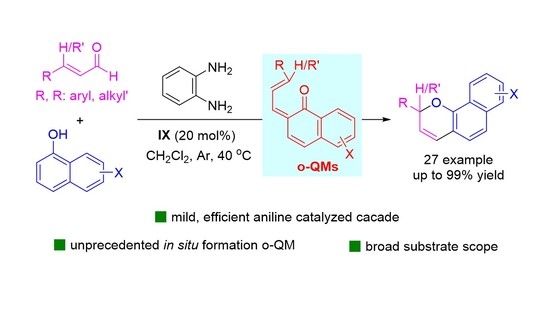
4. General Procedure for the Synthesis of 2H-Benzo[h]Chromene 3
Dry CH2Cl2 (1.0 mL) was added to a mixture of o-phenylenediamine (IX, 0.04 mmol), enal 1 (0.30 mmol) and arylol 2 (0.20 mmol) under Ar. The reaction was stirred at 40 °C until the completion of 2 monitored by TLC. Then, the mixture was applied to column chromatography directly and eluted with ethyl ether and hexane (9/1) to give product 3.
2-Phenyl-2H-benzo[h]chromenes (3a). The title compound was prepared according to the general procedure: 17 h, red oil; 87% yield. 1H NMR (300 MHz, CDCl3) δ (ppm) 8.27–8.24 (m, 1H), 7.81–7.76 (m, 1H), 7.60–7.56 (m, 2H), 7.48–7.39 (m, 6H), 7.22 (d, J = 8.4 Hz, 1H), 6.70 (dd, J = 9.8, 1.7 Hz, 1H), 6.18 (dd, J = 3.6, 1.7 Hz, 1H), 5.92 (dd, J = 9.8, 3.6 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ (ppm) 148.65, 141.21, 134.74, 128.73, 128.35, 127.71, 126.86, 126.41, 125.56, 124.71, 124.64, 123.39, 122.11, 120.51, 115.80, 77.34. HRMS (ESI) calcd for [C19H14O + H]+: 259.1123, found: 259.1128.
2-(2-Nitrophenyl)-2H-benzo[h]chromene (3b). The title compound was prepared according to the general procedure: 19 h, orange oil; 72% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 8.15–8.11 (m, 1H), 8.02 (dd, J = 8.1, 1.2 Hz, 1H), 7.87 (dd, J = 7.9, 1.2 Hz, 1H), 7.79–7.74 (m, 1H), 7.56 (td, J = 7.5, 1.2 Hz, 1H), 7.48–7.39 (m, 4H), 7.17 (d, J = 8.4 Hz, 1H), 6.84 (dd, J = 3.6, 1.8 Hz, 1H), 6.66 (dd, J = 9.9, 1.8 Hz, 1H), 5.92 (dd, J = 9.9, 3.6 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 148.40, 147.09, 136.89, 134.84, 133.82, 128.91, 128.86, 127.82, 126.66, 125.90, 125.13, 124.87, 124.79, 124.28, 121.86, 121.78, 121.03, 115.27, 73.24. HRMS (ESI) calcd for [C19H13NO3 + H]+: 304.0974, found: 304.0971.
2-(4-Nitrophenyl)-2H-benzo[h]chromene (3c). The title compound was prepared according to the general procedure except that 50 mg 4 Å MS powder was added: 60 h, orange oil; 83% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 8.21–8.16 (m, 3H), 7.78–7.72 (m, 1H), 7.66 (d, J = 8.7 Hz, 2H), 7.49–7.39 (m, 3H), 7.17 (d, J = 8.4 Hz, 1H), 6.71 (dd, J = 9.9, 1.8 Hz, 1H), 6.19 (dd, J = 3.9, 1.8 Hz 1H), 5.88 (dd, J = 9.9, 3.9 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 148.16, 148.08, 147.81, 134.86, 127.88, 127.35, 126.76, 125.97, 125.61, 124.65, 124.44, 124.00, 121.82, 121.75, 121.18, 115.67, 75.93. HRMS (ESI) calcd for [C19H13NO3 + H]+: 304.0974, found: 304.0969.
2-(4-Fluorophenyl)-2H-benzo[h]chromene (3d). The title compound was prepared according to the general procedure: 46 h; red oil; 83% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 8.18–8.15 (m, 1H), 7.76–7.73 (m, 1H), 7.53–7.48 (m, 2H), 7.46–7.37 (m, 3H), 7.18 (d, J = 8.1 Hz, 1H), 7.09–7.02 (m, 2H), 6.69 (dd, J = 9.9, 1.8 Hz, 1H), 6.11 (d, J = 2.4 Hz, 1H), 5.86 (dd, J = 9.6, 3.6 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ (ppm) 162.82 (d, J = 245 Hz), 148.38, 136.87, 134.76, 128.91, 128.80, 127.76, 126.50, 125.65, 124.96, 124.68, 123.02, 122.00, 120.66, 115.76, 115.48, 76.58. HRMS (ESI) calcd for [C19H13FO + H]+: 277.1029, found: 277.1032.
2-(4-Chlorophenyl)-2H-benzo[h]chromene (3e). The title compound was prepared according to the general procedure: 17 h; red oil; 81% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 8.21–8.16 (m, 1H), 7.78–7.74 (m, 1H), 7.49–7.39 (m, 5H), 7.36–7.32 (m, 2H), 7.19 (d, J = 8.4 Hz, 1H), 6.69 (dd, J = 9.9, 1.8 Hz, 1H), 6.10 (dd, J = 3.9, 1.8 Hz, 1H), 5.85 (dd, J = 9.9, 3.9 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 148.32, 139.55, 134.75, 134.21, 128.90, 128.33, 127.77, 126.53, 125.70, 125.03, 124.67, 124.60, 122.75, 121.95, 120.73, 115.74, 76.44. HRMS (ESI) calcd for [C19H13ClO + H]+: 293.0733, found: 293.0736.
2-(2-Bromophenyl)-2H-benzo[h]chromene (3f). The title compound was prepared according to the general procedure: 66 h; red oil; 85% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 8.24–8.19 (m, 1H), 7.79–7.74 (m, 1H), 7.66–7.62 (m, 2H), 7.48–7.39 (m, 3H), 7.29 (td, J = 7.5, 0.9 Hz, 1H), 7.19 (d, J = 8.1 Hz, 2H), 6.68 (dd, J = 9.9, 1.8 Hz, 1H), 6.53 (dd, J = 3.6, 1.8 Hz, 1H), 5.91 (dd, J = 9.9, 3.6 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ (ppm) 148.68, 140.00, 134.78, 133.18, 129.75, 128.92, 127.93, 127.72, 126.53, 125.71, 124.97, 124.69, 124.54, 122.19, 122.14, 121.85, 120.70, 115.50, 76.46. HRMS (ESI) calcd for [C19H13BrO + H]+: 337.0228, found: 337.0224.
2-(2-(Trifluoromethyl)phenyl)-2H-benzo[h]chromene (3g). The title compound was prepared according to the general procedure: 42 h, red oil; 71% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 8.15–8.12 (m, 1H), 7.95 (d, J = 7.8 Hz, 1H), 7.78–7.75 (m, 2H), 7.55 (t, J = 7.5 Hz, 1H), 7.47–7.39 (m, 4H), 7.21 (d, J = 8.4 Hz, 1H), 6.66 (dd, J = 9.6, 1.8 Hz, 1H), 6.61 (s, 1H), 5.75 (dd, J = 9.9, 3.3 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ (ppm) 148.59, 139.92, 134.89, 132.57, 129.64, 128.43, 127.71, 126.74 (q, J = 30 Hz), 126.59, 125.97 (q, J = 2.1 Hz), 125.68, 124.69, 124.65, 123.03, 122.14, 120.74, 115.10, 73.71. HRMS (ESI) calcd for [C20H13F3O + H]+: 327.0997, found: 327.0999.
2-(4-Methoxyphenyl)-2H-benzo[h]chromene (3h). The title compound was prepared according to the general procedure: 17 h; red oil; 84% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 8.20–8.17 (m, 1H), 7.76–7.72 (m, 1H), 7.49–7.37 (m, 5H), 7.19 (d, J = 8.4 Hz,1H), 6.93–6.88 (m, 2H), 6.69 (dd, J = 9.8, 1.8 Hz, 1H), 6.10 (dd, J = 3.9, 1.8 Hz, 1H), 5.88 (dd, J = 9.8, 3.6 Hz, 1H), 3.79 (s, 3H). 13C NMR (75 MHz, CDCl3) δ (ppm) 159.82, 148.58, 134.73, 133.23, 128.56, 127.68, 126.35, 125.48, 124.76, 124.67, 123.44, 122.13, 120.39, 115.85, 114.11, 77.02, 55.36. HRMS (ESI) calcd for [C20H16O2 + H]+: 289.1229, found: 289.1235.
2-(2-Methoxyphenyl)-2H-benzo[h]chromene (3i). The title compound was prepared according to the general procedure: 24 h; red oil; 80% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 8.22–8.18 (m, 1H), 7.76–7.53 (m, 1H), 7.51 (dd, J = 7.5, 1.5 Hz, 1H), 7.45–7.39 (m, 2H), 7.37 (d, J = 8.1 Hz, 1H), 7.31–7.25 (m, 1H), 7.16 (d, J = 8.4 Hz, 1H), 6.95–6.89 (m, 2H), 6.58 (dd, J = 9.6, 1.8 Hz, 1H), 6.53 (dd, J = 3.6, 1.8 Hz, 1H), 5.90 (dd, J = 9.9, 3.6 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ (ppm) 155.82, 149.02, 134.71, 129.81, 129.14, 127.73, 127.52, 126.28, 125.50, 124.80, 124.62, 123.78, 123.73, 122.16, 120.94, 120.31, 115.66, 110.70, 72.54, 55.65. HRMS (ESI) calcd for [C20H16O2 + H]+: 289.1229, found: 289.1230.
4-(2H-Benzo[h]chromen-2-yl)-N,N-dimethylaniline (3j). The title compound was prepared according to the general procedure, except that the mixture was stirred at room temperature: 36 h; purple oil; 54% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 8.18–8.14 (m, 1H), 7.3–7.70 (m, 1H), 7.43–7.33 (m, 5H), 7.18 (d, J = 8.4 Hz, 1H), 6.73–6.66 (m, 3H), 6.06 (dd, J = 3.9, 1.8 Hz, 1H), 5.87 (dd, J = 9.6, 3.6 Hz, 1H), 2.94 (s, 6H). 13C NMR (75 MHz, CDCl3) δ (ppm) 150.80, 148.73, 134.65, 128.56, 128.48, 127.61, 126.22, 125.33, 124.84, 124.72, 124.43, 123.73, 122.27, 120.13, 115.92, 112.43, 77.41, 40.59. HRMS (ESI) calcd for [C21H19NO + H]+: 302.1545, found: 302.1544.
4-(2H-Benzo[h]chromen-2-yl)-2-methoxyphenyl acetate (3k). The title compound was prepared according to the general procedure: 46 h; red oil; 99% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 8.20–8.17 (m, 1H), 7.75–7.72 (m, 1H), 7.44–7.36 (m, 3H), 7.19–7.16 (m, 2H), 7.10–7.00 (m, 2H), 6.66 (dd, J = 9.9, 1.8 Hz, 1H), 6.10 (dd, J = 3.6, 1.8 Hz, 1H), 5.87 (dd, J = 9.6, 3.6 Hz, 1H), 3.79 (s, 3H), 2.31 (s, 3H). 13C NMR (75 MHz, CDCl3) δ (ppm) 169.15, 151.28, 148.54, 140.16, 139.70, 134.76, 127.78, 126.50, 125.67, 124.91, 124.68, 124.62, 123.25, 122.93, 122.01, 120.71, 119.16, 115.85, 111.09, 76.97, 55.99, 20.81. HRMS (ESI) calcd for [C22H18O4 + H]+: 347.1283, found: 347.1283.
4-(2H-benzo[h]chromen-2-yl)-2-methoxyphenol (3l). The title compound was prepared according to the general procedure: 68 h; red oil; 77% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 8.19–8.16 (m, 1H), 7.76–7.72 (m, 1H), 7.45–7.36 (m, 3H), 7.19 (d, J = 8.1 Hz, 1H), 7.09–7.01 (m, 2H), 6.91 (d, J = 8.1 Hz, 1H), 6.68 (dd, J = 9.9, 1.8 Hz, 1H), 6.06 (dd, J = 3.3, 1.5 Hz, 1H), 5.87 (dd, J = 9.9, 3.6 Hz, 1H), 5.68 (s, 1H), 3.84 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 148.56, 146.69, 145.90, 134.70, 133.05, 127.71, 126.38, 125.50, 124.79, 124.67, 123.53, 122.06, 120.46, 120.42, 115.88, 114.39, 109.82, 77.32, 55.99. HRMS (ESI) calcd for [C20H16O3 + H]+: 305.1178, found: 305.1177.
6-Chloro-2-phenyl-2H-benzo[h]chromene (3m). The title compound was prepared according to the general procedure: 46 h; red oil; 81% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 1H NMR (300 MHz, CDCl3) δ 8.22–8.13 (m, 2H), 7.57–7.45 (m, 5H), 7.41–7.33 (m, 3H), 7.29 (s, 1H), 6.60 (dd, J = 9.9, 1.8 Hz, 1H), 6.13 (dd, J = 3.6, 1.8 Hz, 1H), 5.91 (dd, J = 9.9, 3.6 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ (ppm) 147.64, 140.68, 131.40, 128.81, 128.58, 127.47, 126.93, 126.29, 125.74, 124.48, 124.17, 123.75, 123.43, 122.47, 116.09, 77.46. HRMS (ESI) calcd for [C19H13ClO + H]+: 293.0733, found: 293.0735.
6-Bromo-2-phenyl-2H-benzo[h]chromene (3n). The title compound was prepared according to the general procedure, except that 50-mg 4Å MS powder was added: 72 h; red oil; 60% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 8.21–8.09 (m, 2H), 7.57–7.43 (m, 5H), 7.41–7.30 (m, 3H), 6.60 (dd, J = 9.9, 1.5 Hz, 1H), 6.14 (dd, J = 3.3, 1.5 Hz, 1H), 5.90 (dd, J = 9.6, 3.6 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ (ppm) 148.34, 140.66, 132.57, 128.83, 128.60, 128.09, 127.76, 127.13, 126.94, 126.31, 125.93, 124.15, 123.62, 122.49, 116.73, 113.45, 77.50. HRMS (ESI) calcd for [C19H13BrO + H]+: 337.0228, found: 337.0232.
6-Nitro-2-phenyl-2H-benzo[h]chromene (3o). The title compound was prepared according to the general procedure, except that 50-mg 4Å MS powder was added: 72 h; red oil; 62% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 8.74 (d, J = 8.7 Hz, 1H), 8.24 (d, J = 8.7 Hz, 1H), 8.18 (s, 1H), 7.68–7.52(m, 1H), 7.52–7.47(m, 3H), 7.43–7.33(m, 3H), 6.65 (dd, J = 9.9, 1.5 Hz, 1H), 6.26 (dd, J = 3.3, 1.5 Hz, 1H), 5.96 (dd, J = 9.9, 3.6 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ (ppm) 154.19, 139.86, 138.92, 130.26, 129.02, 127.18, 127.10, 126.76, 125.01, 124.67, 124.56, 123.74, 122.78, 122.73, 113.63, 78.85. HRMS (ESI) calcd for [C19H13NO3 + H]+: 304.0974, found: 304.0975.
6-Methoxy-2-phenyl-2H-benzo[h]chromene (3p). The title compound was prepared according to the general procedure: 26 h; red oil; 60% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 8.19–8.16 (m, 2H), 7.51–7.53 (m, 2H), 7.50– 7.32 (m, 5H), 6.64 (dd, J = 9.9, 1.8 Hz, 1H), 6.54 (s, 1H), 6.07 (dd, J = 3.6, 1.5 Hz, 1H), 5.93 (dd, J = 9.9, 3.9 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ (ppm) 149.77, 142.45, 141.21, 128.68, 128.26, 126.84, 126.34, 126.27, 125.81, 125.48, 125.05, 123.92, 122.02, 121.84, 115.39, 102.64,76.86, 55.88. HRMS (ESI) calcd for [C20H16O2 + H]+: 289.1229, found: 289.1233.
tert-Butyl (2-phenyl-2H-benzo[h]chromen-6-yl)carbamate (3q). The title compound was prepared according to the general procedure: 41 h; red oil; 81% yield; 1H NMR (300 MHz, CDCl3) δ 7.99 (d, J = 8.4 Hz, 1H), 7.84 (d, J = 7.5 Hz, 1H), 7.52–7.50 (m, 2H), 7.41–7.32 (m, 5H), 7.18 (d, J = 8.4 Hz, 1H), 6.81 (s, 1H), 6.64 (dd, J = 9.6, 1.5 Hz, 1H), 6.13 (dd, J = 3.3, 1.2 Hz, 1H), 5.90 (dd, J = 9.9, 3.9 Hz, 1H), 1.57 (s, 11H). 13C NMR (75 MHz, CDCl3) δ (ppm) 153.60, 149.11, 141.01, 132.92, 128.75, 128.41, 127.69, 126.81, 125.51, 125.33, 124.72, 124.26, 123.78, 119.35, 118.52, 115.84, 113.01, 80.75, 77.39, 28.50. HRMS (ESI) calcd for [C24H23NO3 + H]+: 374.1756, found: 374.1753.
N-(2-Phenyl-2H-benzo[h]chromen-6-yl)acetamide (3r). The title compound was prepared according to the general procedure: 72 h; red oil; 95% yield; 1H NMR (300 MHz, DMSO) δ (ppm) 9.84 (s, 1H), 7.90 (d, J = 8.1 Hz, 1H), 7.66–7.59 (m, 2H), 7.50–7.31 (m, 7H), 6.79 (d, J = 9.6 Hz, 1H), 6.23 (d, J = 3.3 Hz, 1H), 6.11 (dd, J = 9.6, 3.9 Hz, 1H), 2.17 (s, 3H). 13C NMR (75 MHz, DMSO) δ (ppm) 168.84, 147.59, 140.68, 133.77, 128.18, 128.55, 126.37, 125.32, 124.53, 124.32, 124.23, 123.52, 121.99, 118.26, 115.75, 115.22, 75.97, 23.45. HRMS (ESI) calcd for [C21H17NO2 + H]+: 316.1338, found: 316.1340.
2-(Furan-2-yl)-2H-benzo[h]chromene (3s). The title compound was prepared according to the general procedure, except that the mixture was stirred at room temperature: 24 h; red oil; 51% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 8.21–8.18 (m, 1H), 7.74–7.70 (m, 1H), 7.45–7.36 (m, 4H), 7.19 (d, J = 8.4 Hz, 1H), 6.74 (dd, J = 9.6, 1.2 Hz, 1H), 6.41 (d, J = 3.0 Hz, 1H), 6.30 (dd, J = 2.7, 1.8 Hz, 1H), 6.14 (d, J = 3.3 Hz, 1H), 5.89 (dd, J = 9.6, 4.2 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ (ppm) 152.88, 148.20, 143.40, 134.67, 127.65, 126.45, 126.07, 125.57, 124.82, 124.69, 122.16, 120.72, 119.85, 115.88, 110.47, 109.55, 70.04. HRMS (ESI) calcd for [C17H12O2 + H]+: 249.0916, found: 249.0918.
3-(2H-Benzo[h]chromen-2-yl)-2-bromopyridine (3t). The title compound was prepared according to the general procedure: 24 h; red oil; 80% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 8.31 (dd, J = 4.8, 2.1 Hz, 1H), 8.17 (dd, J = 6.0, 3.6 Hz, 1H), 7.87 (dd, J = 7.8, 1.8 Hz, 1H), 7.76–7.33 (m, 1H), 7.46–7.39 (m, 3H), 7.23–7.16 (m, 2H), 6.70 (dd, J = 9.9, 1.8 Hz, 1H), 6.43 (dd, J = 3.6, 1.8 Hz, 1H), 5.90 (dd, J = 9.6, 3.6 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ (ppm) 149.66, 148.23, 141.44, 137.22, 137.13, 134.83, 127.78, 126.73, 125.92, 125.61, 124.60, 124.37, 123.34, 121.96, 121.10, 120.95, 115.37, 75.38. HRMS (ESI) calcd for [C18H12BrNO + H]+: 338.0181, found: 338.0180.
4-(2H-Benzo[h]chromen-2-yl)isoquinoline (3u). The title compound was prepared according to the general procedure: 24 h; red oil; 91% yield; 1H NMR (300 MHz, CDCl3) δ 8.90 (d, J = 4.5 Hz, 1H), 8.27 (d, J = 8.4 Hz, 1H), 8.19 (d, J = 8.4 Hz, 1H), 8.12–8.09 (m, 1H), 7.77–7.73 (m, 2H), 7.68–7.60 (m, 2H), 7.45–7.36 (m, 3H), 7.21 (d, J = 8.4 Hz, 1H), 6.80–6.74 (m, 2H), 5.91 (dd, J = 9.6, 3.6 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ (ppm) 150.55 (s), 148.76, 148.64, 145.01, 134.86, 130.57, 129.40, 127.80, 127.10, 126.70, 126.14, 125.88, 125.61, 124.70, 124.52, 123.62, 121.99, 121.51, 121.17, 119.23, 115.75, 73.67. HRMS (ESI) calcd for [C22H15NO + H]+: 310.1232, found: 310.1236.
2,2-Dimethyl-2H-benzo[h]chromene (3v). The title compound was prepared according to the general procedure, except that 50-mg 4 Å MS powder was added: 72 h; colorless oil; 61% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 8.24–8.21 (m, 1H), 7.76–7.73 (m, 1H), 7.48–7.43 (m, 2H), 7.35 (d, J = 8.4 Hz, 1H), 7.16 (d, J = 8.4 Hz, 1H), 6.46 (d, J = 9.6 Hz, 1H), 5.65 (d, J = 9.6 Hz, 1H), 1.54 (s, 6H). 13C NMR (75 MHz, CDCl3) δ (ppm) 148.20, 134.40, 129.26, 127.52, 126.04, 125.17, 125.03, 124.49, 122.77, 121.93, 119.78, 115.33, 76.75, 27.93. HRMS (ESI) calcd for [C15H14O + H]+: 211.1123, found: 211.1121.
2,2-Diphenyl-2H-benzo[h]chromene (3w). The title compound was prepared according to the general procedure, except that 50-mg 4 Å MS powder was added: 34 h; red oil; 78% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 8.44 (d, J = 7.2 Hz, 1H), 7.77 (d, J = 8.1 Hz, 1H), 7.61–7.46 (m, 6H), 7.40–7.26 (m, 7H), 7.21 (d, J = 8.1 Hz, 1H), 6.78 (d, J = 9.6 Hz, 1H), 6.25 (d, J = 9.6 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ (ppm) 147.70, 145.16, 134.63, 128.12, 127.63, 127.45, 127.25, 126.82, 126.27, 125.54, 124.67, 124.50, 123.83, 121.98, 120.46, 115.42, 83.16. HRMS (ESI) calcd for [C25H18O + H]+: 335.1436, found: 335.1436.
3-Phenyl-3H-benzo[f]chromene (3x). The title compound was prepared according to the general procedure, except that 50-mg 4 Å MS powder was added: 6 days; white solid; 68% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 8.00 (d, J = 9.0 Hz, 1H), 7.76 (d, J = 9.0 Hz, 1H), 7.67 (d, J = 9.0 Hz, 1H), 7.54–7.48 (m, 3H), 7.42–7.26 (m, 4H), 7.29–7.26 (m, 1H), 7.10 (d, J = 9.0 Hz, 1H), 6.01–5.94 (m, 2H). 13C NMR (75 MHz, CDCl3) δ (ppm) 151.47, 140.76, 130.03, 129.87, 129.53, 128.79, 128.72, 128.56, 127.25, 126.83, 123.75, 123.61, 121.41, 120.42, 118.15, 114.29, 77.07. HRMS (ESI) calcd for [C19H14O + H]+: 259.1123, found: 259.1127.
8-Bromo-3-phenyl-3H-benzo[f]chromene (3y). The title compound was prepared according to the general procedure, except that 50-mg 4 Å MS powder was added: 5 days; white solid; 60% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 7.89 (d, J = 1.5 Hz, 1H), 7.83 (d, J = 9.0 Hz, 1H), 7.56–7.50 (m, 4H), 7.42–7.34 (m, 3H), 7.18 (d, J = 9.6 Hz, 1H), 7.09 (d, J = 8.7 Hz, 1H), 6.01–5.94 (m, 2H). 13C NMR (75 MHz, CDCl3) δ (ppm) 151.65, 140.47, 130.63, 130.57, 129.97, 128.84, 128.68, 128.50, 127.23, 124.17, 123.25, 119.96, 119.25, 117.43, 114.43, 77.16. HRMS (ESI) calcd for [C19H13BrO + H]+: 337.0228, found: 337.0229.
3,9-Diphenyl-3,9-dihydrochromeno[8,7-h]chromene (3z). The title compound was prepared according to the general procedure, except that 50-mg 4 Å MS powder was added: 5 days; red oil; 51% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 7.73 (dd, J = 8.4, 1.2 Hz, 2H), 7.55–7.52 (m, 4H), 7.42–7.30 (m, 6H), 7.12 (d, J = 9.0 Hz, 2H), 6.64 (dd, J = 9.6, 1.5 Hz, 2H), 6.14–6.10 (m, 2H), 5.88 (ddd, J = 9.6, 3.6, 2.4 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ (ppm) 148.50, 141.19, 128.73, 128.34, 126.85, 125.60, 124.62, 124.30, 123.63, 123.55, 116.38, 116.30, 114.68, 77.29. HRMS (ESI) calcd for [C28H20O2 + H]+: 389.1542, found: 389.1545.
3-Phenyl-3H-benzo[f]chromen-9-ol (3aa). The title compound was prepared according to the general procedure, except that 50-mg 4 Å MS powder was added: 60 h; orange solid; 95% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 7.62 (d, J = 8.7 Hz, 1H), 7.55 (d, J = 8.7 Hz, 1H), 7.50 (d, J = 6.9 Hz, 2H), 7.39–7.35 (m, 3H), 7.32–7.28 (m, 2H), 7.05 (d, J = 9.9 Hz, 1H), 6.97–6.90 (m, 2H), 5.94 (s, 1H), 5.87 (dd, J = 9.9, 3.0 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ (ppm) 154.92, 152.28, 140.92, 130.79, 129.92, 128.92, 128.69, 127.39, 125.08, 123.17, 120.60, 115.82, 115.63, 113.33, 104.31, 77.16. HRMS (ESI) calcd for [C19H14O2 + H]+: 275.1072, found: 275.1074.
2,8-Diphenyl-2H,8H-pyrano[2,3-f]chromene (3ab). The title compound was prepared according to the general procedure, except that the mixture was heated at 60 °C in sealed tubed with 50-mg 4 Å MS powder: 5 days; orange solid; 60% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 7.53–7.35 (m, 10H), 6.92–6.88 (m, 1H), 6.82 (dd, J = 8.1, 2.1 Hz, 1H), 6.52–6.48 (m, 1H), 6.41 (ddd, J = 8.1, 2.4, 0.6 Hz, 1H), 5.97 (dd, J = 3.3, 1.8 Hz, 1H), 5.90–5.87 (m, 1H), 5.80–5.71 (m, 2H). 13C NMR (75 MHz, CDCl3) δ (ppm) 154.04, 148.86, 148.79, 140.94, 140.65, 140.58, 128.56, 128.29, 128.19, 127.05, 127.03, 126.79, 123.96, 123.87, 123.33, 123.20, 121.75, 121.60, 118.45, 118.29, 115.03, 114.92, 109.98, 109.88, 108.53, 77.07, 76.88. HRMS (ESI) calcd for [C24H18O2 + H]+: 339.1385, found: 339.1384.
6-Methoxy-2,2-dimethyl-2H-benzo[h]chromene (3ac). The title compound was prepared according to the general procedure, with 50-mg 4Å MS powder added: 60 h; colorless; 56% yield; 1H NMR (300 MHz, CDCl3) δ (ppm) 8.16 (d, J = 8.1 Hz, 2H), 7.51–7.41 (m, 2H), 6.52 (s, 1H), 6.41 (d, J = 9.6 Hz, 1H), 5.66 (d, J = 9.6 Hz, 1H), 3.96 (s, 3H), 1.51 (s, 6H). 13C NMR (75 MHz, CDCl3) δ (ppm) 149.42, 142.08, 130.04, 126.19, 126.04, 125.59, 123.19, 121.97, 121.83, 114.90, 102.69, 76.33, 55.92, 27.70. HRMS (ESI) calcd for [C16H16O2 + H]+: 241.1229, found: 241.1233.