Effect of the Amount of Polysorbate 80 and Oregano Essential Oil on the Emulsion Stability and Characterization Properties of Sodium Alginate Microcapsules
Abstract
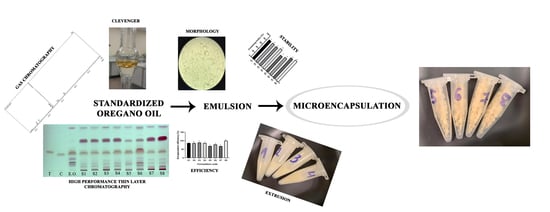
:1. Introduction
2. Results and Discussion
2.1. Identification
2.2. Emulsion Preparation and Characterization
2.3. Physical Parameters of the Oregano Loaded Microcapsules
3. Materials and Methods
3.1. Materials
3.1.1. Chemicals
3.1.2. Plant Material
3.2. Methods
3.2.1. Microscopy
3.2.2. Obtaining of Oregano Essential Oil by Hydro-Distillation
3.2.3. Oregano Essential Oil Emulsion Preparation
3.2.4. Oregano Essential Oil Emulsion Stability
3.2.5. Emulsion Morphology
3.2.6. Characterization of Emulsion Droplet Size
3.2.7. Encapsulation of Oregano Essential Oil by Extrusion Method
3.2.8. Physical Parameters of Microcapsules
3.2.9. Determination of Encapsulation Efficiency
3.2.10. High Performance Thin Layer Chromatography
3.2.11. GC/MS Method
3.2.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bağci, Y.; Kan, Y.; Doğu, S.; Çelik, S.A. The essential Oil compositions of Origanum majorana L. cultivated in Konya and collected from Mersin-Turkey. Indian J. Pharm. Educ. Res. 2017, 51, s463–s469. [Google Scholar] [CrossRef]
- Güner, A.; Aslan, S.; Ekim, T.; Vural, M.; Babaç, M.T.; Yòldòròm, H. Turkey Plant List (Vascular Plants); Publication of Nezahat Gokyigit Botanical Garden and Flora Research Association: Istanbul, Turkey, 2012. [Google Scholar]
- Chávez, M.L.; Rodriguez, R.; Aguilar, C. Essential Oils: A Natural Alternative to Combat Antibiotics Resistance. In Antibiotic Resistance Mechanisms and New Antimicrobial Approaches; Kon, K., Rai, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 227–235. [Google Scholar]
- Baranauskaitė, J.; Jakštas, V.; Ivanauskas, L.; Kopustinskienė, D.M.; Drakšienė, G.; Masteikova, R.; Bernatonienė, J. Optimization of carvacrol, rosmarinic, oleanolic and ursolic acid extraction from oregano herbs (Origanum onites L., Origa-num vulgare spp. hirtum and Origanum vulgare L.). Nat. Prod. Res. 2015, 30, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Kirimer, N. Essential oils of Anatolian Lamiaceae—An update. NVEO 2018, 5, 1–28. [Google Scholar]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food. Sci. 2019, 60, 3042–3053. [Google Scholar] [CrossRef]
- Baser, K.H. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des. 2008, 14, 3106–3119. [Google Scholar] [CrossRef] [PubMed]
- Baranauskaite, J.; Duman, G.; Corapcioglu, G.; Baranauskas, A.; Taralp, A.; Ivanauskas, L.; Bernatoniene, J. Liposomal incorporation to improve dissolution and stability of rosmarinic acid and carvacrol extracted from Oregano (O. onites L.). BioMed Res. Intern. 2018, 2018, 6147315. [Google Scholar] [CrossRef] [Green Version]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef] [Green Version]
- Matulyte, I.; Kasparaviciene, G.; Kasparaviciene, J. Development of New Formula Microcapsules from Nutmeg Essential Oil Using Sucrose Esters and Magnesium Aluminometasilicate. Pharmaceutics 2020, 12, 628. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks and applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef]
- Kumar, S.; Nakka, S.; Rajabalaya, R.; Kumar, H.; Halder, T.; Palanisamy, M.; Nanda, A. Microencapsulation techniques and its practices. IJPST 2011, 6, 1–23. [Google Scholar]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Mousa, L. Microencapsulation of oils: A comprehensive review of benefits, techniques, and applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Kumar, A.; Sahoo, S.K.; Padhee, K.; Kochar, P.S.; Sathapathy, A.; Pathak, N. Review on solubility enhancement techniques for hydrophobic drugs. Pharm. Glob. 2011, 3, 1–7. [Google Scholar]
- Suganya, V.; Anuradha, V. Microencapsulation and nanoencapsulation: A review. IJPCR 2017, 9, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Muthukumarasamy, P.; Allan-Wojtas, P.; Holley, R.A. Stability of Lactobacillus reuteri in different types of microcapsules. J. Food Sci. 2006, 71, M20–M24. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Goli, S.A.H.; Fathi, M. Encapsulation of orange essential oil using cross-linked electrospun gelatin nanofibers. Food Bioprocess Technol. 2018, 1, 427–434. [Google Scholar] [CrossRef]
- Azad, A.K.; Al-Mahmood, S.M.A.; Chatterjee, B.; Wan Sulaiman, W.M.A.; Elsayed, T.M.; Doolaanea, A.A. Encapsulation of Black Seed Oil in Alginate Beads as a pH-Sensitive Carrier for Intestine-Targeted Drug Delivery: In Vitro, In Vivo and Ex Vivo Study. Pharmaceutics 2020, 12, 219. [Google Scholar] [CrossRef] [Green Version]
- Majeed, H.; Bian, Y.Y.; Ali, B.; Jamil, A.; Majeed, U.; Khan, Q.F.; Iqbal, K.J.; Shoemaker, C.F.; Fang, Z. Essential oil encapsulations: Uses, procedures and trends. RSC Adv. 2015, 5, 58449–58463. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Yue, Y.; Wang, X.; Wu, Q.; Han, J.; Jiang, J. Assembly of polyacrylamide-sodium alginate-based organic-inorganic hydrogel with mechanical and adsorption properties. Polymers 2019, 11, 1239. [Google Scholar] [CrossRef] [Green Version]
- Kothale, D.; Verma, U.; Dewangan, N.; Jana, P.; Jain, A.; Jain, D. Alginate as promising natural polymer for pharmaceutical, food, and biomedical applications. Curr. Drug Deliv. 2020, 17, 755–775. [Google Scholar] [CrossRef]
- Woranuch, S.; Yoksan, R. Eugenol-loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through encapsulation. Carbohydr. Polym. 2013, 96, 578–585. [Google Scholar]
- Munhuweyi, K.; Caleb, O.J.; van Reenen, A.J.; Opara, U.L. Physical and antifungal properties of β-cyclodextrin microcapsules and nanofibre films containing cinnamon and oregano essential oils. LWT 2018, 87, 413–422. [Google Scholar] [CrossRef]
- Sedaghat Doost, A.; Dewettinck, K.; Devlieghere, F.; Van der Meeren, P. Influence of non-ionic emulsifier type on the stability of cinnamaldehyde nanoemulsions: A comparison of polysorbate 80 and hydrophobically modified inulin. Food Chem. 2018, 258, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. Oregano—Origani Herba. European Pharmacopoeia, 8th ed.; Council of Europe: Strasbourg, France, 2013; pp. 1342–1344. [Google Scholar]
- Dolca, C.; Ferrandiz, M.; Capablanca, L.; Franco, E.; Mira, E.; Lopez, F.; Garcia, D. Microencapsulation of Rosemary Essential Oil by Co-Extrusion/Gelling Using Alginate as a Wall Material. JEAS 2015, 5, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Gharsallaoui, A.; Saurel, R.; Chambin, O.; Voilley, A. Pea (Pisum sativum L.) protein isolate stabilized emulsions: A novel system for microencapsulation of lipophilic ingredients by spray drying. Food Bioprocess Technol. 2012, 5, 2211–2221. [Google Scholar] [CrossRef]
- Tadros, T.F. Rheology of Dispersions: Principles and Applications; Wiley-VCH: Weinheim, Germany, 2010; p. 218. [Google Scholar]
- McClements, D.J.; Decker, E.A.; Weiss, J. Emulsion-based delivery systems for lipophilic bioactive components. J. Food Sci. 2007, 72, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, D.; Wang, L.-J.; Özkan, N. Effect of concentrated flaxseed protein on the stability and rheological properties of soybean oil-in-water emulsions. J. Food Eng. 2010, 96, 555–561. [Google Scholar] [CrossRef]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Ultrasonic emulsification of food-grade nanoemulsion formulation and evaluation of its bactericidal activity. Ultrason. Sonochem. 2013, 20, 338–344. [Google Scholar] [CrossRef]
- Chirayil, C.J.; Abraham, J.; Mishra, R.K.; George, S.C.; Thomas, S. Instrumental techniques for the characterization of nanoparticles. In Thermal and Rheological Measurement Techniques for Nanomaterials Characterization; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–36. [Google Scholar]
- Calvo, P.; Castaño, Á.L.; Lozano, M.; González-Gómez, D. Influence of the microencapsulation on the quality parameters and shelf-life of extra-virgin olive oil encapsulated in the presence of BHT and different capsule wall components. Food Res. Int. 2012, 45, 256–261. [Google Scholar] [CrossRef]
- Shanmugam, A.; Ashokkumar, M. Ultrasonic preparation of stable flax seed oil emulsions in dairy systems—Physicochemical characterization. Food Hydrocoll. 2014, 39, 151–162. [Google Scholar] [CrossRef]
- Liu, X.; Mäki-Arvela, P.; Aho, A.; Vajglova, Z.; Gun’ko, V.M.; Heinmaa, I.; Kumar, N.; Eränen, K.; Salmi, T.; Murzin, D.Y. Zeta potential of beta zeolites: Influence of structure, acidity, pH, temperature and concentration. Molecules 2018, 23, 946. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.Q. Relationship between the zeta potential and the chemical agglomeration efficiency of fine particles in flue gas during coal combustion. Fuel 2018, 215, 757–765. [Google Scholar] [CrossRef]
- Tonon, R.V.; Pedro, R.B.; Grosso, C.R.F.; Hubinger, M.D. Microencapsulation of Flaxseed Oil by Spray Drying: Effect of Oil Load and Type of Wall Material. Dry. Technol. 2012, 30, 1491–1501. [Google Scholar] [CrossRef]
- Bae, E.K.; Lee, S.J. Microencapsulation of Avocado Oil by Spray Drying Using Whey Protein and Maltodextrin. J. Microencapsul. 2008, 25, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.C.; Tonon, R.V.; Hubinger, M.D. Effect of Homogenization Pressure and Oil Load on the Emulsion Properties and the Oil Retention of Microencapsulated Basil Essential Oil (Ocimum basilicum L.). Dry. Technol. 2012, 30, 1413–1421. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef]
- Belscak-Cvitanovic, A.; Juric, S.; Dordevic, V.; Barisic, L.; Komes, D.; Jezek, D.; Bugarski, B.; Nedovic, V. Chemometric evaluation of binary mixtures of alginate and polysaccharide biopolymers as carriers for microencapsulation of green tea polyphenols. Int. J. Food Prop. 2017, 20, 1971–1986. [Google Scholar] [CrossRef] [Green Version]
- Draget, K.I.; Gaserod, O.; Aune, I.; Andersen, P.O.; Storbakken, B.; Stokke, B.T.; Smidsrod, O. Effects of molecular weight and elastic segment flexibility on syneresis in Ca-alginate gels. Food Hydrocoll. 2001, 15, 485–490. [Google Scholar] [CrossRef]
- Kibici, D.; Kahveci, D. Effect of Emulsifier Type, Maltodextrin, and β-Cyclodextrin on Physical and Oxidative Stability of Oil-In-Water Emulsions. J. Food Sci. 2019, 84, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Aas, T.S.; Oehme, M.; Sorensen, M.; He, G.; Lygren, I.; Asgard, T. Analysis of pellet degradation of extruded high energy fish feeds with different physical qualities in a pneumatic feeding system. Aquacult. Eng. 2011, 44, 25–34. [Google Scholar] [CrossRef]







| Peak | Compounds | Area | RT (min) | % |
|---|---|---|---|---|
| 1 | α-Pinene | 235,069 | 8.055 | 0.19 |
| 2 | Camphene | 136,820 | 8.824 | 0.11 |
| 3 | 4-Cymene | 2,349,093 | 12.968 | 1.87 |
| 4 | 3-Octen-5-yne, 2,7-dimethyl-, (E)- | 2,052,274 | 14.654 | 1.63 |
| 5 | Thymol | 240,379 | 24.472 | 0.19 |
| 6 | Carvacrol | 118,977,011 | 25.016 | 94.65 |
| 7 | (−)-β-Caryophyllene | 761,493 | 28.346 | 0.61 |
| 8 | α-Farnesene | 184,699 | 28.995 | 0.15 |
| 9 | Longipinene epoxide | 269,407 | 33.446 | 0.21 |
| Sample Code | Parameters | Day 0 | Day 3 | Day 6 | Day 14 | Day 28 |
|---|---|---|---|---|---|---|
| S1 | Z-average size (nm) | 806.1 ± 19.3 | 808.1 ± 9.3 | 802.5 ± 20.2 | 804.5 ± 17.6 | 806.5 ± 13.4 |
| PDI | 0.40 ± 0.09 | 0.41 ± 0.07 | 0.42 ± 0.09 | 0.39 ± 0.04 | 0.38 ± 0.05 | |
| Zeta potential (mV) | −0.58 ± 0.02 | −0.56 ± 0.04 | −0.58 ± 0.06 | −0.56 ± 0.01 | −0.55 ± 0.03 | |
| S2 | Z-average size (nm) | 751.2 ± 16.2 | 753.1 ± 15.1 | 753.2 ± 13.1 | 750.9 ± 10.3 | 754.0 ± 9.9 |
| PDI | 0.42 ± 0.08 | 0.44 ± 0.06 | 0.45 ± 0.08 | 0.40 ± 0.07 | 0.43 ± 0.06 | |
| Zeta potential (mV) | −0.49 ± 0.04 | −0.49 ± 0.06 | −0.50 ± 0.07 | −0.48 ± 0.06 | −0.49 ± 0.05 | |
| S3 | Z-average size (nm) | 643.3 ± 16.2 | 657.5 ± 15.8 | 660.3 ± 19.2 | 659.9 ± 12.0 | 658.6 ± 10.2 |
| PDI | 0.40 ± 0.09 | 0.41 ± 0.05 | 0.41 ± 0.08 | 0.40 ± 0.06 | 0.41 ± 0.06 | |
| Zeta potential (mV) | −0.37 ± 0.09 | −0.41 ± 0.02 | −0.42 ± 0.03 | −0.40 ± 0.02 | −0.40 ± 0.05 | |
| S4 | Z-average size (nm) | 700.1 ± 15.6 | 708.2 ± 5.8 | 709.1 ± 8.6 | 706.8 ± 6.5 | 705.1 ± 6.6 |
| PDI | 0.4 ± 0.06 | 0.41 ± 0.1 | 0.42 ± 0.12 | 0.40 ± 0.11 | 0.41 ± 0.12 | |
| Zeta potential (mV) | −0.50 ± 0.05 | −0.50 ± 0.05 | −0.50 ± 0.08 | −0.49 ± 0.07 | −0.50 ± 0.04 | |
| S5 | Z-average size (nm) | 755.2 ± 10.8 | 754.2 ± 11.2 | 750.3 ± 16.3 | 753.7 ± 10.8 | 755.0 ± 10.2 |
| PDI | 0.47 ± 0.06 | 0.48 ± 0.08 | 0.48 ± 0.09 | 0.49 ± 0.05 | 0.49 ± 0.05 | |
| Zeta potential (mV) | −0.54 ± 0.06 | −0.56 ± 0.06 | −0.57 ± 0.05 | −0.58 ± 0.02 | −0.56 ± 0.03 | |
| S6 | Z-average size (nm) | 1100.4 ± 8.3 | 1108.2 ± 9.8 | 1115.3 ± 11.8 | 1119.8 ± 10.2 | 1119.0 ± 8.8 |
| PDI | 0.51 ± 0.05 | 0.48 ± 0.03 | 0.41 ± 0.08 | 0.47 ± 0.02 | 0.41 ± 0.05 | |
| Zeta potential (mV) | −0.42 ± 0.03 | −0.43 ± 0.04 | −0.43 ± 0.07 | −0.40 ± 0.05 | −0.43 ± 0.03 | |
| S7 | Z-average size (nm) | 455 ± 16.5 | 462 ± 22.3 | 453.5 ± 20.4 | 450.9 ± 18.4 | 453.7 ± 15.2 |
| PDI | 0.4 ± 0.03 | 0.41 ± 0.02 | 0.4 ± 0.02 | 0.4 ± 0.02 | 0.4 ± 0.02 | |
| Zeta potential (mV) | −0.58 ± 0.03 | −0.56 ± 0.06 | −0.55 ± 0.04 | −0.57 ± 0.02 | −0.55 ± 0.04 | |
| S8 | Z-average size (nm) | 323.2 ± 10.2 | 325.5 ± 9.3 | 325.7 ±10.9 | 329.7 ±12.0 | 329.2 ± 9.9 |
| PDI | 0.35 ± 0.01 | 0.35 ± 0.03 | 0.35 ± 0.05 | 0.35 ± 0.06 | 0.35 ± 0.04 | |
| Zeta potential (mV) | −0.62 ± 0.02 | −0.63 ± 0.04 | −0.62 ± 0.07 | −0.61 ± 0.05 | −0.62 ± 0.04 |
| Sample Code | Oregano Essential Oil (g/mL) | Polysorbate 80 (g/mL) |
|---|---|---|
| S1 | 0.02 | 0.02 |
| S2 | 0.04 | 0.04 |
| S3 | 0.02 | 0.06 |
| S4 | 0.02 | 0.04 |
| S5 | 0.04 | 0.02 |
| S6 | 0.06 | 0.02 |
| S7 | 0.02 | 0.08 |
| S8 | 0.02 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranauskaite, J.; Ockun, M.A.; Uner, B.; Tas, C.; Ivanauskas, L. Effect of the Amount of Polysorbate 80 and Oregano Essential Oil on the Emulsion Stability and Characterization Properties of Sodium Alginate Microcapsules. Molecules 2021, 26, 6304. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26206304
Baranauskaite J, Ockun MA, Uner B, Tas C, Ivanauskas L. Effect of the Amount of Polysorbate 80 and Oregano Essential Oil on the Emulsion Stability and Characterization Properties of Sodium Alginate Microcapsules. Molecules. 2021; 26(20):6304. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26206304
Chicago/Turabian StyleBaranauskaite, Juste, Mehmet Ali Ockun, Burcu Uner, Cetin Tas, and Liudas Ivanauskas. 2021. "Effect of the Amount of Polysorbate 80 and Oregano Essential Oil on the Emulsion Stability and Characterization Properties of Sodium Alginate Microcapsules" Molecules 26, no. 20: 6304. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26206304