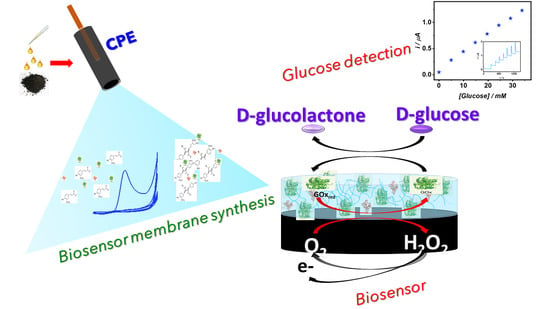
Biosensing Membrane Base on Ferulic Acid and Glucose Oxidase for an Amperometric Glucose Biosensor
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ferulic Acid Electropolymerization
2.2. Ferulic Acid Electropolymerization
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karyakin, A.A. Glucose biosensors for clinical and personal use. Electrochem. Commun. 2021, 125, 106973. [Google Scholar] [CrossRef]
- Yoo, E.-H.; Lee, S.-Y. Glucose Biosensors: An Overview of Use in Clinical Practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef] [Green Version]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, F.; de Falcão, I.A.; Souza, J.D.S.; Rocha, T.; de Sousa, I.; Cavalcante, A.; de Oliveira, A.; de Sousa, M.; dos Santos, J. Designing of nanomaterials-based enzymatic biosensors: Synthesis, properties, and applications. Electrochem 2021, 2, 12. [Google Scholar] [CrossRef]
- Curulli, A. Electrochemical Biosensors in Food Safety: Challenges and Perspectives. Molecules 2021, 26, 2940. [Google Scholar] [CrossRef] [PubMed]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B. Immobilization strategies to develop enzymatic biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J. Screen-Printed Electrodes: Promising Paper and Wearable Transducers for (Bio)Sensing. Biosensors 2020, 10, 76. [Google Scholar] [CrossRef]
- Komathi, S.; Gopalan, A.I.; Muthuchamy, N.; Lee, K.P. Polyaniline nanoflowers grafted onto nanodiamonds via a soft template-guided secondary nucleation process for high-performance glucose sensing. RSC Adv. 2017, 7, 15342–15351. [Google Scholar] [CrossRef] [Green Version]
- Shen, Q.; Zheng, J. Bienzyme system for the biocatalyzed deposition of polyaniline templated by multiwalled carbon nano-tubes: A biosensor design. Biosens. Bioelectron. 2019, 15, 1621–2629. [Google Scholar] [CrossRef]
- Madden, J.; Barret, C.; Laffir, F.; Thompson, M.; Galvin, P.; O’Riordan, A. On-chip glucose detection based on glucose oxidase encapsulated on a platinum modified gold microband electrode. Chemrxiv 2021. [Google Scholar] [CrossRef]
- Ahmad, W.; Verma, S.; Yang, D.-J.; Islam, M.U.; Choudhury, A. Synthesis of silver nanoparticles-decorated poly(m-aminophenol) nanofibers and their application in a non-enzymatic glucose biosensor. J. Macromol. Sci. Part A 2021, 1–17. [Google Scholar] [CrossRef]
- Monti, P.; Bacciu, A.; Arrigo, P.; Marceddu, S.; Migheli, Q.; Serra, P.A.; Rocchitta, G. Chronoamperometry as effective alternative technique for electro-synthesis of ortho - phenylendiamine permselective films for biosensor applications. J. Appl. Polym. Sci. 2020, 137, 49172. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Ahmad, R.; Shrestha, S.; Park, C.H.; Kim, C.S. Globular shaped polypyrrole doped well-dispersed functionalized multiwall carbon nanotubes/nafion composite for enzymatic glucose biosensor application. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Yi, Y.; Zhu, P.; Shen, J.; Wu, K.; Zhang, L.; Liu, J. Polyaniline-based glucose biosensor: A review. J. Electroanal. Chem. 2016, 782, 138–153. [Google Scholar] [CrossRef]
- Windmiller, J.R.; Valdés-Ramírez, G.; Zhou, N.; Zhou, M.; Miller, P.R.; Jin, C.; Brozik, S.M.; Polsky, R.; Katz, E.; Narayan, R.; et al. Bicomponent microneedle array biosensor for minimally-invasive glutamate monitoring. Electroanalysis 2011, 23, 2302–2309. [Google Scholar] [CrossRef]
- Buanafina, M.M.D.O. Feruloylation in grasses: Current and future perspectives. Mol. Plant 2009, 2, 861–872. [Google Scholar] [CrossRef]
- Sohn, Y.T.; Oh, J.H. Characterization of physicochemical properties of ferulic acid. Arch. Pharmacal Res. 2003, 26, 1002–1008. [Google Scholar] [CrossRef]
- Mattila, P.; Kumpulainen, J. Determination of free and total phenolic acids in plant-derived foods by HPLC with diode-array detection. J. Agric. Food Chem. 2002, 50, 3660–3667. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Cappelli, A.; Paolino, M.; Reale, A.; Razzano, V.; Grisci, G.; Giuliani, G.; Donati, A.; Bonechi, C.; Lamponi, S.; Mendichi, R.; et al. Hyaluronan-based graft copolymers bearing aggregation-induced emission fluorogens. RSC Adv. 2018, 8, 5864–5881. [Google Scholar] [CrossRef] [Green Version]
- da Silva, L.V.; Lopes, C.B.; da Silva, W.C.; de Paiva, Y.G.; Silva, F.D.A.D.S.; Lima, P.R.; Kubota, L.T.; Goulart, M.O.F. Electropolymerization of ferulic acid on multi-walled carbon nanotubes modified glassy carbon electrode as a versatile platform for NADH, dopamine and epinephrine separate detection. Microchem. J. 2017, 133, 460–467. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Development of a novel electrochemical biosensor based on carbon nanofibers–gold nanoparticles–tyrosinase for the detection of ferulic acid in cosmetics. Sensors 2020, 20, 6724. [Google Scholar] [CrossRef]
- Matsushita, Y.; Nakamura, A.; Aoki, D.; Yagami, S.; Fukushima, K. Bio-based polymer from ferulic acid by electropolymerization. Bioresources 2016, 11, 9789–9802. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z. Electrochemical determination of ferulic acid in pinellia ternata based on GOs/MWCNTs nanocomposite modified electrode. Int. J. Electrochem. Sci. 2020, 15, 559–566. [Google Scholar] [CrossRef]
- Trabelsi, S.K.; Tahar, N.B.; Trabelsi, B.; Abdelhedi, R. Electrochemical oxidation of ferulic acid in aqueous solutions at gold oxide and lead dioxide electrodes. J. Appl. Electrochem. 2005, 35, 967–973. [Google Scholar] [CrossRef]
- Graf, E. Antioxidant potential of ferulic acid. Free Radic. Biol. Med. 1992, 13, 435–448. [Google Scholar] [CrossRef]
- Barsan, M.M.; Ghica, M.E.; Brett, C.M. Electrochemical sensors and biosensors based on redox polymer/carbon nanotube modified electrodes: A review. Anal. Chim. Acta 2015, 881, 1–23. [Google Scholar] [CrossRef]
- Samet, Y.; Kraiem, D.; Abdelhédi, R. Electropolymerization of phenol, o-nitrophenol and o-methoxyphenol on gold and carbon steel materials and their corrosion protection effects. Prog. Org. Coat. 2010, 69, 335–343. [Google Scholar] [CrossRef]
- Ayankojo, A.G.; Reut, J.; Ciocan, V.; Öpik, A.; Syritski, V. Molecularly imprinted polymer-based sensor for electrochemical detection of erythromycin. Talanta 2020, 209, 120502. [Google Scholar] [CrossRef] [PubMed]
- Fogh-Andersen, N.; Altura, B.M.; Siggaard-Andersen, O. Composition of interstitial fluid. Clin. Chem. 1995, 41, 1522–1525. [Google Scholar] [CrossRef]
- Mader, J.K.; Neubauer, K.M.; Schaupp, L.; Augustin, T.; Beck, P.; Spat, S.; Höll, B.; Treiber, G.M.; Fruhwald, F.M.; Pieber, T.R.; et al. Efficacy, usability and sequence of operations of a workflow-integrated algorithm for basal-bolus insulin therapy in hospitalized type 2 diabetes patients. Diabetes Obes. Metab. 2013, 16, 137–146. [Google Scholar] [CrossRef] [PubMed]


| Material | Detection Limit | Linear Range | Sensitivity | Ref. |
|---|---|---|---|---|
| PANI/GOx | 0.062 mM | 1–10 mM | 0.54 μA mM−1 | [8] |
| Nanodiamond-g-PANI/GOx | 0.018 mM | 1–30 mM | 2.03 μA mM−1 | [8] |
| PANI/MWCNTs/GOx/HRP | 0.02 mM | 0.5–12 mM | 0.94 μA mM−1 | [9] |
| Pt/o-PD/βcyclodextrin/GOx | 0.79 mM | 2.5–15.5 mM | 0.150 nA mM−1 | [10] |
| Ag/p(m-aminophenol) nanofibers | 0.062 μM | 0.1–8.0 mM | 17.45μA mM−1 cm−2 | [11] |
| Pt/poly(o-PD)/GOx | 0.020 mM | 0–2 mM | 0.140 nA mM−1 | [12] |
| Polypyrrole/CNT/GOx | 0.005 mM | 1–4.1 mM | 54.2 μA mM−1 cm−2 | [13] |
| Pt/poly(o-PD)/GOx | 0.100 mM | 0–14 mM | 353 μA mM−1 | [15] |
| CPE/poly-FA–GOx–BSA | 0.025 mM | 0.082–34 mM | 1.1 μAmM−1 cm−2 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdés-Ramírez, G.; Galicia, L. Biosensing Membrane Base on Ferulic Acid and Glucose Oxidase for an Amperometric Glucose Biosensor. Molecules 2021, 26, 3757. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26123757
Valdés-Ramírez G, Galicia L. Biosensing Membrane Base on Ferulic Acid and Glucose Oxidase for an Amperometric Glucose Biosensor. Molecules. 2021; 26(12):3757. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26123757
Chicago/Turabian StyleValdés-Ramírez, Gabriela, and Laura Galicia. 2021. "Biosensing Membrane Base on Ferulic Acid and Glucose Oxidase for an Amperometric Glucose Biosensor" Molecules 26, no. 12: 3757. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26123757