A Formal Rearrangement of Allylic Silanols
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthetic Investigations
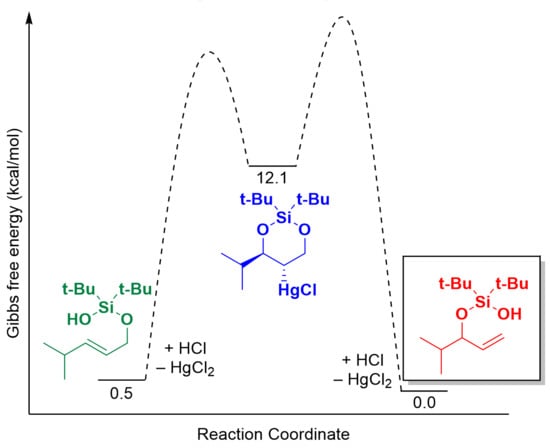
2.2. Mechanistic Studies
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Greer, E.M.; Cosgriff, C.V. Reaction mechanisms: Pericyclic reactions. Annu. Rep. Sect. B (Org. Chem.) 2013, 109, 328–350. [Google Scholar] [CrossRef]
- Graulich, N. The Cope rearrangement—the first born of a great family. WIREs Comput. Mol. Sci. 2011, 1, 172–190. [Google Scholar] [CrossRef]
- Martín Castro, A.M. Claisen Rearrangement over the Past Nine Decades. Chem. Rev. 2004, 104, 2939–3002. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Taguchi, T. Asymmetric Claisen rearrangement. Chem. Soc. Rev. 1999, 28, 43–50. [Google Scholar] [CrossRef]
- Kesava Reddy, N.; Chandrasekhar, S. Total Synthesis of (−)-α-Kainic acid via Chirality Transfer through Ireland–Claisen Rearrangement. J. Org. Chem. 2013, 78, 3355–3360. [Google Scholar] [CrossRef]
- Qin, Y.-c.; Stivala, C.E.; Zakarian, A. Acyclic Stereocontrol in the Ireland–Claisen Rearrangement of α-Branched Esters. Angew. Chem. Int. Ed. 2007, 46, 7466–7469. [Google Scholar] [CrossRef]
- Fulton, T.J.; Cusumano, A.Q.; Alexy, E.J.; Du, Y.E.; Zhang, H.; Houk, K.N.; Stoltz, B.M. Global Diastereoconvergence in the Ireland–Claisen Rearrangement of Isomeric Enolates: Synthesis of Tetrasubstituted α-Amino Acids. J. Am. Chem. Soc. 2020, 142, 21938–21947. [Google Scholar] [CrossRef]
- Li, J.J. Mislow–Evans rearrangement. In Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications; Li, J.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 363–364. [Google Scholar]
- Overman, L.E. Molecular rearrangements in the construction of complex molecules. Tetrahedron 2009, 65, 6432–6446. [Google Scholar] [CrossRef] [Green Version]
- Chabardes, P.; Kuntz, E.; Varagnat, J. Use of oxo-metallic derivatives in isomerisation: Reactions of unsaturated alcohols. Tetrahedron 1977, 33, 1775–1783. [Google Scholar] [CrossRef]
- Volchkov, I.; Lee, D. Recent developments of direct rhenium-catalyzed [1,3]-transpositions of allylic alcohols and their silyl ethers. Chem. Soc. Rev. 2014, 43, 4381–4394. [Google Scholar] [CrossRef]
- Matsubara, S.; Takai, K.; Nozaki, H. Isomerization of primary allylic alcohols to tertiary ones by means of Me3SiOOSiMe3-VO(acac)2 catalyst. Tetrahedron Lett. 1983, 24, 3741–3744. [Google Scholar] [CrossRef]
- Hosogai, T.; Fujita, Y.; Ninagawa, Y.; Nishida, T. Selective Allylic Rearrangement with Tungsten Catalyst. Chem. Lett. 1982, 11, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Belgacem, J.; Kress, J.; Osborn, J.A. On the allylic rearrangements in metal oxo complexes: Mechanistic and catalytic studies on MoO2(allyloxo)2(CH3CN)2 and analogous complexes. J. Am. Chem. Soc. 1992, 114, 1501–1502. [Google Scholar] [CrossRef]
- Narasaka, K.; Kusama, H.; Hayashi, Y. Rearrangement of Allylic and Propargylic Alcohols Catalyzed by the Combined Use of tetrabutylammonium perrhenate (VII) and p-toluenesulfonic acid. Tetrahedron 1992, 48, 2059–2068. [Google Scholar] [CrossRef]
- Tsuji, J. Dawn of organopalladium chemistry in the early 1960s and a retrospective overview of the research on palladium-catalyzed reactions. Tetrahedron 2015, 71, 6330–6348. [Google Scholar] [CrossRef]
- Trost, B.M.; Van Vranken, D.L. Asymmetric Transition Metal-Catalyzed Allylic Alkylations. Chem. Rev. 1996, 96, 395–422. [Google Scholar] [CrossRef] [PubMed]
- Overman, L.E. Mercury(II)- and Palladium(II)-Catalyzed [3,3]-Sigmatropic Rearrangements [New Synthetic Methods (46)]. Angew. Chem. Int. Ed. Engl. 1984, 23, 579–586. [Google Scholar] [CrossRef]
- Overman, L.E.; Knoll, F.M. Palladium (II)—Catalyzed rearrangement of allylic acetates. Tetrahedron Lett. 1979, 20, 321–324. [Google Scholar] [CrossRef]
- Henry, P.M. Palladium(II)-catalyzed exchange and isomerization reactions. III. Allylic esters isomerization in acetic acid catalyzed by palladium(II) chloride. J. Am. Chem. Soc. 1972, 94, 5200–5206. [Google Scholar] [CrossRef]
- Belgacem, J.; Kress, J.; Osborn, J.A. Catalytic oxidation and ammoxidation of propylene. Modelling studies on well-defined molybdenum complexes. J. Mol. Catal. 1994, 86, 267–285. [Google Scholar] [CrossRef]
- Bellemin-Laponnaz, S.; Gisie, H.; Le Ny, J.P.; Osborn, J.A. Mechanistic Insights into the Very Efficient [ReO3OSiR3]-Catalyzed Isomerization of Allyl Alcohols. Angew. Chem. Int. Ed. Engl. 1997, 36, 976–978. [Google Scholar] [CrossRef]
- Morrill, C.; Beutner, G.L.; Grubbs, R.H. Rhenium-Catalyzed 1,3-Isomerization of Allylic Alcohols: Scope and Chirality Transfer. J. Org. Chem. 2006, 71, 7813–7825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrill, C.; Grubbs, R.H. Highly Selective 1,3-Isomerization of Allylic Alcohols via Rhenium Oxo Catalysis. J. Am. Chem. Soc. 2005, 127, 2842–2843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Floreancig, P.E. Stereoselective heterocycle synthesis through a reversible allylic alcohol transposition and nucleophilic addition sequence. Chem. Sci. 2011, 2, 2423–2427. [Google Scholar] [CrossRef]
- Jung, H.H.; Seiders Ii, J.R.; Floreancig, P.E. Oxidative Cleavage in the Construction of Complex Molecules: Synthesis of the Leucascandrolide A Macrolactone. Angew. Chem. Int. Ed. 2007, 46, 8464–8467. [Google Scholar] [CrossRef]
- Xie, Y.; Floreancig, P.E. Cascade Approach to Stereoselective Polycyclic Ether Formation: Epoxides as Trapping Agents in the Transposition of Allylic Alcohols. Angew. Chem. Int. Ed. 2013, 52, 625–628. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, A.T.; Saito, T.; Stivala, C.E.; Tom, J.; Zakarian, A. Regio- and Stereocontrol in Rhenium-Catalyzed Transposition of Allylic Alcohols. J. Am. Chem. Soc. 2010, 132, 5962–5963. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Volchkov, I. Chapter 7—Regio- and Stereoselective Metal-Catalyzed Reactions and Their Application to a Total Synthesis of (−)-Dactylolide. In Strategies and Tactics in Organic Synthesis; Harmata, M., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 8, pp. 171–197. [Google Scholar]
- Volchkov, I.; Park, S.; Lee, D. Ring Strain-Promoted Allylic Transposition of Cyclic Silyl Ethers. Org. Lett. 2011, 13, 3530–3533. [Google Scholar] [CrossRef]
- Volchkov, I.; Lee, D. Asymmetric Total Synthesis of (−)-Amphidinolide V through Effective Combinations of Catalytic Transformations. J. Am. Chem. Soc. 2013, 135, 5324–5327. [Google Scholar] [CrossRef]
- Zheng, H.; Lejkowski, M.; Hall, D.G. Mild and selective boronic acid catalyzed 1,3-transposition of allylic alcohols and Meyer–Schuster rearrangement of propargylic alcohols. Chem. Sci. 2011, 2, 1305–1310. [Google Scholar] [CrossRef]
- Mandal, A.K.; Schneekloth, J.S.; Kuramochi, K.; Crews, C.M. Synthetic Studies on Amphidinolide B1. Org. Lett. 2006, 8, 427–430. [Google Scholar] [CrossRef] [Green Version]
- Trost, B.M.; Toste, F.D. Enantioselective Total Synthesis of (−)-Galanthamine. J. Am. Chem. Soc. 2000, 122, 11262–11263. [Google Scholar] [CrossRef]
- Shinde, A.H.; Sathyamoorthi, S. Tethered Silanoxymercuration of Allylic Alcohols. Org. Lett. 2020, 22, 8665–8669. [Google Scholar] [CrossRef] [PubMed]
- Bonini, C.; Campaniello, M.; Chiummiento, L.; Videtta, V. Stereoselective synthesis of versatile 2-chloromercurium-3,5-syn-dihydroxy esters via intramolecular oxymercuration. Tetrahedron 2008, 64, 8766–8772. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-adjustedab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordwell, F.G.; Douglass, M.L. Reduction of Alkylmercuric Hydroxides by Sodium Borohydride. J. Am. Chem. Soc. 1966, 88, 993–999. [Google Scholar] [CrossRef]











| Entry | Reagent | Solvent | Temp.,Time | P1/P2 a |
|---|---|---|---|---|
| 1 | 1M HCl (aqueous) | THF | RT, 30 min | 60/20 |
| 2 | 1M HCl (aqueous) | THF | 35 °C, 30 min | 60/20 |
| 3 | 1M HCl (aqueous) | THF | 0 °C, 30 min | 50/22 |
| 4 | NaBH4 (2 equiv) | DMF | RT, 30 min | 64/20 |
| 5 | NaBH4 (2 equiv) | DMSO | RT, 30 min | 62/20 |
| 6 | NaBH4 (2 equiv) | MeOH | RT, 30 min | 56/11 |
| 7 | NaBH4 (2 equiv) | DMA | RT, 30 min | 6/4 |
| 8 | NaBH4 (2 equiv) | THF | RT, 30 min | 43/7 |
| 9 | LiBH4 (2 equiv) | THF | RT, 30 min | 10/0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhokale, R.A.; Seidl, F.J.; Sathyamoorthi, S. A Formal Rearrangement of Allylic Silanols. Molecules 2021, 26, 3829. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26133829
Dhokale RA, Seidl FJ, Sathyamoorthi S. A Formal Rearrangement of Allylic Silanols. Molecules. 2021; 26(13):3829. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26133829
Chicago/Turabian StyleDhokale, Ranjeet A., Frederick J. Seidl, and Shyam Sathyamoorthi. 2021. "A Formal Rearrangement of Allylic Silanols" Molecules 26, no. 13: 3829. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26133829