. 2000, 21, 75–77. (In Chinese) [Google Scholar]
- Zhao, G.; Kong, J. Enzymolysis bean dregs biscuit development. Food Res. Dev. 2009, 10, 67–69. [Google Scholar]
- Wu, J.; Shang, Y.; Li, X.; He, P. Study on the crisp bean dregs biscuit. Sichuan Food Ferment. 2006, 42, 32–35. (In Chinese) [Google Scholar]
- Zhang, B. Preparation of sugarless and bean dregs cake. Nongchanpin Jiagong Xuekan 2007, 6, 94–95. (In Chinese) [Google Scholar]
- Wu, S. Preparation of bean dregs cake. Food Ind. 2003, 24, 23–24. [Google Scholar]
- Xie, W.; Cao, L.; Wang, Y.; Tong, Y. Development of the extrusion food of soybean dietary fiber. Nongchanpin Jiagong Xuekan 2005, 11, 37–39. (In Chinese) [Google Scholar]
- You, J.; Liu, D. Study on the development of fiber food from bean-dregs/potato-dregs. Sichuan Food Ferment. 2004, 40, 44–46. (In Chinese) [Google Scholar]
- Yu, H. Development of soybean residue food. China Western Cereals Oils Technol. 2001, 26, 36–37. (In Chinese) [Google Scholar]
- Lu, H.; Li, M. Preparation of fine dried noodle using okara. China Western Cereals Oils Technol. 1998, 23, 48. (In Chinese) [Google Scholar]
- Sun, X.; Yang, Y. Study on cooking quality of noodle of okara fiber. Grain Process. 2010, 1, 57–59. [Google Scholar]
- Bedani, R.; Campos, M.M.; Castro, I.A.; Rossi, E.A.; Saad, S.M. Incorporation of soybean by-product okara and inulin in a probiotic soy yoghurt: Texture profile and sensory acceptance. J. Sci. Food Agric. 2014, 94, 119–125. [Google Scholar] [CrossRef]
- Waliszewski, K.N.; Pardio, V.; Carreon, E. Physicochemical and sensory properties of corn tortillas made from nixtamalized corn flour fortified with spent soymilk residue (okara). J. Food Sci. 2002, 67, 3194–3197. [Google Scholar] [CrossRef]
- Genta, H.D.; Genta, M.L.; Álvarez, N.V.; Santana, M.S. Production and acceptance of a soy candy. J. Food Eng. 2002, 53, 199–202. [Google Scholar] [CrossRef]
- Kong, J. Study on the steam bun with beans dregs. Nongchanpin Jiagong Xuekan 2009, 5, 44–46. (In Chinese) [Google Scholar]
- Li, J.; Wang, Q.; He, M. Development of a soybean residue fiber and vitamin drink. Sci. Technol. Food Ind. 2005, 26, 111–113. [Google Scholar]
- Huang, W.; Cao, L.; Ma, Y.; Wang, J.; Jiang, Y. Preparation of nutritional sausage with soybean fiber. Meat Ind. 2004, 9, 11–13. [Google Scholar]
- Wang, Y.; Wang, J. Preparation of okara vegetable slice. Guangzhou Food Sci Technol. 2003, 19, 64–66. (In Chinese) [Google Scholar]
- Xie, W.; Li, B. Bean Dregs Nutrient Flour and Preparation Method Thereof. CN Patent CN 101507509A, 25 March 2009. [Google Scholar]
- Rotem, I.; Almog, N. Protein-Rich Premix Powders Comprising Okara for Healthy Food Industry. U.S. Patent US 2009/0317530 Al, 24 December 2009. [Google Scholar]
- Vong, W.C.; Liu, S.Q. Biovalorisation of okara (soybean residue) for food and nutrition. Trends Food Sci. Technol. 2016, 52, 139–147. [Google Scholar] [CrossRef]
- Guimarães, R.M.; Silva, T.E.; Lemes, A.C.; Boldrin, M.C.F.; Pereira, M.A.; Silva, F.G.; Egea, M.B. Okara: A soybean by-product as an alternative to enrich vegetable paste. LWT Food Sci. Technol. 2018, 92, 593–599. [Google Scholar] [CrossRef]
- Larrauri, J.A. New approaches in the preparation of high dietary fiber powders from fruit by-products. Trends Food Sci. Technol. 1999, 10, 3–8. [Google Scholar] [CrossRef]
- Li, L.; Zhan, Y.; Xu, K.; Yang, W.; Ning, Z. Study on bleaching technology of dietary fiber from bean dregs. Food Res. Dev. 2007, 28, 113–116. [Google Scholar]
- Zheng, D.; Xie, Q.; Zhang, H. Research on condition for pretreatment of dietary fiber of bean dregs. Food Sci. 2005, 26, 340–346. [Google Scholar]
- Lou, H.; Chi, Y. Optimization of technology for preparing soluble dietary fiber from extruded soybean residue. Trans. Chin. Soc. Agric. Eng. 2009, 25, 285–289. [Google Scholar]
- Sun, Y.; Wu, X.; Wang, Y.; Luo, Y.; Liu, B.; Xu, W. Preparation of soluble dietary fiber from soybean residue. Food Ferment. Ind. 2009, 35, 92–95. [Google Scholar]
- Si, F.; Wang, M. Producing soluble dietary fiber from bean dregs through enzymolysis. Nongchanpin Jiagong Xuekan 2009, 35, 108–110. (In Chinese) [Google Scholar]
- Huang, X.; Qu, W.; Wang, Y.; Wang, C.; Liang, S.; Zhang, X. Producing two dietary fiber from bean dregs through enzymolysis. Food Ferment. Ind. 2004, 30, 25–28. [Google Scholar]
- Tu, Z.; Lin, R.; Liu, C.; Liu, G.; Li, P.; Zheng, M.; Jiang, G. Study on production of high activity dietary fiber from soybean dregs in Neurospora crassa. Food Ferment. Ind. 2008, 34, 68–70. [Google Scholar]
- Yang, C.M.J. Soybean milk residue ensiled with peanut hulls: Fermentation acids, cell wall composition, and silage utilization by mixed ruminal microorganisms. Biores. Technol. 2005, 96, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.H.; Tang, L.Y. The use of enzyme-digested soybean residue for feeding common carp. Biomed. Environ. Sci. 1996, 9, 418–423. [Google Scholar]
- Yang, C.; Gu, J. Study on the active okara for feeding egg chicken. Feed Ind. 1997, 18, 26–27. [Google Scholar]
- Wang, Z.; Wang, L.; Chen, Y.; Wu, Z. Contrast trial of substituting dried tofu pulp for soybean meal in dairy diet. China Dairy Cattle 2003, 2, 24–26. (In Chinese) [Google Scholar]
- Wang, Z.; Jiang, W.; Hu, Z.; Wang, L. Feeding effects on finishing cattle by substituting soybean meal by dry bean curd pulp. J. Yellow Cattle Sci. 2004, 30, 15–17. [Google Scholar]
- Hermann, J.R.; Honeyman, M.S. Okara: A possible high protein feedstuff for organic pig diets. Anim. Ind. Rep. 2004, 650, 124. Available online: http://lib.dr.iastate.edu/ans_air/vol650/iss1/124 (accessed on 17 March 2020).
- Qiao, J.; Zhang, F. Research on the processing of microbial protein feed by mixed culture solid-state fermentation. Feed Ind. 2008, 29, 21–24. [Google Scholar]
- Mo, C. Study on the production of protein feed by mixed bacteria fermentation of soybean dregs. China Feed 2007, 14, 36–38. (In Chinese) [Google Scholar]
- Pan, T.; Zhang, D.; Zhao, C.; Li, K. Study on microbial protein production by mixed culture solid state fermentation on soybean waste. Chem. Bioeng. 2004, 6, 35–41. [Google Scholar]
- Adachi, A.; Hamamoto, H.; Okano, T. Use of lees materials as an adsorbent for removal of organochlorine compounds or benzene from wastewater. Chemosphere 2005, 58, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yuan, X.; Liu, X.; Zeng, G.; Liu, J.; Tong, J. Adsorption of Cd2+ and Zn2+ in water by bean dregs. Environ. Protect. Chem. Ind. 2008, 28, 296–299. [Google Scholar]
- Zhang, H.; Liu, J.; Xu, Z. Preparation and quality analysis of edible paper of bean dregs. Food Sci. 2008, 29, 30–32. [Google Scholar]
- Wen, Z.; Liu, D. Production of edible packaging with bean dregs. Chin. Resour. Compr. Util. 2007, 25, 11–13. (In Chinese) [Google Scholar]
- Li, G.; Cao, C.; Zhao, T.; Yan, J.; Fan, X.; Wang, L. Modification of bean curd residue and preparation of its degradable composite materials. Environ. Protect. Sci. 2007, 33, 30–32. [Google Scholar]
- Chen, Y. Preparation and properties of the corn gluten meal/soybean dreg biodegradable plastics by extrusion. Trans. Chin. Soc. Agric. Machinery 2007, 38, 75–77. (In Chinese) [Google Scholar]
- Vong, W.C.; Lim, X.Y.; Liu, S.Q. Biotransformation with cellulase, hemicellulase and Yarrowia lipolytica boosts health benefits of okara. Appl. Microbiol. Biotechnol. 2017, 101, 1–12. [Google Scholar] [CrossRef]
- Available online: https://www.science.nus.edu.sg/blog/2017/10/26/okara-biotransformed-and-back-in-action/ (accessed on 17 March 2020).
- Fujita, T.; Funako, T.; Hayashi, H. 8-Hydroxydaidzein, an aldose reductase inhibitor from okara fermented with Aspergillus sp. HK-388. Biosci. Biotechnol. Biochem. 2004, 68, 1588–1590. [Google Scholar] [CrossRef] [Green Version]
- Wongkhalaung, C.; Leelawatcharamas, V.; Japakaset, J. Utilisation of soybean residue to produce monacolin K-cholesterol lowering agent. Songklanakarin J. Sci. Technol. 2009, 31, 35–39. [Google Scholar]
- Childress, L.; Gay, A.; Zargar, A.; Ito, M.K. Review of red yeast rice content and current Food and Drug Administration oversight. J. Clin. Lipidol. 2013, 7, 117–122. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products and Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to monacolin K from red yeast rice and maintenance of normal blood LDL-cholesterol concentrations (ID 1648, 1700) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2304. [Google Scholar]
- Li, S.; Chen, Y.; Li, K.; Lei, Z.; Zhang, Z. Characterization of physicochemical properties of fermented soybean curd residue by Morchella esculenta. Int. Biodeter. Biodegr. 2016, 109, 113–118. [Google Scholar] [CrossRef]
- Li, S.; Sang, Y.; Zhu, D.; Yang, Y.; Lei, Z.; Zhang, Z. Optimization of fermentation conditions for crude polysaccharides by Morchella esculenta using soybean curd residue. Ind. Crops Prod. 2013, 50, 666–672. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Sun, H.; Zhang, Z. Ultrasonic-assisted extraction and antioxidant activities of the polysaccharides extracted from soybean curd residue fermented by Flammulina velutipes. Int. J. Biol. 2015, 8, 61. [Google Scholar] [CrossRef]
- Shi, M.; Yang, Y.; Hu, X.; Zhang, Z. Effect of ultrasonic extraction conditions on antioxidative and immunomodulatory activities of a Ganoderma lucidum polysaccharide originated from fermented soybean curd residue. Food Chem. 2014, 155, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Sun, H.; Li, S.; Hu, X.; Yuan, X.; Han, C.; Zhang, Z. Influence of drying methods on antioxidant activities and immunomodulatory of aqueous extract from soybean curd residue fermentated by Grifola frondosa. Int. J. Biol. 2015, 7, 82. [Google Scholar] [CrossRef]
- Xu, X.; Liu, H.; Zhou, Y. Study on the meitauza production from okara by Actinomucor elegans and Zymomonas mobilis. In Information Technology and Agricultural Engineering. Advances in Intelligent and Soft Computing; Zhu, E., Sambath, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 134, pp. 329–336. [Google Scholar]
- Yogo, T.; Ohashi, Y.; Terakado, K.; Nezu, Y.; Hara, Y.; Tagawa, M.; Fujisawa, T. Influence of dried okara-tempeh on the composition and metabolites of faecal microbiota in dogs. Int. J. Appl. Res. Vet. Med. 2011, 9, 181–188. [Google Scholar]
- Matsuo, M. Application of okara koji, okara fermented by Aspergillus oryzae, for cookies and cupcakes. J. Home Econom. Jpn. 1999, 50, 1029–1034. [Google Scholar]
- Matsuo, M.; Takeuchi, T. Preparation of low salt miso-like fermented seasonings using soy-oncom and okara-oncom (fermented soybeans and okara with Neurospora intermedia) and their antioxidant activity and antimutagenicity. Food Sci. Technol. Res. 2003, 9, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, M. Chemical components, palatability, antioxidant activity and antimutagenicity of oncom miso using a mixture of fermented soybeans and okara with Neurospora intermedia. J. Nutr. Sci. Vitaminol. 2006, 52, 216–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, M. In vivo antioxidant activity of okara koji, a fermented okara, by Aspergillus oryzae. Biosci. Biotech. Bioch. 1997, 61, 1968–1972. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M. Low-salt O-miso produced from koji fermentation of oncom improves redox state and cholesterolemia in rats more than low-salt soybean miso. J. Nutr. Sci. Vitaminol. 2004, 50, 362. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Jeong, D. Korean traditional fermented soybean products: Jang. J. Ethnic Foods 2015, 2, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-I.; Lee, Y.-K.; Kim, S.-D.; Lee, J.-E.; Choi, J.; Bak, J.-P.; Lee, I. Effect of fermented soybean curd residue (FSCR; SCR-meju) by Aspergillus oryzae on the anti-obesity and lipids improvement. J. Nutr. Health 2013, 46, 493–502. [Google Scholar] [CrossRef]
- Bhunia, B.; Basak, B.; Dey, A. A review on production of serine alkaline protease by Bacillus spp. J. Biochem. Technol. 2012, 3, 448–457. [Google Scholar]
- Oh, S.-M.; Jang, E.-K.; Seo, J.-H.; Ryu, M.-J.; Lee, S.-P. Characterization of γ-polyglutamic acid produced from the solid-state fermentation of soybean milk cake using Bacillus sp. Food Sci. Biotechnol. 2007, 16, 509–514. [Google Scholar]
- Zhu, Y.P.; Fan, J.F.; Cheng, Y.Q.; Li, L.T. Improvement of the antioxidant activity of Chinese traditional fermented okara (meitauza) using Bacillus subtilis B2. Food Control 2008, 19, 654–661. [Google Scholar] [CrossRef]
- Oh, S.; Kim, C.; Lee, S. Characterization of the functional properties of soymilk cake fermented by Bacillus sp. Food Sci. Biotechnol. 2006, 15, 704. [Google Scholar]
- Zu, X.; Zhang, Z.; Che, H.; Zhang, G.; Yang, Y.; Li, J. Nattokinase’s extraction from Bacillus subtilis fermented soybean curd residue and wet corn distillers’ grain and fibrinolytic activities. Int. J. Biol. 2010, 2, 120. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Cheng, Y.Q.; Wang, L.J.; Fan, J.F.; Li, L.T. Enhanced antioxidative activity of Chinese traditionally fermented okara (meitauza) prepared with various microorganism. Int. J. Food Prop. 2008, 11, 519–529. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Yamaki, K.; Yoshihashi, T.; Ohnishi Kameyama, M.; Li, X.T.; Cheng, Y.Q.; Li, L.T. Purification and identification of 1-deoxynojirimycin (DNJ) in okara fermented by Bacillus subtilis B2 from Chinese traditional food (meitaoza). J. Agric. Food Chem. 2010, 58, 4097–4103. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Mu, S.; Li, H.; Li, Y.; Feng, C.; Jin, J.-M.; Tang, S.Y. Design and application of a novel high-throughput screening technique for 1-deoxynojirimycin. Sci. Rep. 2015, 5, 8563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vichasilp, C.; Nakagawa, K.; Sookwong, P.; Suzuki, Y.; Kimura, F.; Higuchi, O.; Miyazawa, T. Optimisation of 1-deoxynojirimycin extraction from mulberry leaves by using response surface methodology. Biosci. Biotech. Bioch. 2009, 73, 2684–2689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahvaselka, M.; Laakso, S. Production of cis-9, trans-11-conjugated linoleic acid in camelina meal and okara by an oat-assisted microbial process. J. Agric. Food Chem. 2010, 58, 2479–2482. [Google Scholar] [CrossRef] [PubMed]
- Saad, N.; Delattre, C.; Urdaci, M.; Schmitter, J.-M.; Bressollier, P. An overview of the last advances in probiotic and prebiotic field. LWT Food Sci. Technol. 2013, 50, 1–16. [Google Scholar] [CrossRef]
- Bedani, R.; Rossi, E.A.; Isay Saad, S.M. Impact of inulin and okara on Lactobacillus acidophilus La-5 and Bifidobacterium animalis Bb-12 viability in a fermented soy product and probiotic survival under in vitro simulated gastro-intestinal conditions. Food Microbiol. 2013, 34, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Villanueva-Suarez, M.J.; Perez-Cozar, M.L.; Redondo-Cuenca, A. Sequential extraction of polysaccharides from enzymatically hydrolyzed okara byproduct: Physicochemical properties and in vitro fermentability. Food Chem. 2013, 141, 1114–1119. [Google Scholar] [CrossRef]
- Tu, Z.; Chen, L.; Wang, H.; Ruan, C.; Zhang, L.; Kou, Y. Effect of fermentation and dynamic high pressure microfluidization on dietary fibre of soybean residue. J. Food Sci. Technol. 2014, 51, 3285–3292. [Google Scholar] [CrossRef] [Green Version]
- Kitawaki, R.; Takagi, N.; Iwasaki, M.; Asao, H.; Okada, S.; Fukuda, M. Plasma cholesterol-lowering effects of soymilk and okara treated by lactic acid fermentation in rats. J. Jpn. Soc. Food Sci. Technol. (Japan) 2007, 54, 379–382. [Google Scholar]
- Kitawaki, R.; Nishimura, Y.; Takagi, N.; Iwasaki, M.; Tsuzuki, K.; Fukuda, M. Effects of Lactobacillus fermented soymilk and soy yogurt on hepatic lipid accumulation in rats fed a cholesterol-free diet. Biosci. Biotech. Bioch. 2009, 73, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
- Rashad, M.M.; Mahmoud, A.E.; Abou, H.M.; Nooman, M.U. Improvement of nutritional quality and antioxidant activities of yeast fermented soybean curd residue. African J. Biotechnol. 2011, 10, 5504–5513. [Google Scholar]
- Vong, W.C.; Liu, S.Q. Changes in volatile profile of soybean residue (okara) upon solid-state fermentation by yeasts. J. Sci. Food Agric. 2017, 97, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.science.nus.edu.sg/blog/2016/05/13/yeast-fermented-okara-smells-good/ (accessed on 19 March 2020).
- Jing, Y.; Chi, Y.-J. Effects of twin-screw extrusion on soluble dietary fibre and physicochemical properties of soybean residue. Food Chem. 2013, 138, 884–889. [Google Scholar] [CrossRef]
- Perez-Lopez, E.; Mateos-Aparicio, I.; Ruperez, P. Okara treated with high hydrostatic pressure assisted by Ultraflo® L: Effect on solubility of dietary fibre. Innovative Food Sci. Emerg. Technol. 2015, 33, 32–37. [Google Scholar] [CrossRef]
- Huang, S.; He, Y.; Zou, Y.; Liu, Z. Modification of insoluble dietary fibres in Soybean bean okara and their physicochemical properties. Int. J. Food Sci. Technol. 2015, 50, 2606–2613. [Google Scholar] [CrossRef]
- Fierens, E.; Brijs, K.; Delcour, J.A. Emulsifying and foaming properties of okara protein hydrolysates. Cereal Chem. 2015, 93, 71–76. [Google Scholar] [CrossRef]
- Jankowiak, L.; Mendez Sevillano, D.; Boom, R.M.; Ottens, M.; Zondervan, E.; Van der Goot, A.J. A process synthesis approach for isolation of isoflavones from okara. Ind. Eng. Chem. Res. 2015, 54, 691–699. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Chen, M.; Wan, J.-B.; Su, H.; He, C. Review on the extraction, characterization and application of soybean polysaccharide. RSC Advances 2015, 5, 73525–73534. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Tenorio, M.D.; Espinosa-Martos, I.; Rupérez, P. Health-promoting effects of a dietary fiber concentrate from the soybean byproduct okara in rats. J. Agric. Food Chem. 2008, 56, 7495–7501. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Li, D.; Wang, H. Study on separation and purification of genistein in the soybean residue using macroporous resin adsorption. Ind. Eng. Chem. Res. 2011, 51, 44–49. [Google Scholar] [CrossRef]
- IDF Diabetes Atlas, 4th ed.; International Diabetes Federation: Brussels, Belgium, 2009; pp. 1–104. Available online: www.eatlas.idf.org (accessed on 20 March 2020).
- Franz, M.J. Protein controversies in diabetes. Diabetes Spectrum 2000, 13, 132–141. [Google Scholar]
- Xu, H.; Wang, Y.; Liu, H.; Zheng, J.; Xin, Y. Influence of soybean fibers on blood sugar and blood lipid metabolism and hepatic-nephritic histomorphology of mich with STZ-induced diabetes. Acta Nutr. Sinica 2000, 22, 171–174. [Google Scholar]
- Wang, C.; Li, S. Influence of okara fiber on lipid metabolism and hemorheology of rats. Acta Nutr. Sinica 1996, 18, 168–174. [Google Scholar]
- Préstamo, G.; Rupérez, P.; Espinosa-Martos, I.; Villanueva, M.J.; Lasunción, M.A. The effects of okara on rat growth, cecal fermentation, and serum lipid. Eur. Food Res. Technol. 2007, 225, 925–928. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, K.; Watanabe, Y.; Yokoyama, S. Okara, soybean residue, prevents obesity in a diet-induced murine obesity model. Biosci. Biotech. Bioch. 2007, 71, 720–727. [Google Scholar] [CrossRef]
- Ge, F.; Gui, L.; Tao, Y.; Zhu, L.; Huang, Y. DPPH radical scavenging activity of extract from soybean residue and coordination effect. Soybean Sci. 2010, 29, 113–117. [Google Scholar]
- Taruna, I.; Jindal, V.K. Drying of soy pulp (okara) in a bed of inert particles. Drying Technol. 2002, 20, 1035–1051. [Google Scholar] [CrossRef]
- Choi, J.-H.; Kim, M.H.; Kim, J.H.; Choi, Y.C. Drying characteristics of bean-curd refuse. J. Taiwan Inst. Chem. Eng. 2010, 41, 157–161. [Google Scholar] [CrossRef]
- Cui, D.; Luo, L. Drying and production of soybean residue. Modern Agric. 1997, 1, 37. [Google Scholar]
- Wachiraphansakul, S.; Devahastin, S. Drying kinetics and quality of soy residue (okara) dried in a jet spouted bed dryer. Drying Technol. 2005, 23, 1229–1242. [Google Scholar] [CrossRef]
- Cui, G.; Cao, Y.; Pang, S. Experimental study of okara drying with high-voltage electric field. J. Taishan Univ. 2005, 27, 73–75. [Google Scholar]
- Li, F.D.; Li, L.T.; Sun, J.F.; Tatsumi, E. Effect of electrohydrodynamic (EHD) technique on drying process and appearance of okara cake. J. Food Eng. 2006, 77, 275–280. [Google Scholar] [CrossRef]
- Li, B.; Wang, D.; Han, W.; Lu, F. Experimental study on bean curd residue in microwave vacuum drying. Sci. Technol. Food Ind. 2011, 32, 318–320. [Google Scholar]
- Li, B.; Zhang, Y.; Yang, H.; Li, R. Effect of drying methods on functional properties of bean curd dregs. J. Henan Inst. Sci. Technol. 2008, 36, 64–66. (In Chinese) [Google Scholar]
- Wiriyaumpaiwong, S.; Soponronnarit, S.; Prachayawarakorn, S. Comparative study of heating processes for full-fat soybeans. J. Food Eng. 2004, 65, 371–382. [Google Scholar] [CrossRef]
- Marty, B.J.; Chavez, E.R. Ileal digestibilities and urinary losses of amino acids in pigs fed heat processed soybean products. Livestock Prod. Sci. 1995, 43, 37–48. [Google Scholar] [CrossRef]
- Hinks, C.F.; Hupka, D. The effects of feeding leaf sap from oats and wheat, with and without soybean trypsin inhibitor, on feeding behaviour and digestive physiology of adult males of Melanoplus sanguinipes. J. Insect Physiol. 1995, 41, 1007–1015. [Google Scholar] [CrossRef]
- Ao, T.; Cantor, A.H.; Pescatore, A.J.; Pierce, J.L.; Dawson, K.A. Effects of citric acid, alpha-galactosidase and protease inclusion on in vitro nutrient release from soybean meal and trypsin inhibitor content in raw whole soybeans. Animal Feed Sci. Technol. 2010, 162, 58–65. [Google Scholar] [CrossRef]








| Compound | SBs | SBs | SBs | Soy | SB | SP | SP | Soy | Okara 9 |
|---|---|---|---|---|---|---|---|---|---|
| (Amount Unit/100 g) | Raw 1 | Dried 2 | Boiled 3 | Flour 4 | Meal 5 | conc. 6 | Isolate 7 | Milk 8 | |
| Water (g) | 8.54 | 0.8 | 62.6 | 5.2 | 6.94 | 5.8 | 4.98 | 93.3 | 81.6 |
| Energy (kcal/KJ) | 446/1866 | 449/1880 | 172/721 | 434/1816 | 337/1409 | 328/1373 | 335/1401 | 33/138 | 76/320 |
| Protein (g) | 36.5 | 43.3 | 18.2 | 37.8 | 49.2 | 63.6 | 88.3 | 2.8 | 3.52 |
| Total lipid (g) | 19.9 | 21.6 | 8.97 | 206 | 2.39 | 0.46 | 3.39 | 2.0 | 1.73 |
| Tot saturated FA (g) | 2.88 | 3.13 | 1.30 | 2.99 | 0.27 | 0.05 | 0.42 | 0.21 | 0.19 |
| Tot monounsaturated FA (g) | 4.40 | 4.78 | 1.98 | 4.56 | 0.41 | 0.08 | 0.64 | 0.33 | 0.30 |
| Tot polyunsaturated FA (g) | 11.3 | 12.2 | 5.06 | 11.7 | 1.04 | 0.20 | 1.65 | 0.83 | 0.76 |
| Ash (g) | 4.87 | 5.28 | 1.91 | 4.46 | 5.58 | 4.7 | 3.58 | 0.27 | 0.88 |
| Carbohydrate (by diff., g) | 30.2 | 29.0 | 8.36 | 31.9 | 35.9 | 25.4 | 0 | 1.8 | 12.2 |
| Fibre (total dietary, g) | 9.3 | 8.1 | 6 | 9.6 | NR | 5.5 | 0 | 1.3 | NR |
| Sugars (total, g) | 7.33 | NR | 3 | 7.5 | NR | 20 | 0 | NR | NR |
| Minerals | |||||||||
| Calcium (mg) | 277 | 140 | 102 | 206 | 244 | 363 | 178 | 4.0 | 80 |
| Iron (mg) | 15.7 | 3.95 | 5.14 | 6.37 | 13.7 | 10.8 | 14.5 | 0.58 | 1.3 |
| Magnesium (mg) | 280 | 228 | 86 | 429 | 306 | 140 | 39 | 19.0 | 26 |
| Phosphorus (mg) | 704 | 649 | 245 | 494 | 701 | 839 | 776 | 49.0 | 60 |
| Potassium (g) | 1.80 | 1.36 | 0.52 | 2.5 | 2.49 | 0.450 | 0.081 | 0.141 | 0.213 |
| Sodium (mg) | 2 | 2 | 1 | 13 | 3 | 900 | 1005 | 12 | 9 |
| Zinc (mg) | 4.89 | 4.77 | 1.15 | 3.92 | 5.06 | 4.4 | 4.03 | 0.23 | 0.56 |
| Copper (mg) | 1.66 | 1.08 | 0.41 | 2.92 | 2 | 0.98 | 1.60 | 0.12 | 0.2 |
| Manganese (mg) | 2.52 | 2.18 | 0.82 | 2.28 | 3.8 | 4.19 | 1.49 | 0.17 | 0.40 |
| Selenium (µg) | 17.8 | 19.3 | 7.3 | 7.5 | 3.3 | 0.8 | 0.8 | 1.3 | 10.6 |
| Vitamins | |||||||||
| Ascorbic acid (C) (mg) | 6 | 4.6 | 1.7 | 0 | 0 | 0 | 0 | 0 | 0 |
| Thiamine (B1) (mg) | 0.87 | 0.43 | 0.16 | 0.58 | 0.69 | 0.32 | 0.18 | 0.161 | 0.02 |
| Riboflavin (B2) (mg) | 0.87 | 0.78 | 0.28 | 1.16 | 0.25 | 0.14 | 0.1 | 0.07 | 0.02 |
| Niacin (B3) (mg) | 1.62 | 1.06 | 0.40 | 4.32 | 2.59 | 0.72 | 1.44 | 0.15 | 0.1 |
| Pantothenic acid (B5) (mg) | 0.79 | 0.47 | 0.18 | 1.59 | 1.98 | 0.06 | 0.06 | 0.05 | 0.09 |
| Pyridoxine (B6) (mg) | 0.38 | 0.22 | 0.23 | 0.46 | 0.57 | 0.13 | 0.1 | 0.04 | 0.12 |
| Folate (B9) (µg) | 375 | 205 | 54 | 345 | 303 | 340 | 176 | 1.5 | 26 |
| Retinol (A) (IU) * | 22 | 0 | 9 | 120 | 40 | 0 | 0 | 10 | 0 |
| α-Tocopherol (E) (mg) | 0.85 | NR | 0.35 | 1.95 | NR | NR | 0 | 0.01 | NR |
| Phylloquinone (K) (µg) | 47 | 37 | 19.2 | 70 | NR | 0 | NR | NR | NR |
| Compound | Tempeh 1 | Miso 2 | Natto 3 | Soy Sauce 4 | Tofu 5 |
|---|---|---|---|---|---|
| (Amount Unit/100 g) | |||||
| Water (g) | 59.6 | 43.0 | 55.0 | 66 | 70.0 |
| Energy (kcal/KJ) | 192/803 | 198/828 | 211/883 | 60/251 | 116/484 |
| Protein (g) | 20.3 | 12.8 | 19.4 | 10.5 | 8.92 |
| Total lipid (g) | 10.8 | 6.0 | 11 | 0.1 | 8 |
| Tot saturated FA (g) | 2.54 | 1.02 | 1.59 | 0.011 | 1.16 |
| Tot monounsaturated FA (g) | 3.2 | 1.12 | 2.43 | 0.017 | 1.77 |
| Tot polyunsaturated FA (g) | 4.3 | 2.88 | 6.21 | 0.044 | 4.52 |
| Ash (g) | 1.62 | 12.8 | 1.9 | 17.8 | 8.7 |
| Carbohydrate (by diff., g) | 7.64 | 25.4 | 12.7 | 5.6 | 4.38 |
| Fibre (total dietary, g) | NR | 5.4 | 5.4 | 0.8 | NR |
| Sugars (total, g) | NR | 6.2 | 4.9 | 1.7 | NR |
| Minerals | |||||
| Calcium (mg) | 111 | 57 | 217 | 20 | 46 |
| Iron (mg) | 2.7 | 2.49 | 8.6 | 2.38 | 1.98 |
| Magnesium (mg) | 81 | 48 | 115 | 40 | 52 |
| Phosphorus (mg) | 266 | 159 | 174 | 130 | 73 |
| Potassium (g) | 412 | 210 | 729 | 212 | 75 |
| Sodium (mg) | 9 | 3728 | 7 | 5586 | 2873 |
| Zinc (mg) | 1.14 | 2.56 | 3.03 | 0.43 | 1.56 |
| Copper (mg) | 0.56 | 0.42 | 0.67 | 0.14 | 0.38 |
| Manganese (mg) | 1.3 | 0.86 | 1.53 | 0.50 | 1.17 |
| Selenium (µg) | 0 | 7 | 8.8 | 0.8 | 17.3 |
| Vitamins | |||||
| Ascorbic acid (C) (mg) | 0 | 0 | 13 | 0 | 0.2 |
| Thiamine (B1) (mg) | 0.08 | 0.098 | 0.16 | 0.06 | 0.16 |
| Riboflavin (B2) (mg) | 0.36 | 0.23 | 0.19 | 0.152 | 0.10 |
| Niacin (B3) (mg) | 2.64 | 0.091 | 0 | 3.95 | 0.38 |
| Pantothenic acid (B5) (mg) | 0.28 | 0.34 | 0.22 | 0.38 | 0.13 |
| Pyridoxine (B6) (mg) | 0.22 | 0.20 | 0.13 | 0.2 | 0.09 |
| Folate (B9) (µg) | 24 | 19 | 8 | 18 | 29 |
| Cobalamin (B12) (µg) | 0.08 | 0.08 | 0 | 0 | 0 |
| Retinol (A) (IU) * | 0 | 87 | 0 | 0 | 0 |
| α-Tocopherol (E) (mg) | NR | 0.01 | 0.01 | 0 | 0 |
| Phylloquinone (K) (µg) | NR | 29.3 | 23.1 | 0 | 0 |
| Carbohydrates | References | ||
|---|---|---|---|
| [25] | [26] | [27] | |
| Rhamnose | 0.85 | 0.3 ± 0.1 | 1.0 ± 0.1 |
| Fuchose | 0.45 | 0.5 ± 0.1 | 0.1 |
| Arabinose | 6.35 | 5.7 ± 0.1 | - |
| Xylose | 5.14 | 2.7 ± 0.1 | - |
| Mannose | 1.26 | 1.5 ± 0.3 | - |
| Galactose | 10.83 | 10.4 ± 0.2 | 0.2 |
| Glucose | 15.01 | 11.9 ± 0.4 | 0.2 |
| Sucrose | - | - | 0.6 ± 0.1 |
| Amino Acids | Content |
|---|---|
| Aspartic acid | 117 |
| Threonine | 41 |
| Serine | 50 |
| Glutamic acid | 195 |
| Glycine | 46 |
| Alanine | 46 |
| Cysteine + methionine | 26 |
| Valine | 51 |
| Isoleucine | 51 |
| Leucine | 81 |
| Tyrosine + phenylalanine | 95 |
| Lysine | 65 |
| Histidine | 28 |
| Arginine | 75 |
| Proline | 36 |
| Tryptophan | N.D.* |
| Groups | Forms | Content (mg) |
|---|---|---|
| Aglycones | Daidzein | 22 |
| Glycitein | 1.1 | |
| Genistein | 31 | |
| β-glucosides | Daidzein | 48 |
| Glycitein | 2.2 | |
| Genistein | 53 | |
| Malonyl glucosides | Daidzein | 64 |
| Glycitein | 2.8 | |
| Genistein | 130 | |
| Acetyl glucosides | Daidzein | - |
| Glycitein | 3.2 | |
| Genistein | - | |
| Total | 355 |
| Nutrients | Okara | Formulations | ||
|---|---|---|---|---|
| (Amount Unit/100 g) | F1 | F2 | F3 | |
| Humidity | 80.25 ± 0.04 | 81.26 ± 0.04 | 81.42 ± 0.05 | 80.77 ± 0.06 |
| Protein (g) | 7.91 ± 0.25 | 3.07 ± 0.70 | 4.00 ± 0.26 | 4.72 ± 0.19 |
| Lipids (g) | 6.22 ± 0.45 | 5.62 ± 0.86 | 6.20 ± 0.09 | 7.62 ± 0.46 |
| Ash (g) | 0.86 ± 0.00 | 1.98 ± 0.00 | 1.76 ± 0.01 | 1.72 ± 0.00 |
| Total fibre (g) | 13.83 ± 0.49 | 5.79 ± 0.17 | 7.17 ± 0.22 | 8.00 ± 0.25 |
| Soluble fibre (g) | 3.25 ± 0.09 | 1.67 ± 0.03 | 1.99 ± 0.04 | 2.19 ± 0.05 |
| Insoluble fibre (g) | 10.58 ± 0.40 | 4.13c ± 0.14 | 5.18 ± 0.18 | 5.82 ± 0.20 |
| Carbohydrates (g) | 2.44 ± 1.29 | 5.8 ± 0.49 | 4.61 ± 0.32 | 3.50 ± 1.37 |
| Energy (kcal) | 100.17 | 89.65 | 94.20 | 105.81 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colletti, A.; Attrovio, A.; Boffa, L.; Mantegna, S.; Cravotto, G. Valorisation of By-Products from Soybean (Glycine max (L.) Merr.) Processing. Molecules 2020, 25, 2129. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25092129
Colletti A, Attrovio A, Boffa L, Mantegna S, Cravotto G. Valorisation of By-Products from Soybean (Glycine max (L.) Merr.) Processing. Molecules. 2020; 25(9):2129. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25092129
Chicago/Turabian StyleColletti, Alessandro, Andrea Attrovio, Luisa Boffa, Stefano Mantegna, and Giancarlo Cravotto. 2020. "Valorisation of By-Products from Soybean (Glycine max (L.) Merr.) Processing" Molecules 25, no. 9: 2129. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25092129