Evaluation of the Free Radical Scavenging Activities of Ellagic Acid and Ellagic Acid Peracetate by EPR Spectrometry †
Abstract
:Highlights
- EA and EAPA were found to be very good radical scavengers even at 1 μM.
- Rate of radical scavenging reaction by EAPA was found to be slower than that of EA in scavenging the radical, as measured by EPR.
- EA and EAPA decreased ROS production in L-6 myoblasts even at very low concentrations.
- They were both found to inhibit the NADPH catalyzed microsomal lipid peroxidation.
1. Introduction
2. Results and Discussion
2.1. Radical Scavenging Activities of EA and EAPA Measured by EPR
2.2. Radical Scavenging Activities of EA and EAPA by Spectrophotometer
2.3. Antioxidant Activities of EA and EAPA
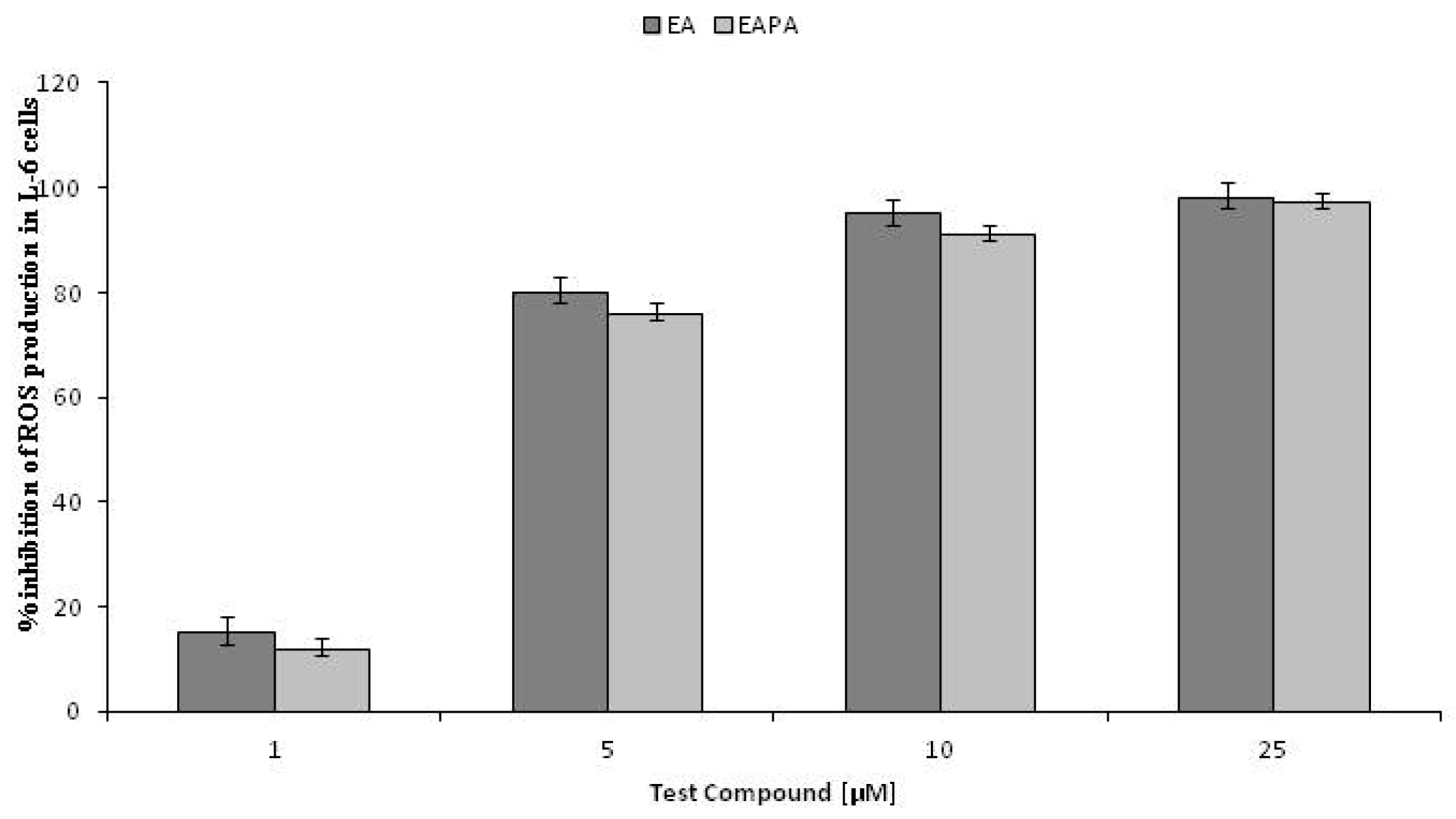
2.4. Intracellular Antioxidant Activities of EA and EAPA
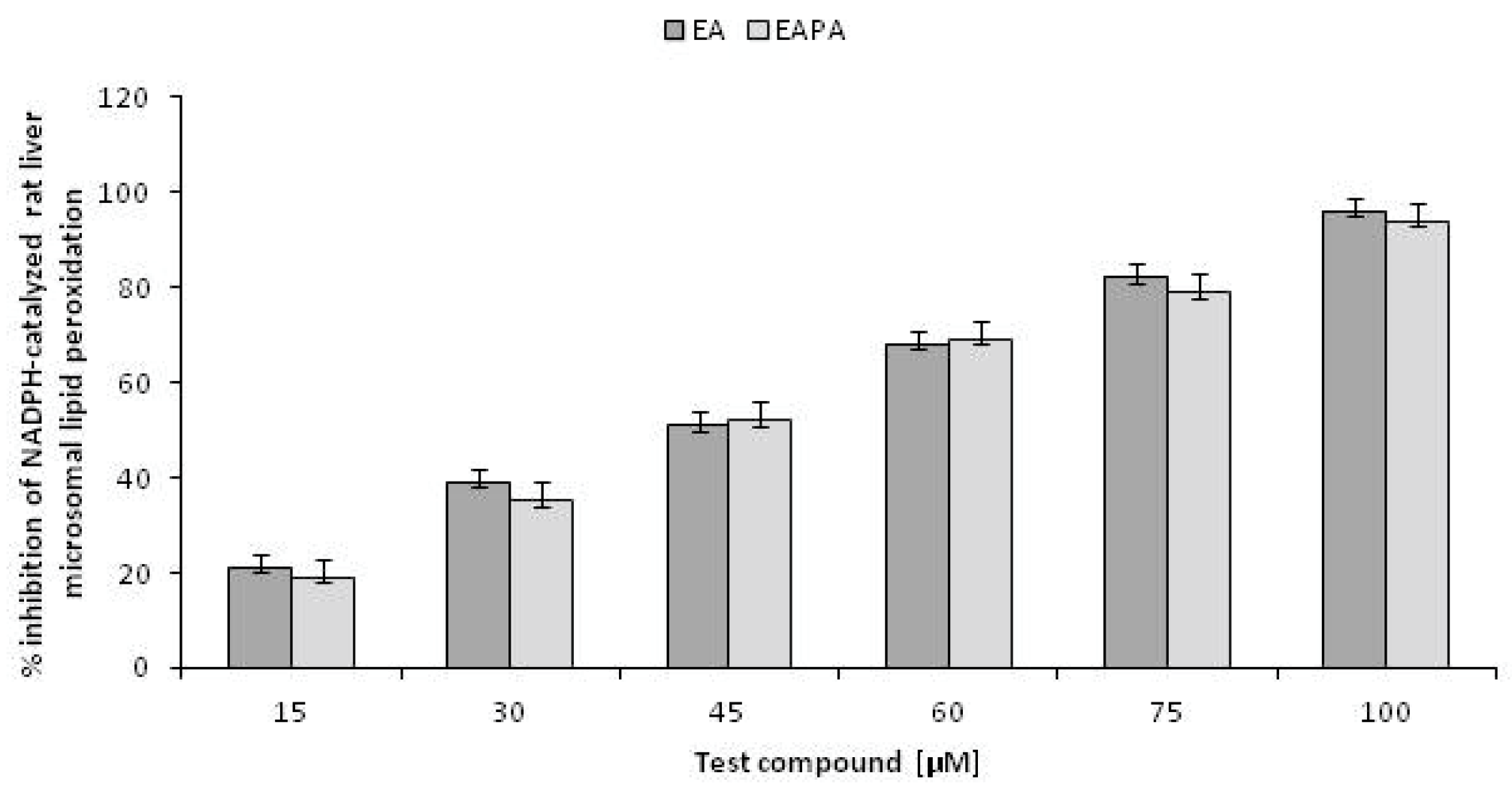
2.5. Inhibition of NADPH Catalysed Rat Liver Microsomal Lipid Peroxidation
2.6. Influence of Different Concentration of EA and EAPA on A549 Cells
3. Materials and Methods
3.1. General Considerations
3.2. Electron Paramagnetic Resonance Spectroscopy
3.3. Spectrophotometric Analysis
3.4. Cell Culture
3.5. Intracellular ROS Determination
3.6. Animals
3.7. Preparation of Rat Liver Microsomes
3.8. Assay for Initiation of Lipid Peroxidation
3.9. Cell Viability Assay
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, C.E.; Kalyanasundaram, B. Oxygen Radicals and Disease Process; Harwood Academic Press: Newark, NJ, USA, 1998. [Google Scholar]
- Chen, H.; Zuo, Y.; Deng, Y. Separation and determination of flavonoides and other phenolic compounds in the cranberry juice by high-performance liquid chromatography. J. Chromatogr. A 2001, 913, 387–395. [Google Scholar] [CrossRef]
- Daniel, E.M.; Krupnick, A.S.; Heur, Y.H.; Blinzler, J.A.; Mims, R.W.; Stoner, G.D. Extraction, stability and quantisation of ellagic acid in various fruits and nuts. J. Food Compos. Anal. 1989, 2, 385–398. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Hoffman, B.; Yang, J.; Soleas, G.J. Phenolic constituents, furans and total antioxidant status of distilled spirits. J. Agric. Food Chem. 1999, 47, 3978–3985. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Grover, I.S.; Kumar, S. Antimutagenic potential of ellagic acid isolated from Terminalia arjuna. Indian J. Exp. Biol. 1997, 35, 478–482. [Google Scholar]
- Loarca-Pina, G.; Kuzicky, P.A.; Demejia, E.G.; Kado, N.Y. Inhibitory effect of ellagic acid on the direct acting muatgenecity of aflatoxin B1 in the Salmonella microsuspension assay. Mutat. Res. 1998, 398, 183–187. [Google Scholar] [CrossRef]
- Singh, K.; Khanna, A.K.; Visen, P.K.; Chander, R. Protective effect of ellagic acid on tert-butyl-hydroperoxide induced lipid peroxidation in isolated rat hepatocytes. Ind. J. Exp. Biol. 1999, 37, 939–940. [Google Scholar]
- Narayanan, B.A.; Geoffroy, O.; Willingham, M.C.; Re, G.G.; Nixon, D.W. P53/p21 (WAF1/CIP1) expression and its possible role in G1 arrest and apoptosis in ellagic acid treated cancer cells. Cancer Lett. 1999, 136, 215–221. [Google Scholar] [CrossRef]
- Whitley, A.C.; Stoner, G.D.; Darby, M.V.; Walle, T. Intestinal epithelial cell accumulation of the cancer preventive polyphenol ellagic acid-extensive binding to protein and DNA. Biochem. Pharmacol. 2003, 66, 907–915. [Google Scholar] [CrossRef]
- Lin, S.S.; Hung, C.F.; Tyan, Y.S.; Yang, C.C.; Hsia, T.C.; Yang, M.D.; Chung, J.G. Ellagic acid inhibits arylamine N-acetyltransferase activity and DNA adducts formation in human bladder tumor cell lines (T24 and TSGH 8301). Urol. Res. 2001, 29, 371–376. [Google Scholar] [CrossRef]
- Priyadarsini, K.I.; Khopdecc, S.M.; Kumar, S.S.; Mohan, H. Free radical studies of ellagic acid, a natural phenolic antioxidant. J. Agric. Food Chem. 2002, 50, 2200–2206. [Google Scholar] [CrossRef]
- Abdurrauf, Y.; Ahmet, A.; Ali, O.C.; Mesut, A. Ellagic acid prevents cisplatin-induced oxidative stress in liver and heart tissue of rats. Basic Clin. Pharmacol. Toxicol. 2007, 101, 345–349. [Google Scholar]
- Devipriya, N.; Sudheer, A.R.; Srinivasan, M.; Menon, V.P. Effect of ellagic acid, a plant polyphenol, on fibrotic markers (mmps and timps) during alcohol-induced hepatotoxicity. Toxicol. Mech. Methods 2007, 17, 349–356. [Google Scholar] [CrossRef]
- Iuliano, L.; Pedersen, J.Z.; Camastra, C.; Bello, V.; Ceccarelli, S.; Violi, F. Protection of low density lipoprotein oxidation by the antioxidant agent IRFI005, a new synthetic hydrophilic vitamin E analogue. Free Radic. Biol. Med. 1999, 26, 858–868. [Google Scholar] [CrossRef]
- Shi, H.; Noguchi, N.; Niki, E. Galvinoxyl method for standardizing electron and proton donation activity. Methods Enzymol. 2001, 335, 157–166. [Google Scholar]
- McPhail, D.B.; Hartley, R.C.; Gardner, P.T.; Duthie, G.G. Kinetic and stoichiometric assessments of the antioxidant activity of flavonoids by electron spin resonance spectroscopy. J. Agric. Food Chem. 2003, 51, 1684–1690. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Chen, C.P.; Dong, E.; Tanaka, T.; Nonaka, G.I.; Nishioka, I. Study on the inhibitory effect of tannins and flavonoids against the 1, 1-diphenyl-2-picrylhydrazyl radical. Biochem. Pharmacol. 1998, 5, 213–222. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. LWT Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Rossi, L.; De Angelis, I.; Pedersen, J.Z.; Marchese, E.; Stammati, A.; Rotilio, G.; Zucco, F. N-[5-Nitro-2-furfurylidene]-3-amino-2-oxazolidinone activation by the human intestinal cell line Caco-2 monitored through noninvasive electron spin resonance spectroscopy. Mol. Pharmacol. 1996, 49, 547–555. [Google Scholar] [PubMed]
- Simic, A.Z.; Verbic, T.Z.; Sentic, M.N.; Vojic, M.P.; Juranic, I.O.; Manojlovic, D.D. Study of ellagic acid electro-oxidation mechanism. Mon. Chem. 2013, 144, 121–128. [Google Scholar] [CrossRef]
- Markovic, Z.; Milenkovic, D.; Dorovic, J.; Markovic, J.M.D.; Amic, D. A DFT and PM6 study of free radical scavenging activity of ellagic acid. Mon. Chem. 2013, 144, 803–812. [Google Scholar] [CrossRef]
- Raj, H.G.; Parmar, V.S.; Jain, S.C.; Goel, S.; Himanshu, P.; Malhotra, S.; Singh, A.; Olsen, C.E.; Wengel, J. Mechanism of biochemical action of substituted 4-methylbenzopyran-2-ones. Part I: Dioxygenated 4-methyl coumarins as superb antioxidant and radical scavenging agents. Bioorg. Med. Chem. 1998, 6, 833–839. [Google Scholar] [CrossRef]
- Raj, H.G.; Sharma, R.K.; Garg, B.S.; Parmar, V.S.; Jain, S.C.; Goel, S.; Tyagi, Y.K.; Singh, A.; Olsen, C.E.; Wengel, J. Mechanism of biochemical action of substituted 4-methylbezopyran-2-ones. Part 3: A novel mechanism for the inhibition of biological membrane lipid peroxidation by dioxygenated 4-methylcoumarins mediated by the formation of a stable ADP-Fe-inhibitor mixed ligand complex. Bioorg. Med. Chem. 1998, 6, 2205–2212. [Google Scholar]
- Sharma, S.D.; Rajor, H.K.; Chopra, S.; Sharma, R.K. Studies on structure activity relationship of some dihydroxy-4-methylcoumarin antioxidants based on their interaction with Fe(III) and ADP. Biometals 2005, 18, 143–154. [Google Scholar] [CrossRef]
- Kumar, A.; Tyagi, Y.K.; Seema; Ponnan, P.; Rohil, V.; Prasad, A.K.; Dwarkanath, B.S.; Parmar, V.S.; Raj, H.G. Ellagic acid peracetate is superior to ellagic acid in the prevention of genotoxicity due to aflatoxin B1 in bone marrow and lung cells. J. Pharm. Pharmacol. 2007, 59, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Kohli, E.; Gaspari, M.; Raj, H.G.; Parmar, V.S.; van der Greef, J.; Gupta, G.; Kumari, R.; Prasad, A.K.; Goel, S.; Pal, G.; et al. Establishment of the enzymatic protein acetylation independent of acetyl CoA: Recombinant glutathione S-transferase 3-3 is acetylated by a novel membrane-bound transacetylase using 7,8-diacetoxy-4-methylcoumarin as the acetyl donor. FEBS Lett. 2002, 530, 139–142. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Singh, B.K.; Tyagi, R.; Jain, S.K.; Sharma, S.K.; Prasad, A.K.; Raj, H.G.; Rastogi, R.C.; Watterson, A.C.; Parmar, V.S. Mechanism of biochemical action of substituted 4-methylcoumarins. Part 11: Comparison of the specificities of acetoxy derivatives of 4-methylcoumarin and 4-phenylcoumarin to acetoxycoumarins: Protein transacetylase. Bioorg. Med. Chem. 2005, 13, 4300–4305. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sushama, A.; Rohil, V.; Manral, S.; Gangopadhyay, S.; Prasad, A.K.; Raj, H.G.; Parmar, V.S. Prevention of benzene-induced genotoxicity in bone marrow and lung cells: Superiority of polyphenolic acetates to polyphenols. Arch. Toxicol. 2011, 85, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Pallottini, V.; Martini, C.; Pascolini, A.; Cavallini, G.; Gori, Z.; Bergamini, E.; Incerpi, S.; Trentalance, A. 3-Hydroxy-3-methylglutaryl coenzyme A reductase deregulation and age related hypercholesterolemia: A new role for ROS. Mech. Ageing Dev. 2005, 126, 845–851. [Google Scholar] [CrossRef] [PubMed]
- D’Arezzo, S.; Incerpi, S.; Davis, F.B.; Acconcia, F.; Marino, M.; Farías, R.N.; Davis, P.J. Rapid nongenomic effects of 3,5,3′-triiodo-L-thyronine on the intracellular pH of L-6 myoblasts are mediated by intracellular calcium mobilization and kinase pathways. Endocrinology 2004, 145, 5694–5703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernster, L.; Nordenbrand, K. Microsomal lipid peroxidation. Meth. Enzymol. 1967, 10, 574–580. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin ohenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Goel, A.; Prasad, A.K.; Parmar, V.S.; Ghosh, B.; Saini, N. Apoptogenic effect of 7,8-diacetoxy-4-methylcoumarin and 7,8-diacetoxy-4-methylthiocoumarin in human lung adenocarcinoma cell line: Role of NF-κB, Akt, ROS and MAP kinase pathway. Chem. Biol. Interact. 2009, 179, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Prasad, A.K.; Parmar, V.S.; Ghosh, B.; Saini, N. 7,8-dihydroxy-4-methylcoumarin induces apoptosis of human lung adenocarcinoma cells by ROS-independent mitochondrial pathway through partial inhibition of ERK/MAPK signaling. FEBS Lett. 2007, 581, 2447–2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]

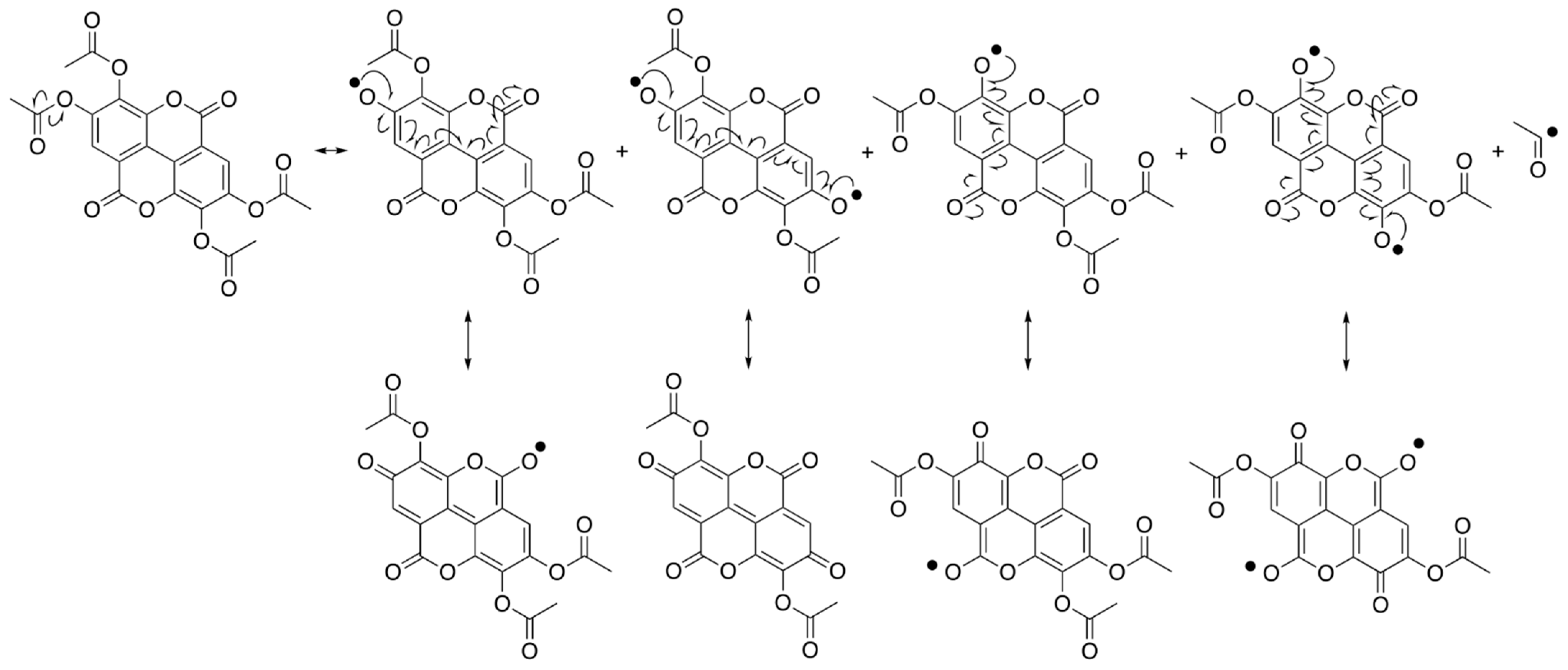

| Preincubation Time (min) | EA | EAPA | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 μM | 2 μM | 5 μM | 10 μM | 1 μM | 2 μM | 5 μM | 10 yM | |
| 1 | 68 ± 4 | 54 ± 4 | 35 ± 5 | 0 | 74 ± 3 | 63 ± 3 | 48 ± 5 | 30 ± 6 |
| 5 | 63 ± 2 | 46 ± 2 | 15 ± 4 | 0 | 70 ± 2 | 59 ± 2 | 37 ± 4 | 22 ± 5 |
| 10 | 59 ± 3 | 42 ± 3 | 06 ± 3 | 0 | 66 ± 4 | 55 ± 3 | 21 ± 3 | 10 ± 3 |
| 30 | 54 ± 2 | 33 ± 4 | 0 | 0 | 63 ± 3 | 51 ± 2 | 02 ± 4 | 06 ± 2 |
| 60 | 49 ± 2 | 27 ± 2 | 0 | 0 | 58 ± 2 | 45 ± 2 | 0 | 02 ± 2 |
| 90 | 44 ± 3 | 21 ± 2 | 0 | 0 | 53 ± 4 | 41 ± 3 | 0 | 0 |
| 120 | 38 ± 4 | 15 ± 3 | 0 | 0 | 47 ± 5 | 36 ± 4 | 0 | 0 |
| 240 | 22 ± 3 | 09 ± 3 | 0 | 0 | 42 ± 3 | 31 ± 4 | 0 | 0 |
| Preincubation Time (min) | EA | EAPA | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 μM | 2.5 μM | 5 μM | 10 μM | 1 μM | 2.5 μM | 5 μM | 10 μM | |
| 1 | 69 ± 2 | 60 ± 4 | 35 ± 5 | 0 | 85 ± 6 | 74 ± 5 | 48 ± 6 | 30 ± 5 |
| 5 | 56 ± 4 | 37 ± 5 | 15 ± 3 | 0 | 82 ± 5 | 68 ± 3 | 37 ± 4 | 22 ± 3 |
| 10 | 44 ± 3 | 26 ± 3 | 06 ± 2 | 0 | 80 ± 3 | 53 ± 2 | 21 ± 2 | 10 ± 4 |
| 30 | 15 ± 4 | 0 | 0 | 0 | 69 ± 2 | 33 ± 4 | 11 ± 4 | 02± 2 |
| 60 | 09 ± 2 | 0 | 0 | 0 | 64 ± 4 | 21 ± 3 | 02 ± 3 | 0 |
| 120 | 0 | 0 | 0 | 0 | 57 ± 3 | 10 ± 2 | 0 | 0 |
| 180 | 0 | 0 | 0 | 0 | 49 ± 5 | 09 ± 2 | 0 | 0 |
| 240 | 0 | 0 | 0 | 0 | 43 ± 4 | 0 | 0 | 0 |
| 300 | 0 | 0 | 0 | 0 | 38 ± 3 | 0 | 0 | 0 |
| 360 | 0 | 0 | 0 | 0 | 29 ± 2 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Kaushik, P.; Incerpi, S.; Pedersen, J.Z.; Goel, S.; Prasad, A.K.; Rohil, V.; Parmar, V.S.; Saso, L.; Len, C. Evaluation of the Free Radical Scavenging Activities of Ellagic Acid and Ellagic Acid Peracetate by EPR Spectrometry. Molecules 2021, 26, 4800. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26164800
Kumar A, Kaushik P, Incerpi S, Pedersen JZ, Goel S, Prasad AK, Rohil V, Parmar VS, Saso L, Len C. Evaluation of the Free Radical Scavenging Activities of Ellagic Acid and Ellagic Acid Peracetate by EPR Spectrometry. Molecules. 2021; 26(16):4800. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26164800
Chicago/Turabian StyleKumar, Ajit, Preeti Kaushik, Sandra Incerpi, Jens Z. Pedersen, Sanjay Goel, Ashok K. Prasad, Vishwajeet Rohil, Virinder S. Parmar, Luciano Saso, and Christophe Len. 2021. "Evaluation of the Free Radical Scavenging Activities of Ellagic Acid and Ellagic Acid Peracetate by EPR Spectrometry" Molecules 26, no. 16: 4800. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26164800







