Aromatic Amines in Organic Synthesis. Part II. p-Aminocinnamaldehydes
Abstract
:1. Introduction
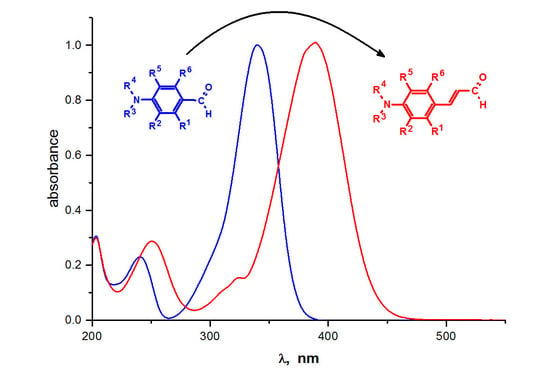
2. Results
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis
3.2.1. Method A
3.2.2. Method B
- 3-[4-(Dimethylamino)phenyl]prop-2-enal (1a)
- 3-[4-(Diethylamino)phenyl]prop-2-enal (1b)
- 3-[4-(Dimethylamino)-2-methylphenyl]prop-2-enal (1c)
- 3-[4-(Dimethylamino)-2,6-dimethylphenyl]prop-2-enal (1d)
- 3-[4-(Pyrrolidin-1-yl)phenyl]prop-2-enal (1e)
- 3-[4-(Piperidin-1-yl)phenyl]prop-2-enal (1f)
- 3-[4-(Morpholin-4-yl)phenyl]prop-2-enal (1g)
- 3-(1-Methyl-2,3-dihydro-1H-indol-5-yl)prop-2-enal (1h)
- 3-(1-Methyl-1,2,3,4-tetrahydroquinolin-6-yl)prop-2-enal (1i)
- 3-(9-Julolidyl)prop-2-enal (1j)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, X.; Gao, X.; Shi, W.; Ma, H. Design strategies for water-soluble small molecular chromogenic and fluorogenic probes. Chem. Rev. 2014, 114, 590–659. [Google Scholar] [CrossRef]
- Jiang, X.; Marinado, T.; Gabrielsson, E.; Hagberg, D.P.; Sun, L.; Hagfeldt, A. Structural modification of organic dyes for efficient coadsorbent-free dye-sensitized solar cells. J. Phys. Chem. C. 2010, 114, 2799–2805. [Google Scholar] [CrossRef]
- Chen, S.-L.; Yang, L.-N.; Li, Z.-S. How to design more efficient organic dyes for dye-sensitized solar cells? Adding more sp2-hybridized nitrogen in the triphenylamine donor. J. Power Sources. 2013, 223, 86–93. [Google Scholar] [CrossRef]
- Lavis, D.L.; Raines, R.T. Bright Ideas for Chemical Biology. ACS Chem. Biol. 2008, 3, 142–155. [Google Scholar] [CrossRef] [Green Version]
- Costero, A.M.; Banuls, M.J.; Aurell, M.J.; Ochando, L.E.; Domenech, A. Cation and anion fluorescent and electrochemical sensors derived from 4,4′-substituted biphenyl. Tetrahedron 2005, 61, 10309–10320. [Google Scholar] [CrossRef]
- Krawczyk, P.; Pietrzak, M.; Janek, T.; Jędrzejewska, B.; Cysewski, P. Spectroscopic and nonlinear optical properties of new chalcone fluorescent probes for bioimaging applications: A theoretical and experimental study. J. Mol. Model. 2016, 22, 125–135. [Google Scholar] [CrossRef]
- Cook, W.D.; Chen, F. Enhanced photopolymerization of dimethacrylates with ketones, amines, and iodonium salts: The CQ system. J. Poym. Sci. Pol. Chem. 2011, 49, 5030–5041. [Google Scholar] [CrossRef]
- Jędrzejewska, B.; Pietrzak, M.; Rafiński, Z. Phenyltrialkylborates as co-initiators with cyanine dyes in visible light polymerization of acrylates. Polymer 2011, 52, 2110–2119. [Google Scholar] [CrossRef]
- Hrobarik, P.; Hrobarikova, V.; Sigmundova, I.; Zahradnik, P.; Fakis, M.; Polyzos, I.; Persephonis, P. Benzothiazoles with tunable electron-withdrawing strength and reverse polarity: A route to triphenylamine-based chromophores with enhanced two-photon absorption. J. Org. Chem. 2011, 76, 8726–8736. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.; Faeh, C.; Diederich, F. Fluorine in pharmaceuticals: Looking beyond intuition. Science. 2007, 317, 1881–1886. [Google Scholar] [CrossRef] [Green Version]
- Grabowski, Z.R.; Rotkiewicz, K.; Rettig, W. Structural changes accompanying intramolecular electron transfer: Focus on twisted intramolecular charge-transfer states and structures. Chem. Rev. 2003, 103, 3899–4031. [Google Scholar] [CrossRef]
- Zakrzewska, A.; Gawinecki, R.; Kolehmainen, E.; Ośmiałowski, B. 13C-NMR based evaluation of the electronic and steric interactions in aromatic amines. Int. J. Mol. Sci. 2005, 6, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Pietrzak, M.; Bajorek, A. 5-Phenyl-1,2,3,4-tetrahydronaphthalene derivatives: Synthesis, spectroscopic and electrochemical investigation. Dyes Pigments 2013, 96, 63–70. [Google Scholar] [CrossRef]
- Heck, R.F.; Nolley, J.P. Palladium-catalyzed vinylic hydrogen substitution reactions with aryl, benzyl, and styryl halides. J. Org. Chem. 1972, 37, 2320–2322. [Google Scholar] [CrossRef]
- Pietrzak, M.; Jędrzejewska, B.; Mądrzejewska, D.; Bajorek, A. Convenient synthesis of p-aminobenzoic acids and their methyl esters. Org. Prep. Proced. Int. 2017, 49, 45–52. [Google Scholar] [CrossRef]
- Gadigennavar, S.; Ranganathan, M.; Sankararaman, S. Which isomer is it, 1,2,5,6- or 1,4,5,8-tetrasubstituted cycloocta-1,3,5,7-tetraene? Synthesis of symmetrically tetrasubstituted cycloocta-1,3,5,7-tetraene derivatives. Org. Biomol. Chem. 2020, 45, 9284–9291. [Google Scholar] [CrossRef]
- Wan, J.; Fan, B.; Thang, S.H. Sonochemical preparation of polymer-metal nanocomposites with catalytic and plasmonic properties. Nanoscale Adv. 2021, 11, 3306–3315. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Yan, H.; Wu, Y.; Li, Z.; Gong, S.; Liu, P.; Liu, Z. Benzothiazole-enamide-based BF2 complexes: Luminophores exhibiting aggregation-induced emission, tunable emission and highly efficient solid-state emission. J. Mater. Chem. C 2015, 3, 2953–2959. [Google Scholar] [CrossRef]
- Ośmiałowski, B.; Petrusevich, E.F.; Antoniak, M.A.; Grela, I.; Bin Jassar, M.A.; Nyk, M.; Luis, J.M.; Jędrzejewska, B.; Zaleśny, R.; Jacquemin, D. Controlling two-photon action cross section by changing a single heteroatom position in fluorescent dyes. J. Phys. Chem. Lett. 2020, 11, 5920–5925. [Google Scholar] [CrossRef] [PubMed]
- Jędrzejewska, B.; Krawczyk, P.; Pietrzak, M.; Gordel, M.; Matczyszyn, K.; Samoć, M.; Cysewski, P. Styryl dye possessing donor-π-acceptor structure-synthesis, spectroscopic and computational studies. Dyes Pigments 2013, 99, 673–685. [Google Scholar] [CrossRef]
- Matsumura, K.; Ono, M.; Yoshimura, M.; Kimura, H.; Watanabe, H.; Okamoto, Y.; Ihara, M.; Takahashi, R.; Saji, H. Synthesis and biological evaluation of novel styryl benzimidazole derivatives as probes for imaging of neurofibrillary tangles in Alzheimer’s disease. Bioorg. Med. Chem. 2013, 21, 3356–3362. [Google Scholar] [CrossRef]
- Shi, P.C.; Jiang, X.D.; Gao, R.N.; Dou, Y.Y.; Zhao, W.L. Synthesis and application of Vis/NIR dialkylaminophenylbuta-1,3-dienyl borondipyrromethene dyes. Chin. Chem. Lett. 2015, 26, 834–838. [Google Scholar] [CrossRef]
- Wang, N.; Wang, C. The Effect of Para Substitution on the Rate of Alkaline Hydrolysis of Ethyl 5-Ethyl-2,4-pentadienoates. J. Org. Chem. 1971, 36, 3178–3179. [Google Scholar] [CrossRef]
- Togninelli, A.; Gevariya, H.; Alongi, M.; Botta, M. An improved general method for palladium catalyzed alkenylations and alkynylations of aryl halides under microwave conditions. Tetrahedron Lett. 2007, 48, 4801–4803. [Google Scholar] [CrossRef]
- Ullrich, F.W.; Breitmaier, E. Vinylogous vilsmeier formylation with 3-(N,N-dimethylamino)-acroleins. Synthesis 1983, 641–645. [Google Scholar] [CrossRef]
- Szukalski, A.; Jędrzejewska, B.; Krawczyk, P.; Bajorek, A. An optical modulator on the pyrazolone-BASED bi-component system. Dyes Pigments 2020, 172, 107805. [Google Scholar] [CrossRef]
- Olmsted, J. Calorimetric determinations of absolute fluorescence quantum yields. J. Phys. Chem. 1979, 83, 2581–2584. [Google Scholar] [CrossRef]




| Abbr. | Ethyl Acetate | Methanol | ||||
|---|---|---|---|---|---|---|
| λmax abs (εmax) | λmax fl (ϕfl) | ∆νSt | λmax abs (εmax) | λmax fl (ϕfl) | ∆νSt | |
| 1a | 372.5 (43,000) | 437 (0.42) | 3962 | 389 (37,700) | 474 (0.33) | 4610 |
| 1b | 379.5 (44,500) | 438 (0.69) | 3519 | 395.5 (45,700) | 477 (0.20) | 4320 |
| 1c | 378 (35,500) | 444 (0.33) | 3933 | 395.5 (36,900) | 474 (0.14) | 4187 |
| 1d | 374 (35,200) | 474 (0.71) | 5641 | 392 (34,900) | not fluorescent | - |
| 1e | 379.5 (43,900) | 440 (0.61) | 3623 | 396.5 (40,200) | 476 (0.30) | 4212 |
| 1f | 368.5 (33,800) | 440 (0.99) | 4410 | 385 (31,500) | 481 (0.28) | 5184 |
| 1g | 357 (31,400) | 438 (0.51) | 5180 | 368 (28,900) | 486 (0.38) | 6598 |
| 1h | 378 (33,800) | 460 (1.19) | 4716 | 395.5 (33,900) | 485 (0.18) | 4666 |
| 1i | 385 (40,500) | 453 (1.14) | 3899 | 402.5 (35,600) | 486 (0.26) | 4269 |
| 1j | 396.5 (41,800) | 460 (1.88) | 3482 | 417.5 (38,300) | 488 (0.24) | 3460 |
| Abbr. | Eox (mV) | Abbr. | Eox (mV) |
|---|---|---|---|
| 1a | 1285 | 1f | 1228 |
| 1b | 1219 | 1g | 1300 |
| 1c | 1194 | 1h | 1093 |
| 1d | 1081 | 1i | 1132 |
| 1e | 1186 | 1j | 870 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrzak, M.; Jędrzejewska, B. Aromatic Amines in Organic Synthesis. Part II. p-Aminocinnamaldehydes. Molecules 2021, 26, 4360. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26144360
Pietrzak M, Jędrzejewska B. Aromatic Amines in Organic Synthesis. Part II. p-Aminocinnamaldehydes. Molecules. 2021; 26(14):4360. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26144360
Chicago/Turabian StylePietrzak, Marek, and Beata Jędrzejewska. 2021. "Aromatic Amines in Organic Synthesis. Part II. p-Aminocinnamaldehydes" Molecules 26, no. 14: 4360. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26144360