Carbon Microsphere-Supported Metallic Nickel Nanoparticles as Novel Heterogeneous Catalysts and Their Application for the Reduction of Nitrophenol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Ni-NP/C Catalysts
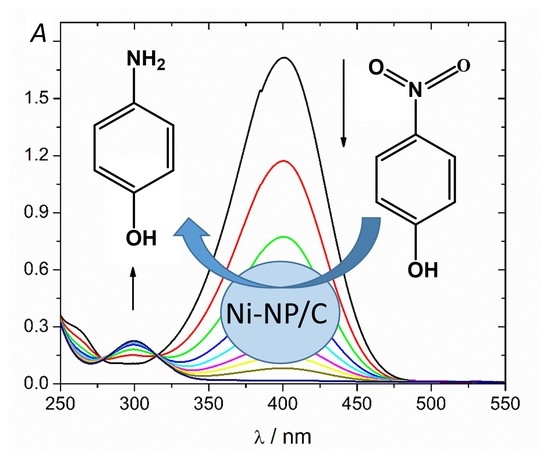
2.2. Catalytic Activity of Ni-NP/C Microspheres for the Reduction of 4-Nitrophenol
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Ni-NP/C Microspheres
3.3. Catalyst Characterization
3.4. Reduction of 4-Nitrophenol (4NP)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armor, J.N. A history of Industrial Catalysis. Catal. Today 2011, 163, 3–9. [Google Scholar] [CrossRef]
- Ghatak, A.; Das, M. The Recent Progress on Supported and Recyclable Nickel Catalysts towards Organic Transformations: A Review. Chem. Select. 2021, 6, 3656–3682. [Google Scholar] [CrossRef]
- Jaji, N.D.; Lee, H.L.; Hussin, M.H.; Akil, H.M.; Zakaria, M.R.; Othman, M.B.H. Advanced nickel nanoparticles technology: From synthesis to applications. Nanotech. Rev. 2020, 9, 1456–1480. [Google Scholar] [CrossRef]
- Bhakta, S.; Ghosh, T. Nickel Nanocatalysis: An Efficient Tool for Heck Reaction. ChemCatChem 2021, 13, 828–835. [Google Scholar] [CrossRef]
- Yu, Y.; Hou, Z. Soluble Nickel Nanoparticles for Catalytic Hydrogenation. Curr. Org. Chem. 2013, 17, 336–347. [Google Scholar] [CrossRef]
- Alonso, F.; Riente, P.; Yus, M. Nickel Nanoparticles in Hydrogen Transfer Reactions. Acc. Chem. Res. 2011, 44, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Alonso, F. Nickel Nanoparticles in the Transfer Hydrogenation of Functional Groups. In RSC Catalysis Series No. 17, Metal Nanoparticles for Catalysis: Advances and Applications; Tao, F., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2014; Chapter 5; pp. 83–98. [Google Scholar] [CrossRef]
- Meyer, J.S.; Lyons-Darden, T.; Garman, E.R.; Middleton, E.T.; Schlekat, C.E. Toxicity of Nanoparticulate Nickel to Aquatic Organisms: Review and Recommendations for Improvement of Toxicity Tests. Envoron. Toxicol. Chem. 2020, 39, 1861–1883. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Kong, L. Advance on toxicity of metal nickel nanoparticles. Environ. Geochem. Health 2020, 42, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kong, L. Research progress on the carcinogenicity of metal nanomaterials. J. Appl. Toxicol. 2021, 1–11. [Google Scholar] [CrossRef]
- Noh, J.; Meijboom, R. Reduction of 4-Nitrophenol as a Model Reaction for Nanocatalysis. In Application of Nanotechnology in Water Research; Mishra, A.K., Ed.; Scrivener Publishing LLC: Beverly, MA, USA, 2014; Chapter 13; pp. 333–406. [Google Scholar]
- Din, M.I.; Khalid, R.; Hussain, Z.; Hussain, T.; Mujahid, A.; Nejeeb, J.; Izhar, F. Nanocatalytic Assemblies for Catalytic Reduction of Nitrophenols: A Critical Review. Crit. Rev. Anal. Chem. 2020, 50, 322–338. [Google Scholar] [CrossRef]
- Hervés, P.; Pérez-Lorenzo, M.; Liz-Marzán, L.M.; Dzubiella, J.; Lu, Y.; Ballauff, M. Catalysis by metallic nanoparticles in aqueous solutions: Model reactions. Chem. Soc. Rev. 2012, 41, 5577–5587. [Google Scholar] [CrossRef]
- Liao, G.; Gong, Y.; Zhong, L.; Fang, J.; Zhang, L.; Xu, Z.; Gao, H.; Fang, B. Unlocking the door to highly efficient Ag-based nanoparticles catalysts for NaBH4-assisted nitrophenol reduction. Nano Res. 2019, 12, 2407–2436. [Google Scholar] [CrossRef]
- Aditya, T.; Pal, A.; Pal, T. Nitroarene reduction: A trusted model reaction to test nanoparticle catalysts. Chem. Commun. 2015, 51, 9410–9431. [Google Scholar] [CrossRef] [PubMed]
- Pasinszki, T.; Krebsz, M.; Lajgut, G.G.; Kocsis, T.; Kótai, L.; Kauthale, S.; Tekale, S.; Pawar, R. Copper nanoparticles grafted on carbon microspheres as novel heterogeneous catalysts and their application for the reduction of nitrophenol and one-pot multicomponent synthesis of hexahydroquinolines. New J. Chem. 2018, 42, 1092–1098. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Kim, C.; Lee, K.; Lee, H. Shaped Ni nanoparticles with an unconventional hcp crystalline structure. Chem. Commun. 2014, 50, 6353–6356. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M.; Kótai, L.; Sajó, I.E.; Homonnay, Z.; Kuzmann, E.; Kiss, L.F.; Váczi, T.; Kovács, I. Nanofurry magnetic carbon microspheres for separation processes and catalysis: Synthesis, phase composition, and properties. J. Mater. Sci. 2015, 50, 7353–7363. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M.; Chand, D.; Kótai, L.; Homonnay, Z.; Sajó, I.E.; Váczi, T. Carbon microspheres decorated with iron sulfide nanoparticles for mercury(II) removal from water. J. Mater. Sci. 2020, 55, 1425–1435. [Google Scholar] [CrossRef] [Green Version]
- Ji, Z.; Shen, X.; Zhu, G.; Zhou, H.; Yuan, A. Reduced graphene oxide/nickel nanocomposites: Facile synthesis, magnetic and catalytic properties. J. Mater. Chem. 2012, 22, 3471–3477. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Wang, Y.; Liu, C.; Jin, M.; Xu, Y.; Li, L.; Guo, X.; Hu, A.; Liu, T.; et al. A thermosensitive hydrogel carrier for nickel nanoparticles. Colloids Interfaces Sci. Commun. 2015, 4, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Sahiner, N.; Ozay, H.; Ozay, O.; Aktas, N. New catalytic route: Hydrogels as templates and reactors for in situ Ni nanoparticle synthesis and usage in the reduction of 2- and 4-nitrophenols. Appl. Catal. A 2010, 385, 201–207. [Google Scholar] [CrossRef]
- Kamal, T.; Asiri, A.M.; Ali, N. Catalytic reduction of 4-nitrophenol and methylene blue pollutants in water by copper and nickel nanoparticles decorated polymer sponges. Spectrochim. Acta Part A 2021, 261, 120019. [Google Scholar] [CrossRef]
- Qiu, H.; Qiu, F.; Han, X.; Li, J.; Yang, J. Microwave-irradiated preparation of reduced graphene oxide-Ni nanostructures and their enhanced performance for catalytic reduction of 4-nitrophenol. Appl. Surf. Sci. 2017, 407, 509–517. [Google Scholar] [CrossRef]
- Ji, Z.; Wang, Y.; Shen, X.; Ma, H.; Yang, J.; Yuan, A.; Zhou, H. Facile synthesis and enhanced catalytic performance of reduced graphene oxide decorated with hexagonal structure Ni nanoparticles. J. Colloids Interfaces Sci. 2017, 487, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Xie, J.; Jiang, D.; Wei, X.; Chen, M. Modifiers-assisted formation of nickel nanoparticles and their catalytic application to p-nitrophenol reduction. CrystEngComm 2013, 15, 560–569. [Google Scholar] [CrossRef]
- Jiang, Z.; Xie, J.; Jiang, D.; Jing, J.; Qin, H. Facile route fabrication of nano-Ni core mesoporous-silica shell particles with high catalytic activity towards 4-nitrophenol reduction. CrystEngComm 2012, 14, 4601–4611. [Google Scholar] [CrossRef]
- Cao, J.; Wang, F.; Liang, S.; Tong, X.; Zhang, Z.; Feng, J.; Wang, H.; Jiang, X. Microwave-Assisted Controllable Synthesis of Nickel Nanoparticles Embedded in Mesoporous Silica for Catalytic Reduction of 4-Nitrophenol. NANO Brief Rep. Rev. 2017, 12, 1750124. [Google Scholar] [CrossRef]
- Xia, J.; He, G.; Zhang, L.; Sun, X.; Wang, X. Hydrogenation of nitrophenols catalyzed by carbon black-supported nickel nanoparticles under mild conditions. Appl. Catal. B Environ. 2016, 180, 408–415. [Google Scholar] [CrossRef]



| Carbonization | hcp-Ni1 | fcc-Ni1 | Graphite 1 | SSA 2 | |
|---|---|---|---|---|---|
| °C | Hours | at% (nm) | at% (nm) | at% (nm) | m2 g−1 |
| 500 | 2 4 8 | 55 (10) 50 (10) 50 (10) | 45 (8) 50 (8) 50 (8) | 3 5 8 | |
| 600 | 2 4 8 | 40 (14) 30 (11) 30 (11) | 60 (11) 70 (14) 70 (17) | 5 7 8 | |
| 700 | 2 4 8 | 15 (11) 8 (11) 5 (11) | 85 (47) 92 (47) 95 (71) | 4 7 9 | |
| 800 | 2 4 8 | 100 (66) 100 (66) 100 (99) | 14 (12) 14 (12) 18 (17) | 7 4 6 | |
| 900 | 2 4 8 | 100 (82) 100 (99) 100 (99) | 25 (12) 25 (12) 33 (17) | 15 15 29 | |
| 1000 | 2 4 8 | 100 (71) 100 (123) 100 (123) | 67 (12) 67 (12) 82 (14) | 39 57 55 | |
| Catalyst | 4NP Conc. mM | NaBH4 Conc. mM | Cat. Amount mg cm−3 | Temp. °C | Rate Constant 1 min−1 g−1 | Reference |
|---|---|---|---|---|---|---|
| Ni-NP | 0.1 | 30 | 0.065 | r.t. | 1.3 | [20] |
| Ni-NP/RGO | 0.1 | 30 | 0.1 | r.t. | 1.5 | [20] |
| Ni-NP/hydrogel | 2.16 | 216 | 0.48 | 27 | 0.9 | [21] |
| Ni-NP/hydrogel | 14 | 288 | 1 | 30 | 1.1 | [22] |
| Ni-NP/polymer-sponge | 0.13 | 200 | 6.7 | r.t. | 7.9 | [23] |
| Ni-NP | 0.1 | 30 | 0.01 | r.t. | 1.8 | [24] |
| Ni-NP/RGO | 0.1 | 30 | 0.01 | r.t. | 6.6 | [24] |
| Ni-NP | 0.05 | 20 | 0.005 | r.t. | 9.4 | [25] |
| Ni-NP/RGO | 0.05 | 20 | 0.005 | r.t. | 25 | [25] |
| Ni-NP/CTAB + gelatin | 0.09 | 18 | 0.9 | 20 | 48 | [26] |
| Ni-NP/CTAB + PEG10000 | 0.09 | 18 | 0.9 | 20 | 54 | [26] |
| Ni-NP/silica | 0.09 | 18 | 0.9 | 20 | 20–56 | [27] |
| Ni-NP/silica | 0.145 | 7.5 | 0.1 | 20 | 13–297 | [28] |
| Ni-NP/carbon black | 0.5 | 53 | 0.02 | 30 | 14–597 | [29] |
| Ni-NP/carbon spheres | 0.1 | 10 | 0.3–0.9 | 20 | 111–113 | this work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krebsz, M.; Kótai, L.; Sajó, I.E.; Váczi, T.; Pasinszki, T. Carbon Microsphere-Supported Metallic Nickel Nanoparticles as Novel Heterogeneous Catalysts and Their Application for the Reduction of Nitrophenol. Molecules 2021, 26, 5680. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26185680
Krebsz M, Kótai L, Sajó IE, Váczi T, Pasinszki T. Carbon Microsphere-Supported Metallic Nickel Nanoparticles as Novel Heterogeneous Catalysts and Their Application for the Reduction of Nitrophenol. Molecules. 2021; 26(18):5680. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26185680
Chicago/Turabian StyleKrebsz, Melinda, László Kótai, István E. Sajó, Tamás Váczi, and Tibor Pasinszki. 2021. "Carbon Microsphere-Supported Metallic Nickel Nanoparticles as Novel Heterogeneous Catalysts and Their Application for the Reduction of Nitrophenol" Molecules 26, no. 18: 5680. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26185680