Fractionation of Regenerated Silk Fibroin and Characterization of the Fractions
Abstract
:1. Introduction
2. Results and Discussion
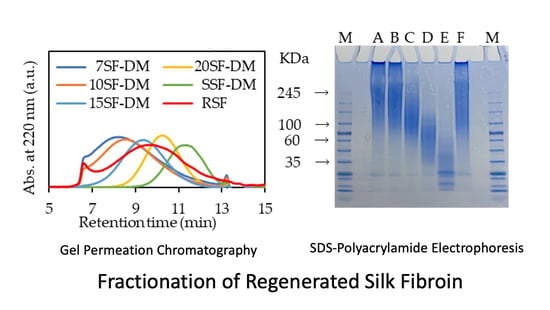
2.1. Influences of Methods on SF Fractionation
2.2. Characterization of Films from Fractionated SFs
2.2.1. Surface Properties
2.2.2. Secondary Structure
2.2.3. Cell Proliferation Test
2.3. Fabrication of Fractionated SFs
2.3.1. Nanofiber Nonwoven Mat
2.3.2. Porous 3D Structure (Sponge)
3. Materials and Methods
3.1. Preparation of RSF Aqueous Solution
3.2. Fractionation with Ammonium Sulfate (AS)
3.3. Fabrication of SF
3.4. Determination of Molecular Weight (MW)
3.5. Amino Acid Compositions Analysis
3.6. Characterizations
3.6.1. FTIR
3.6.2. Mechanical Tests
3.6.3. Viscosity
3.6.4. Water Contact Angle
3.6.5. Zeta Potential
3.6.6. Scanning Electron Microscopy (SEM)
3.6.7. Cell Culture
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Fine, N.A.; Lehfeldt, M.; Gross, J.E.; Downey, S.; Kind, G.M.; Duda, G.; Kulber, D.; Horan, R.; Ippolito, J.; Jewell, M. SERI Surgical Scaffold, Prospective Clinical Trial of a Silk-Derived Biological Scaffold in Two-Stage Breast Reconstruction: 1-Year Data. Plast. Reconstr. Surg. 2015, 135, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Meinel, L.; Hofmann, S.; Malhotra, A.; Volloch, V.; Kaplan, D. Bone morphogenetic protein-2 decorated silk fibroin films induce osteogenic differentiation of human bone marrow stromal cells. J. Biomed. Mater. Res. A 2004, 71, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kluge, J.A.; Leisk, G.G.; Kaplan, D.L. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials 2008, 29, 1054–1064. [Google Scholar] [CrossRef] [Green Version]
- Kambe, Y.; Kojima, K.; Tamada, Y.; Tomita, N.; Kameda, T. Silk fibroin sponges with cell growth-promoting activity induced by genetically fused basic fibroblast growth factor. J. Biomed. Mater. Res. A 2016, 104, 82–93. [Google Scholar] [CrossRef]
- Min, B.M.; Lee, G.; Kim, S.H.; Nam, Y.S.; Lee, T.S.; Park, W.H. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials 2004, 25, 1289–1297. [Google Scholar] [CrossRef]
- Lamboni, L.; Gauthier, M.; Yang, G.; Wang, Q. Silk sericin: A versatile material for tissue engineering and drug delivery. Biotechnol. Adv. 2015, 33, 1855–1867. [Google Scholar] [CrossRef]
- Shimura, K. Chemical composition and biosynthesis of silk proteins. Experientia 1983, 39, 455–461. [Google Scholar] [CrossRef]
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S. Silk Fibroin of Bombyx mori Is Secreted, Assembling a High Molecular Mass Elementary Unit Consisting of H-Chain, L-Chain, and P25, with a 6:6:1 Molar Ratio. J. Biol. Chem. 2000, 275, 40517–40528. [Google Scholar] [CrossRef] [Green Version]
- Dou, H.; Zuo, B. Effect of sodium carbonate concentrations on the degumming and regeneration process of silk fibroin. J. Text. Inst. 2015, 106, 311–319. [Google Scholar] [CrossRef]
- Mathur, A.B.; Tonelli, A.; Rathke, T.; Hudson, S. The Dissolution and Characterization of Bombyx mori Silk Fibroin in Calcium Nitrate–Methanol Solution and the Regeneration of Films. Biopolymers 1997, 42, 61–74. [Google Scholar] [CrossRef]
- Ajisawa, A. Studies on the dissolution of silk fibroin. Sen-i Gakkaishi 1968, 24, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Tsukada, M.; Goto, Y.; Minoura, N. Characterization of the regenerated silk fibroin from Bombyx mori. J. Seric. Sci. Jpn. 1990, 59, 325–330. [Google Scholar]
- Nunes, R.W.; Martin, J.R.; Johnson, J.F. Influence of Molecular Weight and Molecular Weight Distribution on Mechanical Properties of Polymers. Polym. Eng. Sci. 1982, 22, 205–228. [Google Scholar] [CrossRef]
- Montfort, J.P.; Marin, G.; Monge, P. Molecular Weight Distribution Dependence of the Viscoelastic Properties of Linear Polymers: The Coupling of Reptation and Tube-Renewal Effects. Macromolecules 1986, 19, 1979–1988. [Google Scholar] [CrossRef]
- Zhang, J.; Yamagishi, N.; Gotoh, Y.; Potthast, A.; Rosenau, T. High performance cellulose fibers regenerated from 1-butyl-3-methylimidazolium chloride solution: Effects of viscosity and molecular weight. J. Appl. Polym. Sci. 2019, 137, 48681. [Google Scholar] [CrossRef]
- Michud, A.; Hummel, M.; Sixta, H. Influence of molar mass distribution on the final properties of fibers regenerated from cellulose dissolved in ionic liquid by dry-jet wet spinning. Polymer 2015, 75, 1–9. [Google Scholar] [CrossRef]
- Shen, H.W.; Xie, B.H.; Yang, W.; Yang, M.B. Thermal and Rheological Properties of Polyethylene Blends with Bimodal Molecular Weight Distribution. J. Appl. Polym. Sci. 2013, 129, 2145–2151. [Google Scholar] [CrossRef]
- Tanaka, K.; Takahara, A.; Kajiyama, T. Effect of Polydispersity on Surface Molecular Motion of Polystyrene Films. Macromolecules 1997, 30, 6626–6632. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.; Kim, C. Effects of molecular weight distribution on the spinodal temperature of polymer mixtures. Polym. Int. 2004, 53, 2059–2065. [Google Scholar] [CrossRef]
- Xu, S.; Trujillo, F.J.; Xu, J.; Boyer, C.; Corrigan, N. Influence of Molecular Weight Distribution on the Thermoresponsive Transition of Poly(N-isopropylacrylamide). Macromol. Rapid Commun. 2021, 42, e2100212. [Google Scholar] [CrossRef]
- Nadgorny, M.; Gentekos, D.T.; Xiao, Z.; Singleton, S.P.; Fors, B.P.; Connal, L.A. Manipulation of Molecular Weight Distribution Shape as a New Strategy to Control Processing Parameters. Macromol. Rapid Commun. 2017, 38, 1700352. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Morikawa, H.; Yamanaka, S.; Tamada, Y. Electrospinning of silk fibroin from all aqueous solution at low concentration. Mater. Sci. Eng. C 2017, 73, 498–506. [Google Scholar] [CrossRef]
- Cho, H.J.; Yoo, Y.J.; Kim, J.W.; Park, Y.H.; Bae, D.G.; Um, I.C. Effect of molecular weight and storage time on the wet- and electro-spinning of regenerated silk fibroin. Polym. Degrad. Stab. 2012, 97, 1060–1066. [Google Scholar] [CrossRef]
- Zeng, D.M.; Pan, J.J.; Wang, Q.; Liu, X.F.; Wang, H.; Zhang, K.Q. Controlling silk fibroin microspheres via molecular weight distribution. Mater. Sci. Eng. C 2015, 50, 226–233. [Google Scholar] [CrossRef]
- Kim, H.H.; Song, D.W.; Kim, M.J.; Ryu, S.J.; Um, I.C.; Ki, C.S.; Park, Y.H. Effect of silk fibroin molecular weight on physical property of silk hydrogel. Polymer 2016, 90, 26–33. [Google Scholar] [CrossRef]
- Hofmeister, F. Zur Lehre von der Wirkung der Salze. Arch. Exp. Pathol. Pharmakol. 1888, 24, 247–260. [Google Scholar] [CrossRef] [Green Version]
- Kunz, W.; Henle, J.; Ninham, B.W. ‘Zur Lehre von der Wirkung der Salze’ (about the science of the effect of salts): Franz Hofmeister’s historical papers. Curr. Opin. Colloid Interface Sci. 2004, 9, 19–37. [Google Scholar] [CrossRef]
- Eursakun, S.; Simsiriwong, P.; Ratanabanangkoon, K. Studies on the fractionation of equine antivenom IgG by combinations of ammonium sulfate and caprylic acid. Toxicon 2012, 60, 1022–1029. [Google Scholar] [CrossRef]
- Sha, X.M.; Tu, Z.C.; Liu, W.; Wang, H.; Shi, Y.; Huang, T.; Man, Z.Z. Effect of ammonium sulfate fractional precipitation on gel strength and characteristics of gelatin from bighead carp (Hypophthalmichthys nobilis) scale. Food Hydrocoll. 2014, 36, 173–180. [Google Scholar] [CrossRef]
- Shimura, K.; KIKUCHI, A.; Ohtomo, K.; Katagata, Y.; Hyodo, A. Studies on Silk Fibroin of Bombyx mori. J. Biochem. 1976, 80, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Schein, C.H. Solubility as a Function of Protein Structure and Solvent Components. Nat. Biotechnol. 1990, 8, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Ohmi, S. Saibou Kougaku shi; Gakken Medical Shujunsha Co., Ltd.: Tokyo, Japan, 1996; pp. 856–861. [Google Scholar]
- Zhou, C.Z.; Confalonieri, F.; Jacquet, M.; Perasso, R.; Li, Z.G.; Janin, J. Silk Fibroin: Structural Implications of a Remarkable Amino Acid Sequence. Proteins 2001, 44, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Marsh, R.E.; Corey, R.B.; Pauling, L. An Investigation of the Structure of Silk Fibroin. Biochim. Biophys. Acta 1955, 16, 1–34. [Google Scholar] [CrossRef]
- Asakura, T.; Kuzuhara, A.; Tabeta, R.; Saito, H. Conformation Characterization of Bombyx mori Silk Fibroin in the Solid State by High-Frequency 13C Cross Polarization-Magic Angle Spinning NMR, X-ray Diffraction, and Infrared Spectroscopy. Macromolecules 1985, 18, 1841–1845. [Google Scholar] [CrossRef]
- Saito, H.; Iwanaga, Y.; Tabeta, R.; Narita, M.; Asakura, T. A High Resolution 13C NMR Study of Silk Fibroin in Solid State by the Cross Polarization-magic Angle Spinning Method: Conformational Characterization Utilizing Conformation-dependent 13C Chemical Shifts. Chem. Lett. 1983, 12, 427–430. [Google Scholar] [CrossRef]
- Zhao, C.; Asakura, T. Structure of Silk studied with NMR. Prog. Nucl. Magn. Reson. Spectrosc. 2001, 39, 301–352. [Google Scholar] [CrossRef]
- Lu, Q.; Hu, X.; Wang, X.; Kluge, J.A.; Lu, S.; Cebe, P.; Kaplan, D.L. Water-Insoluble Silk Films with Silk I Structure. Acta Biomater. 2010, 6, 1380–1387. [Google Scholar] [CrossRef] [Green Version]
- Tretinnikov, O.N.; Tamada, Y. Influence of Casting Temperature on the Near-Surface Structure and Wettability of Cast Silk Fibroin Films. Langmuir 2001, 17, 7406–7413. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Vepari, C.; Kaplan, D.L. Silk as a Biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliver. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- Park, B.K.; Um, I.C. Effect of molecular weight on electro-spinning performance of regenerated silk. Int. J. Biol. Macromol. 2018, 106, 1166–1172. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Shao, H.; Hu, X. Electrospun ultra-fine silk fibroin fibers from aqueous solutions. J. Mater. Sci. 2005, 40, 5359–5363. [Google Scholar] [CrossRef]
- Tamada, Y. New Process to Form a Silk Fibroin Porous 3-D Structure. Biomacromolecules 2005, 6, 3100–3106. [Google Scholar] [CrossRef]
- Navas, A.A. Use of a Programmable Pocket Calculator for Data Reduction in GPC: Calculation of Molecular Weights and Molecular Weight Distribution. J. Liq. Chromatogr. Relat. Technol. 2006, 5, 413–423. [Google Scholar] [CrossRef]
- Gavin, W. GPC-Gel Permeation Chromatography aka Size Exclusion Chromatography–SEC. Available online: https://crf.uml.edu/gc.php?u=%2Ffmi%2Fxml%2Fcnt%2Fgpc-Training-2.pdf%3F-db%3DUML_CoreResearchFacilities%26-lay%3DPHP_Resource%26-recid%3D1696%26-field%3Dresource_DOCUMENT%3A%3ADocument%281%29.1639 (accessed on 25 September 2021).
- DJK Corp. Available online: https://www.djklab.com/parts/service/pdf/GPC-1.pdf (accessed on 25 September 2021).
- Tamada, Y.; Ikada, Y. Cell Attachment to Various Polymer Surfaces. In Polymers in Medicine II; Chiellini, E., Giusti, P., Migliaresi, C., Nicolais, L., Eds.; Springer: Boston, MA, USA, 1986; Volume 34, pp. 101–115. [Google Scholar]







| (A) | ||||||||
| Sample | Mn | Mw | PDI | Yield (%) | ||||
| Avg. | Std. | Avg. | Std. | Avg. | Std. | Avg. | Std. | |
| 7SF-AM | 75,000 | 9600 | 190,000 | 20,000 | 2.5 | 0.44 | 17.0 | 8.1 |
| 10SF-AM | 70,000 | 8900 | 140,000 | 24,000 | 2.0 | 0.14 | 13.5 | 8.9 |
| 15SF-AM | 46,000 | 4400 | 76,000 | 8100 | 1.7 | 0.10 | 8.16 | 8.6 |
| 20SF-AM | 32,000 | 5600 | 44,000 | 8300 | 1.4 | 0.09 | 3.76 | 1.3 |
| SSF-AM | 15,000 | 6000 | 19,000 | 8700 | 1.2 | 0.08 | 0.98 | 0.56 |
| RSF | 52,000 | 1500 | 140,000 | 12,000 | 2.6 | 0.29 | ||
| Total | 43.4 | 13.9 | ||||||
| (B) | ||||||||
| Sample | Mn | Mw | PDI | Yield (%) | ||||
| Avg. | Std. | Avg. | Std. | Avg. | Std. | Avg. | Std. | |
| 7SF-DM | 66,000 | 2900 | 160,000 | 11,000 | 2.3 | 0.13 | 15.8 | 2.0 |
| 10SF-DM | 70,000 | 9000 | 140,000 | 11,000 | 2.1 | 0.13 | 25.5 | 15.1 |
| 15SF-DM | 51,000 | 11,000 | 82,000 | 15,000 | 1.6 | 0.14 | 11.1 | 3.7 |
| 20SF-DM | 35,000 | 4300 | 48,000 | 3700 | 1.4 | 0.12 | 9.11 | 2.83 |
| SSF-DM | 17,000 | 4500 | 21,000 | 6400 | 1.2 | 0.06 | 1.66 | 0.63 |
| RSF | 54,000 | 9100 | 130,000 | 15,000 | 2.4 | 0.14 | ||
| Total | 67.7 | 16.5 | ||||||
| 7SF-DM | 10SF-DM | 15SF-DM | 20SF-DM | RSF | |
|---|---|---|---|---|---|
| Gly | 3.48 | 3.33 | 3.80 | 4.72 | 3.52 |
| Ala | 1.64 | 1.57 | 1.72 | 1.96 | 1.68 |
| Ser + Tyr | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Sample | 7SF-DM2, MPa | 20SF-DM2, MPa | RSF, MPa |
|---|---|---|---|
| SF 2% | 1.2 ± 0.09 | 1.1 ± 0.06 | 1.1 ± 0.1 |
| SF 4% | 1.3 ± 0.1 | 1.5 ± 0.05 | 1.2 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoki, M.; Masuda, Y.; Ishikawa, K.; Tamada, Y. Fractionation of Regenerated Silk Fibroin and Characterization of the Fractions. Molecules 2021, 26, 6317. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26206317
Aoki M, Masuda Y, Ishikawa K, Tamada Y. Fractionation of Regenerated Silk Fibroin and Characterization of the Fractions. Molecules. 2021; 26(20):6317. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26206317
Chicago/Turabian StyleAoki, Masaaki, Yu Masuda, Kota Ishikawa, and Yasushi Tamada. 2021. "Fractionation of Regenerated Silk Fibroin and Characterization of the Fractions" Molecules 26, no. 20: 6317. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26206317