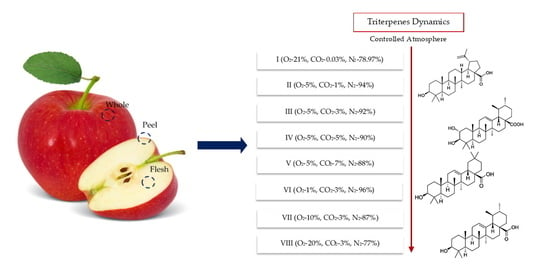
Variation of Triterpenes in Apples Stored in a Controlled Atmosphere
Abstract
:1. Introduction
2. Results
2.1. Triterpenes Variation in Whole Apple Samples before and after Storage CA
2.2. Triterpenes Variation in Apple Peel and Apple Flesh Samples before and after Storage CA
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Chemicals and Solvents
4.3. Controlled Atmosphere (CA) Conditions during Storage of Apple
4.4. Preparation of Samples
4.5. Preparation of Extracts
4.6. Qualitative and Quantitative Analysis by HPLC-PDA Method
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yanrong, L. Triterpenes and phenolic compounds in apple fruit (Malus domestica Borkh). Acta Uni. Agric. Suec. Agrar. 2016, 5, 13–24. [Google Scholar]
- Food and Agriculture Organization. FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QL (accessed on 23 January 2021).
- Thewes, F.R.; Brackmann, A.; Both, V.; Weber, A.; Anese, R.O.; Ferrão, T.S.; Wagner, R. The different impacts of dynamic controlled atmosphere and controlled atmosphere storage in the quality attributes of ‘Fuji Suprema’ apples. Postharvest Biol. Technol. 2017, 130, 7–20. [Google Scholar] [CrossRef]
- Klein, B.; Falk, R.B.; Thewes, F.R.; Anese, R.O.; Santos, I.D.; Ribeiro, S.R.; Donadel, J.Z.; Brackmann, A.; Barin, J.S.; Cichoski, A.J.; et al. Dynamic controlled atmosphere: Effects on the chemical composition of cuticular wax of ‘Cripps pink’ apples after long-term storage. Postharvest Biol. Technol. 2020, 164, 111170. [Google Scholar] [CrossRef]
- Mditshwa, A. Recent developments on dynamic controlled atmosphere storage of apples—A review. Food Packag. Shelf Life. 2018, 16, 59–68. [Google Scholar] [CrossRef]
- Wu, W.; Cronjé, P.; Nicolai, B.; Verboven, P.; Opara, U.L.; Defraeye, T. Virtual cold chain method to model the postharvest temperature history and quality evolution of fresh fruit—A case study for citrus fruit packed in a single carton. Comput. Electron. Agric. 2018, 144, 199–208. [Google Scholar] [CrossRef]
- Bessemans, N.; Verboven, P.; Verlinden, B.E.; Nicolaï, B.M. A novel type of dynamic controlled atmosphere storage based on the respiratory quotient (RQ-DCA). Postharvest Biol. Technol. 2016, 115, 91–102. [Google Scholar] [CrossRef]
- Konopacka, D.; Plocharski, W.J. Effect of storage conditions on the relationship between apple firmness and texture acceptability. Postharvest Biol. Technol. 2004, 32, 205–211. [Google Scholar] [CrossRef]
- Gwanpua, S.G.; Hertog, M.; Nicolai, B.; Verlinden, B.E.; Impe, J.F.M.; Geeraerd, A. Towards flexible management of postharvest variation in fruit firmness of three apple cultivars. Postharvest Biol. Technol. 2013, 85, 18–29. [Google Scholar] [CrossRef]
- Dobrzański, B.; Rabcewicz, J.; Rybczyński, R.; Bylica, T. Handling of Apple: Transport Techniques and Efficiency Vibration, Damage and Bruising Texture, Firmness and Quality; Institute of Agrophysics Polish Academy of Sciences: Lublin, Poland, 2006; pp. 33–177. [Google Scholar]
- Brizzolara, S.; Santucci, C.; Tenori, L.; Hertog, M.; Nicolai, B.; Stürz, S.; Zanella, A.; Tonutti, P. A metabolomics approach to elucidate apple fruit responses to static and dynamic controlled atmosphere storage. Postharvest Biol. Technol. 2017, 127, 76–87. [Google Scholar] [CrossRef]
- Costa, F.; Cappellina, L.; Fontanaria, M.; Longhia, S.; Guerrab, W.; Magnagoa, P.; Gasperia, F.; Biasioli, F. Texture dynamics during postharvest cold storage ripening in apple (Malus × domestica Borkh.). Postharvest Biol. Technol. 2012, 69, 54–63. [Google Scholar] [CrossRef]
- Drzyzga, O. Diphenylamine and derivatives in the environment: A review. Chemosphere 2003, 53, 809–818. [Google Scholar] [CrossRef]
- Saba, K.M.; Watkins, C.B. Flesh browning development of ‘Empire’ apple during a shelf-life period after 1-methylcyclopropene (1-MCP) treatment and controlled atmosphere storage. Sci. Hortic. 2020, 261, 108938. [Google Scholar] [CrossRef]
- Thewes, F.R.; Brackmann, A.; Neuwald, D.A. Dynamics of sugars, anaerobic metabolism enzymes and metabolites in apples stored under dynamic controlled atmosphere. Sci. Hortic. 2019, 255, 145–152. [Google Scholar] [CrossRef]
- Hoang, N.T.T.; Golding, J.B.; Wilkes, M.A. The effect of postharvest 1-MCP treatment and storage atmosphere on ‘Cripps Pink’ apple phenolics and antioxidant activity. Food Chem. 2011, 127, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Stanger, M.C.; Steffens, C.; Soethe, C.; Moreira, M.A.; Amarante, C.; Both, V.; Brackmann, A. Phenolic compounds content and antioxidant activity of ‘Galaxy’ apples stored in dynamic controlled atmosphere and ultralow oxygen conditions. Postharvest Biol. Technol. 2018, 144, 70–76. [Google Scholar] [CrossRef]
- Anese, R.O.; Brackmann, A.; Wendt, L.M.; Thewes, F.R.; Schultz, E.E.; Ludwig, V.; Pasquetti Berghetti, M.R. Interaction of 1-methylcyclopropene, temperature and dynamic controlled atmosphere by respiratory quotient on ‘Galaxy’ apples storage. Food Packag. Shelf Life. 2019, 20, 100246. [Google Scholar] [CrossRef]
- Vondráková, Z.; Trávníčková, A.; Malbeck, J.; Haisel, D.; Černý, R.; Cvikrová, M. The effect of storage conditions on the carotenoid and phenolic acid contents of selected apple cultivars. Eur. Food Res. Technol. 2020, 246, 1783–1794. [Google Scholar] [CrossRef]
- Anese, R.O.; Thewes, F.R.; Brackmann, A.; Eliseu Schultz, E.; Wagner, R.; Klein, B.; Roberto, M.; Berghetti, P.; Mallmann Wendt, L. Growth regulators on quality traits and volatile organic compounds profile of ‘Royal Gala’ apple at harvest and after dynamic controlled atmosphere storage. Postharvest Biol. Technol. 2020, 164, 111158. [Google Scholar] [CrossRef]
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical compositional characterization of some apple cultivars. Food Chem. 2007, 103, 88–93. [Google Scholar] [CrossRef]
- Lv, Y. Triterpenes and Phenolic Compounds in Apple Fruit (Malus domestica Borkh.). PhD Thesis, Swedish University of Agricultural Sciences, Alnarp, Sweden, 2016; pp. 23–24. [Google Scholar]
- Poirier, B.C.; Buchanan, D.A.; Rudell, D.R.; Mattheis, J.P. Differential partitioning of triterpenes and triterpene esters in apple peel. J. Agric. Food Chem. 2018, 66, 1800–1806. [Google Scholar] [CrossRef]
- Allouche, Y.; Beltrán, G.; Gaforio, J.J.; Uceda, M.; Mesa, M.D. Antioxidant and antiatherogenic activities of pentacyclic triterpenic diols and acids. Food Chem. Toxicol. 2010, 48, 2885–2890. [Google Scholar] [CrossRef] [PubMed]
- Camer, D.; Yu, Y.; Szabo, A.; Huang, X.F. The molecular mechanisms underpinning the therapeutic properties of oleanolic acid, its isomer and derivatives for type 2 diabetes an associated complication. Mol. Nutr. Food Res. 2014, 58, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Hamida, A.K.; Kama, A.; Wonga, K.H.; Abdelhaka, Z.; Naumovskia, V.R.; Chana, K.; Lic, K.M.; Groundwatera, P.W.; Li, G.Q. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr. Med. Chem. 2013, 20, 908–993. [Google Scholar]
- Abdullah, N.H.; Thomas, N.F.; Sivasothy, Y.; Lee, V.S.; Liew, S.Y.; Noorbatcha, I.A.; Awang, K. hyaluronidase inhibitory activity of pentacylic triterpenoids from Prismatomeris tetrandra (Roxb.) K. Schum: Isolation, synthesis and QSAR study. Int. J. Mol. Sci. 2016, 17, 143. [Google Scholar] [CrossRef]
- Han, N.; Bakovic, M. Biologically active triterpenoids and their cardioprotective and anti-inflammatory effects. J. Bioanal. Biomed. 2015, 12, 005. [Google Scholar]
- Andre, C.M.; Greenwood, J.M.; Walker, E.G.; Rassam, M.; Sullivan, M.; Evers, D.; Perry, N.B.; Laing, W.A. Anti–inflammatory procyanidins and triterpenes in 109 apple varieties. J. Agric. Food Chem. 2012, 60, 10546–10554. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Komohara, Y.; Ikeda, T.; Takeya, M. Corosolic acid inhibits glioblastoma cell proliferation by suppressing the activation of signal transducer and activator of transcription-3 and nuclear factor-kappa B in tumor cells and tumor-associated macrophages. Cancer Sci. 2011, 102, 206–211. [Google Scholar] [CrossRef]
- Yadav, V.R.; Prasad, S.; Sung, B.; Kannappan, R.; Aggarwal, B.B. Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins 2010, 2, 2428–2466. [Google Scholar] [CrossRef] [Green Version]
- Nile, S.H.; Nile, A.; Liu, J.; Kim, D.H.; Kai, G. Exploitation of apple pomace towards extraction of triterpenic acids, antioxidant potential, cytotoxic effects, and inhibition of clinically important enzymes. Food Chem. Toxicol. 2019, 131, 110563. [Google Scholar] [CrossRef] [PubMed]
- Oloyede, H.O.B.; Ajiboye, H.O.; Salawu, M.O.; Ajiboye, T.O. Influence of oxidative stress on the antibacterial activity of betulin, betulinic acid and ursolic acid. Microb. Pathog. 2017, 111, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, H.; Lee, S.; Yoon, Y.; Choi, K.H. Antimicrobial action of oleanolic acid on Listeria monocytogenes, Enterococcus faecium, and Enterococcus faecalis. PLoS ONE 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Wu, P.; Tu, B.; Liang, J.; Guo, S.; Cao, N.; Chen, S.; Luo, Z.; Li, J.; Zheng, W.; Tang, X.; et al. Synthesis and biological evaluation of pentacyclic triterpenoid derivatives as potential novel antibacterial agents. Bioorg. Chem. 2021, 109, 104692. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Aruna, A.; Lee, J.S.; Kim, M.; Shivakumar, M.S.; Natarajan, D. Antioxidant and antiproliferative potential of bioactive molecules ursolic acid and thujone isolated from Memecylon edule and Elaeagnus indica and their inhibitory effect on topoisomerase II by molecular docking approach. BioMed Res. Int. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habtemariam, S. Antioxidant and anti-inflammatory mechanisms of neuroprotection by ursolic acid: Addressing brain injury, cerebral ischemia, cognition deficit, anxiety, and depression. Oxid. Med. Cell Longev. 2019, 2019, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Watkins, C.B. Dynamic controlled atmosphere storage—A new technology for the New York storage industry. NY Fruit Quarterly 2008, 16, 23–27. [Google Scholar]
- Oszmiański, J.; Lachowicz, S.; Gławdel, E.; Cebulak, T.; Ochmian, I. Determination of phytochemical composition and antioxidant capacity of 22 old apple cultivars grown in Poland. Eur. Food Res. Technol. 2018, 244, 647–662. [Google Scholar] [CrossRef] [Green Version]
- Patocka, J.; Bhardwaj, K.; Klimova, B.; Nepovimova, E.; Wu, Q.; Landi, M.; Kuca, K.; Valis, M.; Wu, W. A review on nutritional features, chemical composition, traditional and medicinal value. Plants 2020, 9, 1408. [Google Scholar] [CrossRef]
- Sut, S.; Zengin, G.; Maggi, F.; Malagoli, M.; Dall’Acqua, S. Triterpenic acid and phenolics from ancient apples of Friuli Venezia Giulia as nutraceutical ingredients: LC-MS study and in vitro activities. Molecules 2019, 24, 1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jemmali, Z.; Chartiern, A.; Dufresne, C.; Elfakir, C. Optimization of the derivatization protocol of pentacyclic triterpenes prior to their gas chromatography—mass spectrometry analysis in plant extracts. Talanta 2016, 147, 35–43. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpenic distribution in various plants rich sources for a new group of multi potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [Green Version]
- Hyson, D.A. A comprehensive review of apples and apple components and their relationship to human health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef]
- Farneti, B.; Masuero, D.; Costa, F.; Magnago, P.; Malnoy, M.; Costa, G.; Vrhovsek, U.; Mattivi, F. Is there room for improving the nutraceutical composition of apple? J. Agric. Food Chem. 2015, 63, 2750–2759. [Google Scholar] [CrossRef]
- Siani, A.C.; Nakamura, M.J.; Santos, D.S.; Mazzei, J.L.; Nascimento, A.C.; Valente, L.M.M. Efficiency and selectivity of triterpenic acid extraction from decoctions and tinctures prepared from apple peels. Pharmacogn. Mag. 2014, 10, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Viškelis, J.; Uselis, N.; Liaudanskas, M.; Janulis, V.; Bielicki, P.; Univer, T.; Lepsis, J.; Kviklys, D. Triterpenic acid content in the fruit peel of Malus × domestica Borkh. depends on the growing technology. Zemdirbyste-Agriculture 2018, 105, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Chai, Y.; Li, A.; Wai, S.C.; Song, C.; Zhao, Y.; Duan, Y.; Zhang, B.; Lin, Q. Cuticular wax composition changes of 10 apple cultivars during postharvest storage. Food Chem. 2020, 324, 126903. [Google Scholar] [CrossRef] [PubMed]
- Frighetto, R.T.S.; Welendorf, R.M.; Nigro, E.N.; Frighetto, N.; Siani, A.C. Isolation of ursolic acid from apple peels by high speed counter-current chromatography. Food Chem. 2008, 106, 767–771. [Google Scholar] [CrossRef]
- Klein, B.; Ribeiro, Q.M.; Thewes, F.R.; Anese, R.O.; Oliveira, F.C.O.; Santos, I.D.; Ribeiro, S.R.; Donadel, J.Z.D.; Brackmann, A.; Barin, J.S.; et al. The isolated or combined effects of dynamic controlled atmosphere (DCA) and 1-MCP on the chemical composition of cuticular wax and metabolism of ‘Maxi Gala’ apples after long-term storage. Int. Food Res. J. 2021, 140, 109900. [Google Scholar] [CrossRef] [PubMed]
- Dashbaldan, S.; Paczkowski, C.; Szakiel, A. Variations in triterpenoid deposition in cuticular waxes during development and maturation of selected fruits of Rosaceae family. Int. J. Mol. Sci. 2020, 21, 9762. [Google Scholar] [CrossRef] [PubMed]
- Butkevičiūtė, A.; Liaudanskas, M.; Kviklys, D.; Zymonė, Z.; Raudonis, R.; Viškelis, J.; Uselis, N.; Janulis, V. Detection and analysis of triterpenic compounds in apple extracts. Int. J. Food Prop. 2018, 21, 1716–1727. [Google Scholar] [CrossRef] [Green Version]
- Sabban-Amin, R.; Feygenberg, O.; Belausov, E.; Pesis, E. Low oxygen and 1-MCP pretreatments delay superficial scald development by reducing reactive oxygen species (ROS) accumulation in stored ‘Granny Smith’ apples. Postharvest Biol. Technol. 2011, 62, 295–304. [Google Scholar] [CrossRef]
- Wright, A.; Delong, J.; Arul, J.; Prange, R. The trend toward lower oxygen levels during apple (Malus × domestica Borkh) storage. J. Hortic. Sci. Biotech. 2015, 90, 1–13. [Google Scholar] [CrossRef]
- Falagán, N.; Terry, L.A. Recent advances in controlled and modified atmosphere of fresh produce. Technol. Rev. 2018, 62, 107–117. [Google Scholar] [CrossRef]
- Zevallos, L.C. The use of controlled postharvest abiotic stresses as a tool for enhancing the nutraceutical content and adding-value of fresh fruits and vegetables. J. Food Sci. 2003, 68, 1560–1565. [Google Scholar] [CrossRef]
- Senica, M.; Bavec, M.; Stampar, F.; Mikulic-Petkovsek, M. Blue honeysuckle (Lonicera caerulea subsp. edulis (Turcz. Ex Herder) Hultén.) berries and changes in their ingredients across different locations. J. Sci. Food Agric. 2018, 98, 3333–3342. [Google Scholar] [CrossRef]
- Harb, J.; Saleh, O.; Kittemann, D.; Neuwald, D.; Hoffmann, T.; Reski, R.; Schwab, W. Changes in polyphenols and expression levels of related genes in “Duke” blueberries stored under high CO2 levels. J. Agric. Food Chem. 2014, 62, 7460–7467. [Google Scholar] [CrossRef]
- Khorshidi, S.; Davarynejad, G.; Tehranifar, A.; Fallahi, E. Effect of modified atmosphere packaging on chemical composition, antioxidant activity, anthocyanin, and total phenolic content of cherry fruits. Hortic. Environ. Biotechnol. 2011, 52, 471–481. [Google Scholar] [CrossRef]
- Veraverbeke, E.A.; Lammertyn, J.; Saevels, S.; Nicolaï, B.M. Changes in chemical wax composition of three different apple (Malus domestica Borkh.) cultivars during storage. Postharvest Biol. Technol. 2001, 23, 197–208. [Google Scholar] [CrossRef]
- Kevers, C.; Pincemail, J.; Tabart, J.; Defraigne, J.O.; Dommes, J. Influence of cultivar, harvest time, storage conditions, and peeling on the antioxidant capacity and phenolic and ascorbic acid contents of apples and pears. J. Agric. Food Chem. 2011, 59, 6165–6171. [Google Scholar] [CrossRef]
- Sluis, A.A.; Dekker, M.; Jager, A.; Jongen, W.M. Activity and concentration of polyphenolic antioxidants in apple: Effect of cultivar, harvest year, and storage conditions. J. Agric. Food Chem. 2001, 49, 3606–3613. [Google Scholar] [CrossRef] [PubMed]












| Variant | Amount of Oxygen (O2), % | Amount of Carbon Dioxide (CO2), % | Amount of Nitrogen (N2), % | Relative Humidity, % | Temperature, oC |
|---|---|---|---|---|---|
| I | 21 | 0.03 | 78.97 | 95 ± 3 | +1.5 ± 0.5 |
| II | 5 | 1 | 94 | ||
| III | 5 | 3 | 92 | ||
| IV | 5 | 5 | 90 | ||
| V | 5 | 7 | 88 | ||
| VI | 1 | 3 | 96 | ||
| VII | 10 | 3 | 87 | ||
| VIII | 20 | 3 | 77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butkeviciute, A.; Viskelis, J.; Liaudanskas, M.; Viskelis, P.; Bobinas, C.; Janulis, V. Variation of Triterpenes in Apples Stored in a Controlled Atmosphere. Molecules 2021, 26, 3639. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26123639
Butkeviciute A, Viskelis J, Liaudanskas M, Viskelis P, Bobinas C, Janulis V. Variation of Triterpenes in Apples Stored in a Controlled Atmosphere. Molecules. 2021; 26(12):3639. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26123639
Chicago/Turabian StyleButkeviciute, Aurita, Jonas Viskelis, Mindaugas Liaudanskas, Pranas Viskelis, Ceslovas Bobinas, and Valdimaras Janulis. 2021. "Variation of Triterpenes in Apples Stored in a Controlled Atmosphere" Molecules 26, no. 12: 3639. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26123639