Behavioral and Antennal Responses of Tribolium confusum to Varronia globosa Essential Oil and Its Main Constituents: Perspective for Their Use as Repellent
Abstract
:1. Introduction
2. Results
2.1. Chemical Analysis of V. globosa Essential Oil
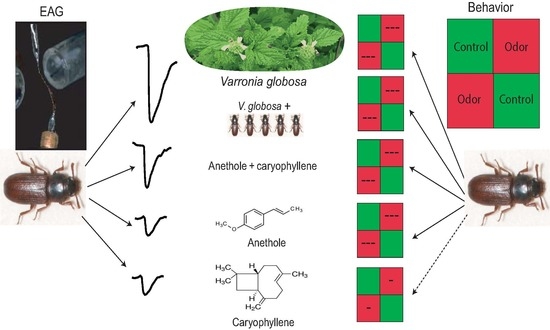
2.2. Strong Repulsive Behavioral Effect of V. globosa Essential oil on T. confusum
2.3. Differential Repulsive Behavioral Effects of Main Constituents of V. globosa Essential Oil and Their Mixture
2.4. Inhibition of Behavioral Aggregation Pheromone Attractiveness by V. globosa Essential Oil
2.5. Differential Antennal Responses to V. globosa Essential Oil, Individual Constituents and Their Mixture
3. Discussion
4. Materials and Methods
4.1. Insects
4.2. Plants, Essential Oils and Individual Compounds
4.3. Gas Chromatography-Mass Spectrometry
4.4. Olfactometer Tests
4.4.1. Behavioral Experiments for Single Odors
4.4.2. Behavioral Experiments for Odor Combinations
4.5. Electroantennogram Recordings
4.6. Data Analysis
4.6.1. Behavioral Experiments
4.6.2. Electroantennogram Recordings
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohammed, H.H. Repellency of Ethanolic Extract of Some Indigenous Plants against Tribolium confusum (Coleoptera: Tenebrionidae. J. Agric. Vet. Sci. 2013, 2, 27–31. [Google Scholar]
- Javadzadeh, M.; Sheikhi-Garjan, A.; Hosseini-Gharalari, A. Susceptibility of Different Populations of Tribolium confusum (Coleoptera: Tenebrionidae) to Malathion (EC 57%) in Flour Mills of Iran. Acta Phytopathol. Entomol. Hung. 2017, 52, 111–115. [Google Scholar] [CrossRef]
- Baker, T.C. Sex Pheromone Communication in the Lepidoptera: New Research Progress. Experientia 1989, 45, 248–262. [Google Scholar] [CrossRef]
- Byers, J.A. Chemical Ecology of Bark Beetles. Experientia 1989, 45, 271–283. [Google Scholar] [CrossRef]
- Verheggen, F.; Ryne, C.; Olsson, P.O.C.; Arnaud, L.; Lognay, G.; Högberg, H.E.; Persson, D.; Haubruge, E.; Löfstedt, C. Electrophysiological and Behavioral Activity of Secondary Metabolites in the Confused Flour Beetle, Tribolium confusum. J. Chem. Ecol. 2007, 33, 525–539. [Google Scholar] [CrossRef]
- Gadenne, C.; Barrozo, R.B.; Anton, S. Plasticity in Insect Olfaction: To Smell or Not to Smell? Annu. Rev. Entomol. 2016, 61, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Renou, M.; Anton, S. Insect Olfactory Communication in a Complex and Changing World. Curr. Opin. Insect Sci. 2020, 42, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, B.; Grossi, G.; Falabella, P.; Liu, Y.; Yan, S.; Lu, J.; Xi, J.; Wang, G. Molecular Basis of Alarm Pheromone Detection in Aphids. Curr. Biol. 2017, 27, 55–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kheloul, L.; Kellouche, A.; Bréard, D.; Gay, M.; Gadenne, C.; Anton, S. Trade-off between Attraction to Aggregation Pheromones and Repellent Effects of Spike Lavender Essential Oil and Its Main Constituent Linalool in the Flour Beetle Tribolium confusum. Entomol. Exp. Appl. 2019, 167, 826–834. [Google Scholar] [CrossRef]
- Rodrigues, F.A.; Pimenta, V.d.S.C.; Braga, K.M.d.S.; de Araújo, E.G. Obtenção de extratos de plantas do cerrado. Enciclopédia Biosf. 2016, 13, 870–887. [Google Scholar] [CrossRef]
- De Oliveira, J.V.; de França, S.M.; Barbosa, D.R.eS.; Dutra, K.d.A.; de Araujo, A.M.N.; Navarro, D.M.d.A.F.; de Oliveira, J.V.; de França, S.M.; Barbosa, D.R.eS.; Dutra, K.d.A.; et al. Fumigation and Repellency of Essential Oils against Callosobruchus maculatus (Coleoptera: Chrysomelidae: Bruchinae) in Cowpea. Pesqui. Agropecuária Bras. 2017, 52, 10–17. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Barreca, S.; La Bella, S.; Maggio, A.; Licata, M.; Buscemi, S.; Leto, C.; Pace, A.; Tuttolomondo, T. Flavouring Extra-Virgin Olive Oil with Aromatic and Medicinal Plants Essential Oils Stabilizes Oleic Acid Composition during Photo-Oxidative Stress. Agriculture 2021, 11, 266. [Google Scholar] [CrossRef]
- Gaire, S.; Scharf, M.E.; Gondhalekar, A.D. Synergistic Toxicity Interactions between Plant Essential Oil Components Against the Common Bed Bug (Cimex lectularius L.). Insects 2020, 11, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, K.; Guleria, S.; Razdan, V.K.; Babu, V. Synergistic Antioxidant and Antimicrobial Activities of Essential Oils of Some Selected Medicinal Plants in Combination and with Synthetic Compounds. Ind. Crops Prod. 2020, 154, 112569. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential Oils in Insect Control: Low-Risk Products in a High-Stakes World. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Bougherra, H.H.; Bedini, S.; Flamini, G.; Cosci, F.; Belhamel, K.; Conti, B. Pistacia entiscus Essential Oil Has Repellent Effect against Three Major Insect Pests of Pasta. Ind. Crops Prod. 2015, 63, 249–255. [Google Scholar] [CrossRef]
- Chiluwal, K.; Kim, J.; Bae, S.D.; Park, C.G. Essential Oils from Selected Wooden Species and Their Major Components as Repellents and Oviposition Deterrents of Callosobruchus chinensis (L.). J. Asia-Pac. Entomol. 2017, 20, 1447–1453. [Google Scholar] [CrossRef]
- Oza, M.J.; Kulkarni, Y.A. Traditional Uses, Phytochemistry and Pharmacology of the Medicinal Species of the Genus Cordia (Boraginaceae). J. Pharm. Pharmacol. 2017, 69, 755–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miguel, M.; Garcia-Bores, A.; Meraz, S.; Piedra, E.; Avila, M.; Serrano, R.; Orozco, J.; Jimenez-Estrada, M.; Chavarria, J.C.; Penalosa, I.; et al. Antimicrobial Activity of Essential Oil of Cordia globosa. Afr. J. Pharm. Pharmacol. 2016, 10, 179–184. [Google Scholar] [CrossRef]
- de Menezes, J.E.S.A.; Lemos, T.L.G.; Silveira, E.R.; Pessoa, O.D.L.; Santiago, G.M.P.; Nascimento, R.F. Chemical Composition and Larvicidal Activity of the Essential Oil From Leaves of Cordia globosa (Jacq.) H.B.K. from Northeastern Brazil. J. Essent. Oil Res. 2006, 18, 253–255. [Google Scholar] [CrossRef]
- de Carvalho, P.M.; Rodrigues, R.F.O.; Sawaya, A.C.H.F.; Marques, M.O.M.; Shimizu, M.T. Chemical Composition and Antimicrobial Activity of the Essential Oil of Cordia verbenacea D.C. J. Ethnopharmacol. 2004, 95, 297–301. [Google Scholar] [CrossRef]
- Hernandez, T.; Canales, M.; Teran, B.; Avila, O.; Duran, A.; Garcia, A.M.; Hernandez, H.; Angeles-Lopez, O.; Fernandez-Araiza, M.; Avila, G. Antimicrobial Activity of the Essential Oil and Extracts of Cordia curassavica (Boraginaceae). J. Ethnopharmacol. 2007, 111, 137–141. [Google Scholar] [CrossRef]
- Mhamdi, B.; Wannes, W.A.; Dhiffi, W.; Marzouk, B. Volatiles From Leaves and Flowers of Borage (Borago officinalis L.). J. Essent. Oil Res. 2009, 21, 504–506. [Google Scholar] [CrossRef]
- Zribi, I.; Bleton, J.; Moussa, F.; Abderrabba, M. GC-MS Analysis of the Volatile Profile and the Essential Oil Compositions of Tunisian Borago officinalis L.: Regional Locality and Organ Dependency. Ind. Crops Prod. 2019, 129, 290–298. [Google Scholar] [CrossRef]
- Fiori, J.; Hudaib, M.; Valgimigli, L.; Gabbanini, S.; Cavrini, V. Determination of Trans-Anethole in Salvia sclarea Essential Oil by Liquid Chromatography and GC-MS. J. Sep. Sci. 2002, 25, 703–709. [Google Scholar] [CrossRef]
- Senatore, F.; Oliviero, F.; Scandolera, E.; Taglialatela-Scafati, O.; Roscigno, G.; Zaccardelli, M.; De Falco, E. Chemical Composition, Antimicrobial and Antioxidant Activities of Anethole-Rich Oil from Leaves of Selected Varieties of Fennel [Foeniculum vulgare Mill. Ssp. vulgare Var. azoricum (Mill.) Thell]. Fitoterapia 2013, 90, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Tunç, I.; Erler, F. Fumigant Activity of Anethole, a Major Component of Essential Oil of Anise Pimpinella anisum L. Integr. Prot. Stored Prod. IOBC Bull. 2000, 23, 221–225. [Google Scholar]
- Alkan, M.; Ertürk, S. Insecticidal Efficacy and Repellency of Trans-Anethole Against Four Stored-Product Insect Pests. J. Agric. Sci. 2020, 26, 64–70. [Google Scholar] [CrossRef]
- Cao, J.-Q.; Guo, S.-S.; Wang, Y.; Pang, X.; Geng, Z.-F.; Du, S.-S. Contact Toxicity and Repellency of the Essential Oils of Evodia lenticellata Huang and Evodia rutaecarpa (Juss.) Benth. Leaves against Three Stored Product Insects. J. Oleo Sci. 2018, 67, 1027–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoma, M.; Hansson, B.S.; Knaden, M. Compound Valence Is Conserved in Binary Odor Mixtures in Drosophila melanogaster. J. Exp. Biol. 2014, 217, 3645–3655. [Google Scholar] [CrossRef] [Green Version]
- Koul, O.; Singh, R.; Kaur, B.; Kanda, D. Comparative Study on the Behavioral Response and Acute Toxicity of Some Essential Oil Compounds and Their Binary Mixtures to Larvae of Helicoverpa armigera, Spodoptera litura and Chilo partellus. Ind. Crops Prod. 2013, 49, 428–436. [Google Scholar] [CrossRef]
- Jiang, Z.; Akhtar, Y.; Bradbury, R.; Zhang, X.; Isman, M.B. Comparative Toxicity of Essential Oils of Litsea pungens and Litsea pubeba and Blends of Their Major Constituents against the Cabbage Looper, Trichoplusia ni. J. Agric. Food Chem. 2009, 57. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, J.; Zhang, N.; Yu, W.; Jiang, J.; Dai, G. Insecticidal Activities of Salvia hispanica L. Essential Oil and Combinations of Their Main Compounds against the Beet Armyworm Spodoptera exigua. Ind. Crops Prod. 2021, 162, 113271. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Chi, D. Electrophysiological and Behavioral Responses of Tenebrio molitor L. to Fourteen Kinds of Plant Volatiles. J. Asia-Pac. Entomol. 2016, 19, 261–267. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Holighaus, G.; Weißbecker, B.; Schütz, S. Electroantennographic Responses of Red Flour Beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) to Volatile Organic Compounds. J. Appl. Entomol. 2017, 141, 477–486. [Google Scholar] [CrossRef]
- Badji, C.A.; Eiras, A.E.; Cabrera, A.; Jaffe, K. Avaliação Do Feromônio Sexual de Neoleucinodes elegantalis Guenée (Lepidoptera: Crambidae). Neotrop. Entomol. 2003, 32, 221–229. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Wadhams, L.J.; Woodcock, C.M. Insect Host Location: A Volatile Situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef]
- Hieu, T.T.; Jung, J.; Kim, S.-I.; Ahn, Y.-J.; Kwon, H.W. Behavioural and Electroantennogram Responses of the Stable Fly (Stomoxys calcitrans L.) to Plant Essential Oils and Their Mixtures with Attractants. Pest Manag. Sci. 2014, 70, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Destro, B.G.I.; Jorge, R.M.M.; Mathias, A.L. Optimization of High-Concentration Trans-Anethole Production through Hydrodistillation of Star Anise. Braz. J. Chem. Eng. 2019, 36, 823–830. [Google Scholar] [CrossRef] [Green Version]
- Arthur, F.H. Grain Protectants: Current Status and Prospects for the Future. J. Stored Prod. Res. 1996, 32, 293–302. [Google Scholar] [CrossRef]
- Suzuki, T.; Sugawara, R. Isolation of an Aggregation Pheromone from the Flour Beetles, Tribolium castaneum and T. confusum (Coleoptera: Tenebrionidae). Appl. Entomol. Zool. 1979, 14, 228–230. [Google Scholar] [CrossRef] [Green Version]
- Pettersson, J. An Aphid Sex Attractant. Insect Syst. Evol. 1970, 1, 63–73. [Google Scholar] [CrossRef]




| 1 Compounds Similarity Index > 90% (NIST) | Retention Time (min) | Relative Percentage (%) |
|---|---|---|
| β-Pinene | 8.73 | 0.17 |
| Estragole | 27.95 | 0.89 |
| Anethole | 38.28 | 41.53 |
| Δ-Elemene | 43.59 | 1.77 |
| α-Copaene | 47.9 | 0.59 |
| β-Elemene | 50.19 | 0.64 |
| Caryophyllene | 52.83 | 7.72 |
| α-Humulene | 56.90 | 2.6 |
| γ-Gurjunene | 59.96 | 0.56 |
| β-Cubebene | 60.36 | 3.04 |
| β-Selinene | 60.86 | 0.49 |
| 2 NI | 62.04 | 1 |
| Elixene | 62.28 | 4.94 |
| α-Guaiene | 63.19 | 1.15 |
| γ-Cadinene | 64.56 | 0.57 |
| 2 NI | 64.93 | 0.17 |
| 2 NI | 65.08 | 1.77 |
| Δ-Cadinene | 65.92 | 1.2 |
| Germacrene B | 69.09 | 0.58 |
| Spathulenol | 71.86 | 7.06 |
| 2 NI | 72.51 | 0.56 |
| 2 NI | 74.50 | 2.08 |
| Humulene epoxide II | 75.14 | 0.32 |
| 2 NI | 76.24 | 0.27 |
| 2 NI | 76.89 | 1.58 |
| 2 NI | 77.48 | 0.86 |
| 2 NI | 78.18 | 6.69 |
| 2 NI | 79.04 | 1.47 |
| 2 NI | 79.98 | 0.54 |
| 2 NI | 80.47 | 1 |
| Cadin-4-en-10-ol | 80.80 | 1.01 |
| Shyobunol | 84.36 | 3.53 |
| 2 NI | 84.81 | 0.6 |
| 2 NI | 88.17 | 0.62 |
| (E,E)-Geranyllinalool | 119.56 | 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badji, C.A.; Dorland, J.; Kheloul, L.; Bréard, D.; Richomme, P.; Kellouche, A.; Azevedo de Souza, C.R.; Bezerra, A.L.; Anton, S. Behavioral and Antennal Responses of Tribolium confusum to Varronia globosa Essential Oil and Its Main Constituents: Perspective for Their Use as Repellent. Molecules 2021, 26, 4393. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26154393
Badji CA, Dorland J, Kheloul L, Bréard D, Richomme P, Kellouche A, Azevedo de Souza CR, Bezerra AL, Anton S. Behavioral and Antennal Responses of Tribolium confusum to Varronia globosa Essential Oil and Its Main Constituents: Perspective for Their Use as Repellent. Molecules. 2021; 26(15):4393. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26154393
Chicago/Turabian StyleBadji, Cesar Auguste, Jean Dorland, Lynda Kheloul, Dimitri Bréard, Pascal Richomme, Abdellah Kellouche, Claudio Roberto Azevedo de Souza, Antônio Lourenço Bezerra, and Sylvia Anton. 2021. "Behavioral and Antennal Responses of Tribolium confusum to Varronia globosa Essential Oil and Its Main Constituents: Perspective for Their Use as Repellent" Molecules 26, no. 15: 4393. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26154393