Prospects for More Efficient Multi-Photon Absorption Photosensitizers Exhibiting Both Reactive Oxygen Species Generation and Luminescence
Abstract
:1. Introduction
1.1. TPA and Its Applications
1.2. Evaluation of Parameters Relevant for the Use of Photosensitizers
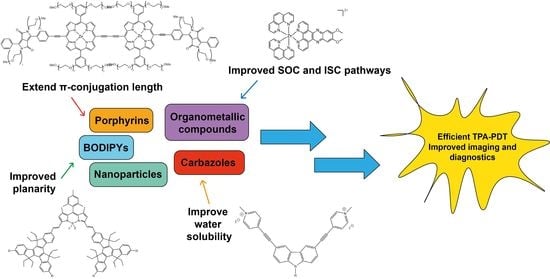
2. Modifications of Photosensitizers for Improved Two-Photon Absorption
- The presence of a long π-conjugated system with enforced co-planarity ensures large conjugation lengths to provide ease of delocalization of π-electrons;
- Donor (D) and acceptor (A) groups located at the ends of the molecule and also possibly at its center (giving e.g., A-π-D or A-π-D-π-A or D-π-A-π-D structures where -π- denotes the conjugated linker) to provide dipolar or multipolar structures (quadrupolar, octupolar etc.) and large transition dipole moments;
- Narrow one-photon and two-photon absorption bands.
3. Two-Photon Absorption Molecules: Small Molecules
3.1. Highly Branched Molecules
3.2. Zwitterion Molecules
3.3. Small Molecule-Based Salts
3.4. Organometallic Molecules
4. Two-Photon Absorption Molecules: Large Molecules
4.1. Dimers
4.2. Dendrimers
4.3. BODIPYs
4.4. FRET Systems
4.5. Large PEGylated Photosensitizers
5. Nanoparticles as Two-Photon Absorption Species
5.1. Polymer Nanoparticles
5.2. Gold Nanoparticles
5.2.1. Gold Nanorods
5.2.2. Gold Nanoclusters
5.2.3. Gold Nanobipyramids
5.3. Hybrid Nanoparticles
5.4. Nanocontainers with Nanoparticles and Photosensitizers
6. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rumi, M.; Perry, J.W. Two-photon absorption: An overview of measurements and principles. Adv. Opt. Photonics 2010, 2. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Mutoh, K.; Abe, J. Stepwise two-photon absorption processes utilizing photochromic reactions. J. Photochem. Photobiol. C Photochem. Rev. 2018, 34, 2–28. [Google Scholar] [CrossRef]
- Pawlicki, M.; Collins, H.A.; Denning, R.G.; Anderson, H.L. Two-photon absorption and the design of two-photon dyes. Angew. Chem. Int. Ed. Engl. 2009, 48, 3244–3266. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, W.; Garrett, C.G.B. Two-Photon Excitation in CaF2:Eu2+. Phys. Rev. Lett. 1961, 7, 229–231. [Google Scholar] [CrossRef]
- Shen, Y.; Shuhendler, A.J.; Ye, D.; Xu, J.J.; Chen, H.Y. Two-photon excitation nanoparticles for photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6725–6741. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, L.K.; Bryant, H.E.; Weinstein, J.A. Transition metal complexes as photosensitisers in one-and two-photon photodynamic therapy. Coord. Chem. Rev. 2019, 379, 2–29. [Google Scholar] [CrossRef] [Green Version]
- Gu, B.; Wu, W.; Xu, G.; Feng, G.; Yin, F.; Chong, P.H.J.; Qu, J.; Yong, K.T.; Liu, B. Precise two-photon photodynamic therapy using an efficient photosensitizer with aggregation-induced emission characteristics. Adv. Mater. 2017, 29, 1701076. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, B.H.; Ananthavel, S.P.; Barlow, S.; Dyer, D.L.; Ehrlich, J.E.; Erskine, L.L.; Heikal, A.A.; Kuebler, S.M.; Lee, I.Y.S.; McCord-Maughon, D.; et al. Two-photon polymerization initiators for three-dimensional optical data storage and microfabrication. Nature 1999, 398, 51–54. [Google Scholar] [CrossRef]
- Zhang, Q.; Yue, S.; Sun, H.; Wang, X.; Hao, X.; An, S. Nondestructive up-conversion readout in Er/Yb co-doped Na0.5Bi2.5Nb2O9-based optical storage materials for optical data storage device applications. J. Mater. Chem. C 2017, 5, 3838–3847. [Google Scholar] [CrossRef]
- Liu, J.-C.; Li, X.-Z.; Zhang, Y. Two-photon absorption induced optical power limiting behavior of strong femtosecond hyper-Gaussian pulses. In Proceedings of the SPIE/COS Photonics Asia, Beijing, China, 3 November 2016; Proc. SPIE: San Diego, CA, USA, 2016; p. 100291H. [Google Scholar] [CrossRef]
- Chang, Z.F.; Jing, L.M.; Chen, B.; Zhang, M.; Cai, X.; Liu, J.J.; Ye, Y.C.; Lou, X.; Zhao, Z.; Liu, B.; et al. Rational design of asymmetric red fluorescent probes for live cell imaging with high AIE effects and large two-photon absorption cross sections using tunable terminal groups. Chem. Sci. 2016, 7, 4527–4536. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Tian, X.; Zhou, H.; Wu, J.; Tian, Y. Lighting the Way to See Inside Two-Photon Absorption Materials: Structure-Property Relationship and Biological Imaging. Materials 2017, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Arita, Y.; Matsuo, R.; Kawaguchi, H.; Miyamoto, K.; Dholakia, K.; Omatsu, T. Photopolymerization with Light Fields Possessing Orbital Angular Momentum: Generation of Helical Microfibers. In Proceedings of the Lasers and Electro-Optics Europe & European Quantum Electronics Conference (CLEO/Europe-EQEC), Munich, Germany, 23–27 June 2019; ACS Photonics: Washington, DC, USA, 2019; p. 11. [Google Scholar] [CrossRef]
- Xu, X.; Madrigal, J.B.; Broussier, A.; Lio, G.E.; Geoffray, F.; Issa, A.; Jradi, S.; Bachelot, R.; Couteau, C.; Blaize, S. Quantum emitters based on polymeric structures embedded with quantum dots fabricated via photo-polymerization. In Advanced Fabrication Technologies for Micro/Nano Optics and Photonics XIII, Proceeding of SPIE OPTO, San Francisco, CA, USA, 28 February 2020; Proc. SPIE: San Francisco, CA, USA, 2020; p. 112920O. [Google Scholar] [CrossRef]
- Niesler, F.; Hermatschweiler, M. Two-Photon Polymerization - A Versatile Microfabrication Tool. Opt. Photonik. 2016, 11, 54–57. [Google Scholar] [CrossRef]
- Lemma, E.D.; Spagnolo, B.; De Vittorio, M.; Pisanello, F. Studying Cell Mechanobiology in 3D: The Two-Photon Lithography Approach. Trends Biotechnol. 2019, 37, 358–372. [Google Scholar] [CrossRef]
- Scarpa, E.; Lemma, E.D.; Fiammengo, R.; Cipolla, M.P.; Pisanello, F.; Rizzi, F.; De Vittorio, M. Microfabrication of pH-responsive 3D hydrogel structures via two-photon polymerization of high-molecular-weight poly(ethylene glycol) diacrylates. Sens. Actuators B Chem. 2019, 279, 418–426. [Google Scholar] [CrossRef]
- Kikuchi, K. Highly sensitive interferometric autocorrelator using Si avalanche photodiode as two-photon absorber. Electron. Lett. 1998, 34. [Google Scholar] [CrossRef]
- Hayakawa, R.; Ishikura, N.; Nguyen, H.C.; Baba, T. Two-photon-absorption photodiodes in Si photonic-crystal slow-light waveguides. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef] [Green Version]
- Homann, C.; Krebs, N.; Riedle, E. Convenient pulse length measurement of sub-20-fs pulses down to the deep UV via two-photon absorption in bulk material. Appl. Phys. B 2011, 104, 783–791. [Google Scholar] [CrossRef]
- Fan, W.; Huang, P.; Chen, X. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6488–6519. [Google Scholar] [CrossRef] [PubMed]
- Mazur, L.M.; Roland, T.; Leroy-Lhez, S.; Sol, V.; Samoc, M.; Samuel, I.D.W.; Matczyszyn, K. Efficient Singlet Oxygen Photogeneration by Zinc Porphyrin Dimers upon One- and Two-Photon Excitation. J Phys. Chem. B 2019, 123, 4271–4277. [Google Scholar] [CrossRef] [Green Version]
- Frochot, C.; Mordon, S. Update of the situation of clinical photodynamic therapy in Europe in the 2003–2018 period. J. Porphyr. Phthalocyanines 2019, 23, 347–357. [Google Scholar] [CrossRef]
- dos Santos, A.F.; de Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic therapy in cancer treatment-an update review. J. Cancer Metastasis Treat. 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Sun, J.; Kormakov, S.; Liu, Y.; Huang, Y.; Wu, D.; Yang, Z. Recent progress in metal-based nanoparticles mediated photodynamic therapy. Molecules 2018, 23, 1704. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.-K.; Park, C.-H. Clinical efficacy of photodynamic therapy. Obstet. Gynecol. Sci. 2016, 59, 479. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy–mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Makarov, N.S.; Drobizhev, M.; Wicks, G.; Makarova, E.A.; Lukyanets, E.A.; Rebane, A. Alternative selection rules for one-and two-photon transitions in tribenzotetraazachlorin: Quasi-centrosymmetrical π-conjugation pathway of formally non-centrosymmetrical molecule. J. Chem. Phys. 2013, 138, 214314. [Google Scholar] [CrossRef] [Green Version]
- Bhawalkar, J.; Kumar, N.; Zhao, C.-F.; Prasad, P. Two-photon photodynamic therapy. J. Clin. Laser Med. Surg. 1997, 15, 201–204. [Google Scholar] [CrossRef]
- Rocha, L.B.; Soares, H.T.; Mendes, M.I.P.; Cabrita, A.; Schaberle, F.A.; Arnaut, L.G. Necrosis Depth and Photodynamic Threshold Dose with Redaporfin-PDT. Photochem. Photobiol. 2020, 96, 692–698. [Google Scholar] [CrossRef]
- Paschotta, R. Encyclopedia of Laser Physics and Technology, Vol. 1; Wiley Online Library: Weinheim, Germany, 2008. [Google Scholar]
- Vivas, M.G.; De Boni, L.; Mendonça, C.R. Molecular and Laser Spectroscopy: Advances and Applications; Gupta, V.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 165–191. [Google Scholar]
- GeorgeáTruscott, T.; Edward, J. Effect of oxygen-enhanced intersystem crossing on the observed efficiency of formation of singlet oxygen. J. Chem. Soc. Faraday Trans. 1990, 86, 3075–3080. [Google Scholar] [CrossRef]
- McClure, D.S. Spin-orbit interaction in aromatic molecules. J. Chem. Phys. 1952, 20, 682–686. [Google Scholar] [CrossRef]
- Marian, C.M. Spin–orbit coupling and intersystem crossing in molecules. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 187–203. [Google Scholar] [CrossRef]
- Koziar, J.C.; Cowan, D.O. Photochemical heavy-atom effects. Acc. Chem. Res. 1978, 11, 334–341. [Google Scholar] [CrossRef]
- Ohulchanskyy, T.Y.; Donnelly, D.J.; Detty, M.R.; Prasad, P.N. Heteroatom substitution induced changes in excited-state photophysics and singlet oxygen generation in chalcogenoxanthylium dyes: Effect of sulfur and selenium substitutions. J. Phys. Chem. B 2004, 108, 8668–8672. [Google Scholar] [CrossRef]
- Wang, C.; Abbas, M.; Wantz, G.; Kawabata, K.; Takimiya, K. “Heavy-atom effects” in the parent [1] benzochalcogenopheno [3, 2-b][1] benzochalcogenophene system. J. Mater. Chem. C 2020, 8, 15119–15127. [Google Scholar] [CrossRef]
- Martinez, V.; Henary, M. Nile red and Nile blue: Applications and syntheses of structural analogues. Chem.-A Eur. J. 2016, 22, 13764–13782. [Google Scholar] [CrossRef]
- Petri, A.; Yova, D.; Alexandratou, E.; Kyriazi, M.; Rallis, M. Comparative characterization of the cellular uptake and photodynamic efficiency of Foscan® and Fospeg in a human prostate cancer cell line. Photodiagnosis Photodyn. Ther. 2012, 9, 344–354. [Google Scholar] [CrossRef]
- da Silva, C.L.; Del Ciampo, J.O.; Rossetti, F.C.; Bentley, M.V.L.B.; Pierre, M.B.R. PLGA nanoparticles as delivery systems for protoporphyrin IX in topical PDT: Cutaneous penetration of photosensitizer observed by fluorescence microscopy. J. Nanosci. Nanotechnol. 2013, 13, 6533–6540. [Google Scholar] [CrossRef]
- Chen, K. Photophysical Characterization and Optimization of Novel Polymer Based Photosensitizer Carrier Systems for PDT. Ph.D. Thesis, Humboldt University of Berlin, Berlin, Germany, 22 June 2010. [Google Scholar] [CrossRef]
- Yoon, I.; Li, J.Z.; Shim, Y.K. Advance in photosensitizers and light delivery for photodynamic therapy. Clin. Endosc. 2013, 46, 7–23. [Google Scholar] [CrossRef]
- Xu, C.; Webb, W.W. Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm. JOSA B 1996, 13, 481–491. [Google Scholar] [CrossRef]
- Mertz, J.; Xu, C.; Webb, W. Single-molecule detection by two-photon-excited fluorescence. Opt. Lett. 1995, 20, 2532–2534. [Google Scholar] [CrossRef]
- Drobizhev, M.; Tillo, S.; Makarov, N.; Hughes, T.; Rebane, A. Absolute two-photon absorption spectra and two-photon brightness of orange and red fluorescent proteins. J. Phys. Chem. B 2009, 113, 855–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makarov, N.S.; Drobizhev, M.; Rebane, A. Two-photon absorption standards in the 550-1600 nm excitation wavelength range. Opt Express. 2008, 16, 4029–4047. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sheik-Bahae, M.; Said, A.A.; Hagan, D.J.; Van Stryland, E.W. Time-resolved Z-scan measurements of optical nonlinearities. J. Opt. Soc. Am. B 1994, 11. [Google Scholar] [CrossRef] [Green Version]
- Sheik-Bahae, M.; Said, A.A.; Wei, T.-H.; Wu, Y.-Y.; Hagan, D.J.; Soileau, M.; Van Stryland, E.W. Z-Scan: A Simple and Sensitive Technique for Nonlinear Refraction Measurements. In Nonlinear Optical Properties of Materials, Proceeding of the 33rd Annual Technical Symposium, San Jose, CA, USA, 4 January 1990; Proc. SPIE: San Jose, CA, USA, 1990; pp. 41–51. [Google Scholar] [CrossRef]
- DeSalvo, R.; Sheik-Bahae, M.; Said, A.; Hagan, D.J.; Van Stryland, E.W. Z-scan measurements of the anisotropy of nonlinear refraction and absorption in crystals. Opt. Lett. 1993, 18, 194–196. [Google Scholar] [CrossRef]
- Sheik-Bahae, M.; Wang, J.; DeSalvo, R.; Hagan, D.; Van Stryland, E. Measurement of nondegenerate nonlinearities using a two-color Z scan. Opt. Lett. 1992, 17, 258–260. [Google Scholar] [CrossRef] [Green Version]
- Dinesh Babu, K.; Murali, K.; Karthikeyan, N.; Karuppusamy, S. Investigation of optical limiting and third-order optical nonlinear properties of 2-Nitroaniline by Z-scan and f-scan techniques. Laser Phys. 2019, 29. [Google Scholar] [CrossRef]
- Ajami, A.; Husinsky, W.; Tromayer, M.; Gruber, P.; Liska, R.; Ovsianikov, A. Measurement of degenerate two-photon absorption spectra of a series of developed two-photon initiators using a dispersive white light continuum Z-scan. Appl. Phys. Lett. 2017, 111. [Google Scholar] [CrossRef]
- Gu, B.; Fan, Y.-X.; Chen, J.; Wang, H.-T.; He, J.; Ji, W. Z-scan theory of two-photon absorption saturation and experimental evidence. J. Appl. Phys. 2007, 102. [Google Scholar] [CrossRef]
- Hu, Y.; Gu, B.; Wen, B.; Lv, C.; Rui, G.; He, J.; Cui, Y. Anisotropic two-photon absorbers measured by the Z-scan technique and its application in laser beam shaping. J. Opt. Soc. Am. B 2020, 37, 756–761. [Google Scholar] [CrossRef]
- Garcia, H.; Serna, J.; Rueda, E. Bulk ZnSe and CdS two-photon absorption measurement with an F-scan nonlinear absorption spectrometer. OSA Continuum. 2020, 3, 498–504. [Google Scholar] [CrossRef]
- Kolkowski, R.; Samoc, M. Modified Z-scan technique using focus-tunable lens. J. Optics. 2014, 16. [Google Scholar] [CrossRef]
- Rueda, E.; Serna, J.H.; Hamad, A.; Garcia, H. Two-photon absorption coefficient determination using the differential F-scan technique. Opt. Laser Technol. 2019, 119, 105584. [Google Scholar] [CrossRef] [Green Version]
- Cronstrand, P.; Luo, Y.; Ågren, H. Multi-photon absorption of molecules. Adv. Quantum Chem. 2005, 50, 1–21. [Google Scholar] [CrossRef]
- Wang, C.-K.; Macak, P.; Luo, Y.; Ågren, H. Effects of π centers and symmetry on two-photon absorption cross sections of organic chromophores. J. Chem. Phys. 2001, 114, 9813–9820. [Google Scholar] [CrossRef]
- Salem, M.; Gedik, M.; Brown, A. Two photon absorption in biological molecules. In Handbook of Computational Chemistry; Leszczynski, J., Kaczmarek-Kedziera, A., Puzyn, T., Papadopoulos, M.G., Reis, H., Shukla, M.K., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Nifosì, R.; Luo, Y. Predictions of novel two-photon absorption bands in fluorescent proteins. J. Phys. Chem. B 2007, 111, 14043–14050. [Google Scholar] [CrossRef]
- Salem, M.A.; Brown, A. Two-photon absorption in fluorescent protein chromophores: TDDFT and CC2 results. J. Chem. Theory Comput. 2014, 10, 3260–3269. [Google Scholar] [CrossRef] [PubMed]
- Karotki, A.; Kruk, M.; Drobizhev, M.; Rebane, A.; Nickel, E.; Spangler, C.W. Efficient singlet oxygen generation upon two-photon excitation of new porphyrin with enhanced nonlinear absorption. IEEE J. Sel. Top. Quantum Electron. 2001, 7, 971–975. [Google Scholar] [CrossRef]
- Arnbjerg, J.; Johnsen, M.; Frederiksen, P.K.; Braslavsky, S.E.; Ogilby, P.R. Two-photon photosensitized production of singlet oxygen: Optical and optoacoustic characterization of absolute two-photon absorption cross sections for standard sensitizers in different solvents. J. Phys. Chem. A. 2006, 110, 7375–7385. [Google Scholar] [CrossRef]
- Poulsen, T.D.; Frederiksen, P.K.; Jørgensen, M.; Mikkelsen, K.V.; Ogilby, P.R. Two-Photon Singlet Oxygen Sensitizers: Quantifying, Modeling, and Optimizing the Two-Photon Absorption Cross Section. J. Phys. Chem. A 2001, 105, 11488–11495. [Google Scholar] [CrossRef]
- Ishi-i, T.; Taguri, Y.; Kato, S.-i.; Shigeiwa, M.; Gorohmaru, H.; Maeda, S.; Mataka, S. Singlet oxygen generation by two-photon excitation of porphyrin derivatives having two-photon-absorbing benzothiadiazole chromophores. J. Mater. Chem. 2007, 17. [Google Scholar] [CrossRef]
- Pitre, S.P.; McTiernan, C.D.; Vine, W.; DiPucchio, R.; Grenier, M.; Scaiano, J.C. Visible-Light Actinometry and Intermittent Illumination as Convenient Tools to Study Ru(bpy)3Cl2 Mediated Photoredox Transformations. Sci. Rep. 2015, 5, 16397. [Google Scholar] [CrossRef]
- Darmanyan, A.P. Generation of 1O2 and the mechanism of internal conversion in 9,10-diphenylanthracene. Chem. Phys. Lett. 1982, 91, 396–400. [Google Scholar] [CrossRef]
- Schmitz, C.; Aubry, J.M.; Rigaudy, J. A new access to the anthracene core. Tetrahedron 1982, 38, 1425–1430. [Google Scholar] [CrossRef]
- Fatima, K.; Masood, N.; Luqman, S. Quenching of singlet oxygen by natural and synthetic antioxidants and assessment of electronic UV/Visible absorption spectra for alleviating or enhancing the efficacy of photodynamic therapy. Biomed. Res. Ther. 2016, 3. [Google Scholar] [CrossRef]
- Hartman, P.E.; Hartman, Z.; Ault, K.T. Scavenging of singlet molecular oxygen by imidazole compounds: High and sustained activities of carboxy terminal histidine dipeptides and exceptional activity of imidazole-4-acetic acid. Photochem. Photobiol. 1990, 51, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Linetsky, M.; Ortwerth, B.J. Quantitation of the singlet oxygen produced by UVA irradiation of human lens proteins. Photochem. Photobiol. 1997, 65, 522–529. [Google Scholar] [CrossRef]
- Lion, Y.; Delmelle, M.; Van de Vorst, A. New method of detecting singlet oxygen production. Nature 1976, 263, 442–443. [Google Scholar] [CrossRef]
- Abbas, K.; Babić, N.; Peyrot, F. Use of spin traps to detect superoxide production in living cells by electron paramagnetic resonance (EPR) spectroscopy. Methods 2016, 109, 31–43. [Google Scholar] [CrossRef]
- Nakamura, K.; Ishiyama, K.; Ikai, H.; Kanno, T.; Sasaki, K.; Niwano, Y.; Kohno, M. Reevaluation of analytical methods for photogenerated singlet oxygen. J. Clin. Biochem. Nutr. 2011, 1107120096. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Liu, Y.; Wamer, W.G.; Yin, J.-J. Electron spin resonance spectroscopy for the study of nanomaterial-mediated generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 49–63. [Google Scholar] [CrossRef]
- Fan, J.; Qin, H.; Jiang, S. Mn-doped g-C3N4 composite to activate peroxymonosulfate for acetaminophen degradation: The role of superoxide anion and singlet oxygen. Chem. Eng. J. 2019, 359, 723–732. [Google Scholar] [CrossRef]
- Igarashi, T.; Sakurai, K.; Oi, T.; Obara, H.; Ohya, H.; Kamada, H. New sensitive agents for detecting singlet oxygen by electron spin resonance spectroscopy. Free Radic. Biol. Med. 1999, 26, 1339–1345. [Google Scholar] [CrossRef]
- Jung, M.Y.; Choi, D.S. Electron spin resonance and luminescence spectroscopic observation and kinetic study of chemical and physical singlet oxygen quenching by resveratrol in methanol. J. Agric. Food Chem. 2010, 58, 11888–11895. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prasad, A.; Pospíšil, P. Formation of α-tocopherol hydroperoxide and α-tocopheroxyl radical: Relevance for photooxidative stress in Arabidopsis. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Marchand, G.; Calliste, C.A.; Williams, R.M.; McLure, C.; Leroy-Lhez, S.; Villandier, N. Acetylated lignins: A potential bio-sourced photosensitizer. ChemistrySelect 2018, 3, 5512–5516. [Google Scholar] [CrossRef]
- Ando, T.; Yoshikawa, T.; Tanigawa, T.; Kohno, M.; Yoshida, N.; Kondo, M. Quantification of singlet oxygen from hematoporphyrin derivative by electron spin resonance. Life Sci. 1997, 61, 1953–1959. [Google Scholar] [CrossRef]
- Matsumura, Y.; Iwasawa, A.; Kobayashi, T.; Kamachi, T.; Ozawa, T.; Kohno, M. Detection of high-frequency ultrasound-induced singlet oxygen by the ESR spin-trapping method. Chem. Lett. 2013, 42, 1291–1293. [Google Scholar] [CrossRef]
- Myung Kim, H.; Rae Cho, B. Two-photon materials with large two-photon cross sections. Structure-property relationship. Chem. Commun. 2009, 153–164. [Google Scholar] [CrossRef]
- Terenziani, F.; Katan, C.; Badaeva, E.; Tretiak, S.; Blanchard-Desce, M. Enhanced Two-Photon Absorption of Organic Chromophores: Theoretical and Experimental Assessments. Adv. Mater. 2008, 20, 4641–4678. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Zhang, J.; Yin, L.; Long, X.; Zhang, W.; Zhang, Q. Recent progress in efficient organic two-photon dyes for fluorescence imaging and photodynamic therapy. J. Mater. Chem. C 2020, 8, 6342–6349. [Google Scholar] [CrossRef]
- Mongin, O.; Hugues, V.; Blanchard-Desce, M.; Merhi, A.; Drouet, S.; Yao, D.; Paul-Roth, C. Fluorenyl porphyrins for combined two-photon excited fluorescence and photosensitization. Chem. Phys. Lett. 2015, 625, 151–156. [Google Scholar] [CrossRef]
- Oar, M.A.; Serin, J.M.; Dichtel, W.R.; Fréchet, J.M.J.; Ohulchanskyy, T.Y.; Prasad, P.N. Photosensitization of Singlet Oxygen via Two-Photon-Excited Fluorescence Resonance Energy Transfer in a Water-Soluble Dendrimer. Chem. Mater. 2005, 17, 2267–2275. [Google Scholar] [CrossRef]
- Huang, H.; Yu, B.; Zhang, P.; Huang, J.; Chen, Y.; Gasser, G.; Ji, L.; Chao, H. Highly Charged Ruthenium(II) Polypyridyl Complexes as Lysosome-Localized Photosensitizers for Two-Photon Photodynamic Therapy. Angew. Chem. Int. Ed. Engl. 2015, 54, 14049–14052. [Google Scholar] [CrossRef]
- McKenzie, L.K.; Sazanovich, I.V.; Baggaley, E.; Bonneau, M.; Guerchais, V.; Williams, J.A.; Weinstein, J.A.; Bryant, H.E. Metal Complexes for Two-Photon Photodynamic Therapy: A Cyclometallated Iridium Complex Induces Two-Photon Photosensitization of Cancer Cells under Near-IR Light. Chemistry 2017, 23, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Dahlstedt, E.; Collins, H.A.; Balaz, M.; Kuimova, M.K.; Khurana, M.; Wilson, B.C.; Phillips, D.; Anderson, H.L. One- and two-photon activated phototoxicity of conjugated porphyrin dimers with high two-photon absorption cross sections. Org. Biomol. Chem. 2009, 7, 897–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dy, J.T.; Ogawa, K.; Satake, A.; Ishizumi, A.; Kobuke, Y. Water-soluble self-assembled butadiyne-bridged bisporphyrin: A potential two-photon-absorbing photosensitizer for photodynamic therapy. Chemistry 2007, 13, 3491–3500. [Google Scholar] [CrossRef]
- Reinhardt, B.A.; Brott, L.L.; Clarson, S.J.; Dillard, A.G.; Bhatt, J.C.; Kannan, R.; Yuan, L.; He, G.S.; Prasad, P.N. Highly Active Two-Photon Dyes: Design, Synthesis, and Characterization toward Application. Chem. Mater. 1998, 10, 1863–1874. [Google Scholar] [CrossRef]
- Albota, M.; Beljonne, D.; Bredas, J.L.; Ehrlich, J.E.; Fu, J.Y.; Heikal, A.A.; Hess, S.E.; Kogej, T.; Levin, M.D.; Marder, S.R.; et al. Design of organic molecules with large two-photon absorption cross sections. Science 1998, 281, 1653–1656. [Google Scholar] [CrossRef] [Green Version]
- Ohta, K.; Kamada, K. Theoretical Approach to Large Two-Photon Absorption Cross Section in Extended π-Conjugated Systems. AIP Conf. Proc. 2007, 963, 389–405. [Google Scholar] [CrossRef]
- Karotki, A.; Kruk, M.; Rebane, A.; Nickel, E.; Charles, W.S. Strong two-photon absorption and singlet oxygen photogeneration in near-IR with new porphyrin molecule. In Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy XI, Proceedings of International Symposium on Biomedical Optics, San Jose, CA, USA, 6 June 2002; Dougherty, T.J., Ed.; Proc. SPIE: San Jose, CA, USA, 2002; Volume 4612, pp. 143–151. [Google Scholar] [CrossRef]
- Wielgus, M.; Bartkowiak, W.; Samoc, M. Two-photon solvatochromism. I. Solvent effects on two-photon absorption cross section of 4-dimethylamino-4′-nitrostilbene (DANS). Chem. Phys. Lett. 2012, 554, 113–116. [Google Scholar] [CrossRef]
- Wielgus, M.; Zalesny, R.; Murugan, N.A.; Kongsted, J.; Agren, H.; Samoc, M.; Bartkowiak, W. Two-photon solvatochromism II: Experimental and theoretical study of solvent effects on the two-photon absorption spectrum of Reichardt’s dye. Chemphyschem 2013, 14, 3731–3739. [Google Scholar] [CrossRef] [PubMed]
- Wielgus, M.; Michalska, J.; Samoc, M.; Bartkowiak, W. Two-photon solvatochromism III: Experimental study of the solvent effects on two-photon absorption spectrum of p-nitroaniline. Dye. Pigment. 2015, 113, 426–434. [Google Scholar] [CrossRef]
- Wielgus, M.; Samoć, M.; Bartkowiak, W. Two-photon absorption of Crystal Violet in solutions: Analysis of the solvent effect and aggregation process based on linear and nonlinear absorption spectra. J. Mol. Liq. 2016, 222, 125–132. [Google Scholar] [CrossRef]
- Hornum, M.; Reinholdt, P.; Zareba, J.K.; Jensen, B.B.; Wustner, D.; Samoc, M.; Nielsen, P.; Kongsted, J. One- and two-photon solvatochromism of the fluorescent dye Nile Red and its CF3, F and Br-substituted analogues. Photochem. Photobiol. Sci. 2020, 19, 1382–1391. [Google Scholar] [CrossRef]
- Sharmoukh, W.; Attanzio, A.; Busatto, E.; Etienne, T.; Carli, S.; Monari, A.; Assfeld, X.; Beley, M.; Caramori, S.; Gros, P.C. 2,5-Dithienylpyrrole (DTP) as a donor component in DTP–π–A organic sensitizers: Photophysical and photovoltaic properties. RSC Adv. 2015, 5, 4041–4050. [Google Scholar] [CrossRef]
- Sengul, O.; Marazzi, M.; Monari, A.; Catak, S. Photophysical Properties of Novel Two-Photon Absorbing Dyes: Assessing Their Possible Use for Singlet Oxygen Generation. J. Phys. Chem. C 2018, 122, 16315–16324. [Google Scholar] [CrossRef]
- Sengul, O.; Boydas, E.B.; Pastore, M.; Sharmouk, W.; Gros, P.C.; Catak, S.; Monari, A. Probing optical properties of thiophene derivatives for two-photon absorption. Theor. Chem. Acc. 2017, 136. [Google Scholar] [CrossRef]
- Turan, H.T.; Eken, Y.; Marazzi, M.; Pastore, M.; Aviyente, V.; Monari, A. Assessing One- and Two-Photon Optical Properties of Boron Containing Arenes. J. Phys. Chem. C 2016, 120, 17916–17926. [Google Scholar] [CrossRef]
- Gallavardin, T.; Armagnat, C.; Maury, O.; Baldeck, P.L.; Lindgren, M.; Monnereau, C.; Andraud, C. An improved singlet oxygen sensitizer with two-photon absorption and emission in the biological transparency window as a result of ground state symmetry-breaking. Chem. Commun. 2012, 48, 1689–1691. [Google Scholar] [CrossRef]
- Hu, W.; Xie, M.; Zhao, H.; Tang, Y.; Yao, S.; He, T.; Ye, C.; Wang, Q.; Lu, X.; Huang, W.; et al. Nitric oxide activatable photosensitizer accompanying extremely elevated two-photon absorption for efficient fluorescence imaging and photodynamic therapy. Chem. Sci. 2018, 9, 999–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; He, T.; Jiang, R.; Yin, J.; Li, L.; Lu, X.; Zhao, H.; Zhang, L.; Huang, L.; Sun, H.; et al. Inner salt-shaped small molecular photosensitizer with extremely enhanced two-photon absorption for mitochondrial-targeted photodynamic therapy. Chem. Commun. 2017, 53, 1680–1683. [Google Scholar] [CrossRef]
- Zheng, M.L.; Fujita, K.; Chen, W.Q.; Smith, N.I.; Duan, X.M.; Kawata, S. Comparison of staining selectivity for subcellular structures by carbazole-based cyanine probes in nonlinear optical microscopy. ChemBioChem 2011, 12, 52–55. [Google Scholar] [CrossRef]
- Zheng, Y.-C.; Zheng, M.-L.; Chen, S.; Zhao, Z.-S.; Duan, X.-M. Biscarbazolylmethane-based cyanine: A two-photon excited fluorescent probe for DNA and selective cell imaging. J. Mater. Chem. B. 2014, 2, 2301–2310. [Google Scholar] [CrossRef]
- Zheng, Y.-C.; Zheng, M.-L.; Li, K.; Chen, S.; Zhao, Z.-S.; Wang, X.-S.; Duan, X.-M. Novel carbazole-based two-photon photosensitizer for efficient DNA photocleavage in anaerobic condition using near-infrared light. RSC Adv. 2015, 5, 770–774. [Google Scholar] [CrossRef]
- Sajewicz, W.; Dlugosz, A. Cytotoxicity of some potential DNA intercalators (carbazole, acridine and anthracene derivatives) evaluated through neutrophil chemiluminescence. J. Appl. Toxicol. 2000, 20, 305–312. [Google Scholar] [CrossRef]
- Gu, J.; Yulan, W.; Chen, W.-Q.; Dong, X.-Z.; Duan, X.-M.; Kawata, S. Carbazole-based 1D and 2D hemicyanines: Synthesis, two-photon absorption properties and application for two-photon photopolymerization 3D lithography. New J. Chem. 2007, 31, 63–68. [Google Scholar] [CrossRef]
- Taima, H.; Okubo, A.; Yoshioka, N.; Inoue, H. DNA-binding properties and photocleavage activity of cationic water-soluble chlorophyll derivatives. Chemistry 2006, 12, 6331–6340. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Zheng, M.L.; Zheng, Y.C.; Jin, F.; Chai, Q.Q.; Zhao, Y.Y.; Meng, X.W.; Liu, Y.H.; Duan, X.M. Laser-Induced Antibacterial Activity of Novel Symmetric Carbazole-Based Ethynylpyridine Photosensitizers. ACS Omega 2018, 3, 3737–3743. [Google Scholar] [CrossRef]
- Gluszynska, A. Biological potential of carbazole derivatives. Eur. J. Med. Chem. 2015, 94, 405–426. [Google Scholar] [CrossRef]
- Knolker, H.J.; Reddy, K.R. Isolation and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2002, 102, 4303–4427. [Google Scholar] [CrossRef]
- Chen, Y.; Yamamura, T.; Igarashi, K. Photosensitization of carbazole derivatives in cationic polymerization with a novel sensitivity to near-UV light. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 90–100. [Google Scholar] [CrossRef]
- Grigalevicius, S. 3,6(2,7),9-Substituted carbazoles as electroactive amorphous materials for optoelectronics. Synth. Met. 2006, 156, 1–12. [Google Scholar] [CrossRef]
- Wasielewski, M.R.; Svec, W.A. Synthesis of covalently linked dimeric derivatives of chlorophyll a, pyrochlorophyll a, chlorophyll b, and bacteriochlorophyll a. J. Org. Chem. 1980, 45, 1969–1974. [Google Scholar] [CrossRef]
- Lower, S.K.; El-Sayed, M.A. The Triplet State and Molecular Electronic Processes in Organic Molecules. Chem. Rev. 1966, 66, 199–241. [Google Scholar] [CrossRef]
- Susumu, K.; Fisher, J.A.; Zheng, J.; Beratan, D.N.; Yodh, A.G.; Therien, M.J. Two-photon absorption properties of proquinoidal D-A-D and A-D-A quadrupolar chromophores. J. Phys. Chem. A 2011, 115, 5525–5539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, J.; Heitz, V.; Sour, A.; Bolze, F.; Ftouni, H.; Nicoud, J.F.; Flamigni, L.; Ventura, B. Diketopyrrolopyrrole-porphyrin conjugates with high two-photon absorption and singlet oxygen generation for two-photon photodynamic therapy. Angew. Chem. Int. Ed. Engl. 2015, 54, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-T.; Yin, X.; Lai, X.-Y.; Wang, X. The photophysical properties of three [M(phen)2dppz]2+ (M=Ru and Zn) derivatives for two-photon photodynamic therapy: Insights from theoretical investigations. Dye. Pigment. 2020, 176. [Google Scholar] [CrossRef]
- Hess, J.; Huang, H.; Kaiser, A.; Pierroz, V.; Blacque, O.; Chao, H.; Gasser, G. Evaluation of the Medicinal Potential of Two Ruthenium(II) Polypyridine Complexes as One- and Two-Photon Photodynamic Therapy Photosensitizers. Chemistry 2017, 23, 9888–9896. [Google Scholar] [CrossRef]
- Mazzone, G.; Russo, N.; Sicilia, E. Theoretical investigation of the absorption spectra and singlet-triplet energy gap of positively charged tetraphenylporphyrins as potential photodynamic therapy photosensitizers. Can. J. Chem. 2013, 91, 902–906. [Google Scholar] [CrossRef]
- Alberto, M.E.; De Simone, B.C.; Mazzone, G.; Sicilia, E.; Russo, N. The heavy atom effect on Zn(ii) phthalocyanine derivatives: A theoretical exploration of the photophysical properties. Phys. Chem. Chem. Phys. 2015, 17, 23595–23601. [Google Scholar] [CrossRef]
- Schmitt, J.; Heitz, V.; Jenni, S.; Sour, A.; Bolze, F.; Ventura, B. π-extended porphyrin dimers as efficient near-infrared emitters and two-photon absorbers. Supramol. Chem. 2017, 29, 769–775. [Google Scholar] [CrossRef]
- Alam, M.M.; Bolze, F.; Daniel, C.; Flamigni, L.; Gourlaouen, C.; Heitz, V.; Jenni, S.; Schmitt, J.; Sour, A.; Ventura, B. π-Extended diketopyrrolopyrrole-porphyrin arrays: One- and two-photon photophysical investigations and theoretical studies. Phys. Chem. Chem. Phys. 2016, 18, 21954–21965. [Google Scholar] [CrossRef] [PubMed]
- Gouterman, M. Optical spectra and electronic structure of porphyrins and related rings. In The Porphyrins; Dolphin, D., Ed.; Elsevier: Amsterdam, The Netherlands, 1978; Chapter 1; pp. 1–168. [Google Scholar]
- Yao, D.; Zhang, X.; Triadon, A.; Richy, N.; Mongin, O.; Blanchard-Desce, M.; Paul, F.; Paul-Roth, C.O. New Conjugated meso-Tetrafluorenylporphyrin-Cored Derivatives as Fluorescent Two-Photon Photosensitizers for Singlet Oxygen Generation. Chem.-A Eur. J. 2017, 23, 2635–2647. [Google Scholar] [CrossRef]
- Zhang, X.; Abid, S.; Shi, L.; Sun, Z.; Mongin, O.; Blanchard-Desce, M.; Paul, F.; Paul-Roth, C.O. New conjugated meso-tetrathienylporphyrin-cored derivatives as two-photon photosensitizers for singlet oxygen generation. Dye. Pigment. 2018, 153, 248–255. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheung, Y.K.; Ma, C.; Zhao, S.; Gao, D.; Lo, P.C.; Fong, W.P.; Wong, K.S.; Ng, D.K.P. Endoplasmic Reticulum-Localized Two-Photon-Absorbing Boron Dipyrromethenes as Advanced Photosensitizers for Photodynamic Therapy. J. Med. Chem. 2018, 61, 3952–3961. [Google Scholar] [CrossRef]
- Awuah, S.G.; You, Y. Boron dipyrromethene (BODIPY)-based photosensitizers for photodynamic therapy. RSC Adv. 2012, 2. [Google Scholar] [CrossRef]
- Li, M.; Tian, R.; Fan, J.; Du, J.; Long, S.; Peng, X. A lysosome-targeted BODIPY as potential NIR photosensitizer for photodynamic therapy. Dye. Pigment. 2017, 147, 99–105. [Google Scholar] [CrossRef]
- Tang, Q.; Si, W.; Huang, C.; Ding, K.; Huang, W.; Chen, P.; Zhang, Q.; Dong, X. An aza-BODIPY photosensitizer for photoacoustic and photothermal imaging guided dual modal cancer phototherapy. J. Mater. Chem. B 2017, 5, 1566–1573. [Google Scholar] [CrossRef]
- Yue, Y.; Huo, F.; Cheng, F.; Zhu, X.; Mafireyi, T.; Strongin, R.M.; Yin, C. Functional synthetic probes for selective targeting and multi-analyte detection and imaging. Chem. Soc. Rev. 2019, 48, 4155–4177. [Google Scholar] [CrossRef]
- Kang, J.; Huo, F.; Chao, J.; Yin, C. Nitroolefin-based BODIPY as a novel water-soluble ratiometric fluorescent probe for detection of endogenous thiols. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 195, 16–20. [Google Scholar] [CrossRef]
- Kang, J.; Huo, F.; Ning, P.; Meng, X.; Chao, J.; Yin, C. Two red-emission single and double ‘arms’ fluorescent materials stemed from ‘one-pot’ reaction for hydrogen sulfide vivo imaging. Sens. Actuators B Chem. 2017, 250, 342–350. [Google Scholar] [CrossRef]
- Zhao, Q.; Yin, C.; Kang, J.; Wen, Y.; Huo, F. A viscosity sensitive azide-pyridine BODIPY-based fluorescent dye for imaging of hydrogen sulfide in living cells. Dye. Pigment. 2018, 159, 166–172. [Google Scholar] [CrossRef]
- Yang, J.; Rousselin, Y.; Bucher, L.; Desbois, N.; Bolze, F.; Xu, H.J. Two-photon absorption properties of BODIPYs: Units’ number from 1 to 4 versus geometry. ChemPlusChem 2018, 83, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sun, D.; Yang, J.; Zhu, G.; Fang, Y.; Gros, C.P.; Bolze, F.; Xu, H.-J. Truxene-BODIPY dyads and triads: Synthesis, spectroscopic characterization, one and two-photon absorption properties and electrochemistry. Dye. Pigment. 2020, 179. [Google Scholar] [CrossRef]
- Sun, J.; Tian, M.; Lin, W. A two-photon excited red-emissive probe for imaging mitochondria with high fidelity and its application in monitoring mitochondrial depolarization via FRET. Analyst 2019, 144, 2387–2392. [Google Scholar] [CrossRef]
- Wang, H.; Fang, B.; Kong, L.; Li, X.; Feng, Z.; Wu, Y.; Uvdal, K.; Hu, Z. A novel Schiff base derivative: Synthesis, two-photon absorption properties and application for bioimaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 198, 304–308. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, S.; Xu, L.; Ni, S.; Liu, W.; Huang, B.; Huang, Q.; Zhang, Q.; Lu, F.; Li, M.-D. Arylamine-coumarin based donor-acceptor dyads: Unveiling the relationship between two-photon absorption cross-section and lifetime of singlet excited state intramolecular charge separation. Dye. Pigment. 2019, 165, 301–307. [Google Scholar] [CrossRef]
- Cai, Z.-B.; Liu, S.-S.; Li, B.; Dong, Q.-J.; Liu, Z.-L.; Zheng, M.; Li, S.-L.; Tian, Y.-P.; Chen, L.-J.; Ye, Q. Linear and V-shaped carbazole-based molecules functionalized by cyano acceptors and diversified donors: Synthesis, single- and two-photon related photophysical properties. Dye. Pigment. 2019, 165, 200–211. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Liu, D.; Wang, Y.; Wang, G.; Hua, J. Ultrafast responses of two V-shaped compounds with a reverse conjugated structural configuration: An investigation of the reason for the enhanced two-photon absorption cross-section. Appl. Phys. B 2018, 124. [Google Scholar] [CrossRef]
- Xu, S.; Zhu, Y.; Li, R.; Su, J.; Li, S.; Zhou, H.; Wu, J.; Tian, Y. Thiophene-based pyridine derivatives: Synthesis, crystal structures, two-photon absorption properties and bio-imaging applications in the near-IR region. New J. Chem. 2016, 40, 8809–8814. [Google Scholar] [CrossRef]
- Tao, J.; Sun, D.; Sun, L.; Li, Z.; Fu, B.; Liu, J.; Zhang, L.; Wang, S.; Fang, Y.; Xu, H. Tuning the photo-physical properties of BODIPY dyes: Effects of 1, 3, 5, 7- substitution on their optical and electrochemical behaviours. Dye. Pigment. 2019, 168, 166–174. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Y.-Z.; Cao, J.-H.; Yang, Q.-Z.; Wu, L.-Z.; Tung, C.-H.; Wu, D.-Y. Dicyanoboron diketonate dyes: Synthesis, photophysical properties and bioimaging. Dye. Pigment. 2015, 112, 162–169. [Google Scholar] [CrossRef]
- Xue, P.; Wang, P.; Chen, P.; Yao, B.; Gong, P.; Sun, J.; Zhang, Z.; Lu, R. Bright persistent luminescence from pure organic molecules through a moderate intermolecular heavy atom effect. Chem. Sci. 2017, 8, 6060–6065. [Google Scholar] [CrossRef] [Green Version]
- Xiong, T.; Li, M.; Zhao, X.; Zou, Y.; Du, J.; Fan, J.; Peng, X. Functional two-photon cationic targeted photosensitizers for deep-seated tumor imaging and therapy. Sens. Actuators B Chem. 2020, 304. [Google Scholar] [CrossRef]
- Morgan, J.; Oseroff, A.R. Mitochondria-based photodynamic anti-cancer therapy. Adv. Drug Del. Rev. 2001, 49, 71–86. [Google Scholar] [CrossRef]
- Kessel, D.; Luo, Y. Mitochondrial photodamage and PDT-induced apoptosis. J. Photochem. Photobiol. B Biol. 1998, 42, 89–95. [Google Scholar] [CrossRef]
- Qi, J.; Sun, C.; Li, D.; Zhang, H.; Yu, W.; Zebibula, A.; Lam, J.W.; Xi, W.; Zhu, L.; Cai, F. Aggregation-induced emission luminogen with near-infrared-II excitation and near-infrared-I emission for ultradeep intravital two-photon microscopy. Acs Nano 2018, 12, 7936–7945. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, J.; Zhou, Y.; Xing, P.; Gong, L.; Su, C.; Qi, D.; Du, H.; Bian, Y.; Jiang, J. Two-Photon Excited FRET Dyads for Lysosome-Targeted Imaging and Photodynamic Therapy. Inorg. Chem. 2018, 57, 11537–11542. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Jiang, X.F.; Hu, D.; Liu, P.; Li, S.; Huang, F.; Ma, Y.; Xu, Q.H.; Cao, Y. Red emitting conjugated polymer based nanophotosensitizers for selectively targeted two-photon excitation imaging guided photodynamic therapy. Nanoscale 2018, 11, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Redmond, R.W.; Gamlin, J.N. A Compilation of Singlet Oxygen Yields from Biologically Relevant Molecules. Photochem. Photobiol. 1999, 70, 391–475. [Google Scholar] [CrossRef]
- Jiang, J.; Hu, D.; Hanif, M.; Li, X.; Su, S.; Xie, Z.; Liu, L.; Zhang, S.; Yang, B.; Ma, Y. Twist Angle and Rotation Freedom Effects on Luminescent Donor-Acceptor Materials: Crystal Structures, Photophysical Properties, and OLED Application. Adv. Opt. Mater. 2016, 4, 2109–2118. [Google Scholar] [CrossRef]
- Han, C.; Jiang, S.; Qiu, J.; Guo, H.; Yang, F. A diphenylacrylonitrile conjugated porphyrin with near-infrared emission by AIE–FRET. New J. Chem. 2019, 43, 3317–3322. [Google Scholar] [CrossRef]
- Zhang, L.P.; Li, X.; Liu, T.; Kang, L.; Huang, X.; Zhao, Y. A water-soluble pyrazino[2,3-g]quinoxaline photosensitizer for high-efficiency one- and two-photon excited bioimaging and photodynamic therapy. Chem. Commun. 2020, 56, 5544–5547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-P.; Jiang, K.-J.; Li, G.; Zhang, Q.-Q.; Yang, L.-M. Pyrazino[2,3-g]quinoxaline dyes for solar cell applications. J. Mater. Chem. A 2014, 2, 14852–14857. [Google Scholar] [CrossRef]
- Peng, Q.; Liu, X.; Qin, Y.; Xu, J.; Li, M.; Dai, L. Pyrazino[2,3-g]quinoxaline-based conjugated copolymers with indolocarbazole coplanar moieties designed for efficient photovoltaic applications. J. Mater. Chem. 2011, 21. [Google Scholar] [CrossRef]
- Mastalerz, M.; Fischer, V.; Ma, C.Q.; Janssen, R.A.; Bauerle, P. Conjugated oligothienyl dendrimers based on a pyrazino[2,3-g]quinoxaline core. Org. Lett. 2009, 11, 4500–4503. [Google Scholar] [CrossRef]
- Hu, B.L.; Zhang, K.; An, C.; Schollmeyer, D.; Pisula, W.; Baumgarten, M. Layered Thiadiazoloquinoxaline-Containing Long Pyrene-Fused N-Heteroacenes. Angew. Chem. Int. Ed. Engl. 2018, 57, 12375–12379. [Google Scholar] [CrossRef]
- Wu, W.; Guo, H.; Wu, W.; Ji, S.; Zhao, J. Organic triplet sensitizer library derived from a single chromophore (BODIPY) with long-lived triplet excited state for triplet-triplet annihilation based upconversion. J. Org. Chem. 2011, 76, 7056–7064. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Fowley, C.; Thapaliya, E.R.; McCaughan, B.; Tang, S.; Fraix, A.; Captain, B.; Sortino, S.; Callan, J.F.; Raymo, F.M. Supramolecular nanoreactors for intracellular singlet-oxygen sensitization. Nanoscale 2015, 7, 14071–14079. [Google Scholar] [CrossRef]
- Li, S.; Jiang, X.-F.; Xu, Q.-H. Polyfluorene based conjugated polymer nanoparticles for two-photon live cell imaging. Sci. China Chem. 2017, 61, 88–96. [Google Scholar] [CrossRef]
- Duan, X.; Liu, L.; Feng, F.; Wang, S. Cationic conjugated polymers for optical detection of DNA methylation, lesions, and single nucleotide polymorphisms. Acc Chem. Res. 2010, 43, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhu, C.; Yuan, H.; Liu, L.; Lv, F.; Wang, S. Conjugated polymer nanoparticles: Preparation, properties, functionalization and biological applications. Chem. Soc. Rev. 2013, 42, 6620–6633. [Google Scholar] [CrossRef] [PubMed]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Li, L.; Wu, H.; Yao, S.Q.; Xu, Q.-H. Photosensitizer-doped conjugated polymer nanoparticles for simultaneous two-photon imaging and two-photon photodynamic therapy in living cells. Nanoscale 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, D.; Demir, H.V. Conjugated polymer nanoparticles. Nanoscale 2010, 2, 484–494. [Google Scholar] [CrossRef]
- Hashim, Z.; Howes, P.; Green, M. Luminescent quantum-dot-sized conjugated polymernanoparticles—nanoparticle formation in a miniemulsion system. J. Mater. Chem. 2011, 21, 1797–1803. [Google Scholar] [CrossRef]
- Kandel, P.K.; Fernando, L.P.; Ackroyd, P.C.; Christensen, K.A. Incorporating functionalized polyethylene glycol lipids into reprecipitated conjugated polymer nanoparticles for bioconjugation and targeted labeling of cells. Nanoscale 2011, 3, 1037–1045. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.; Yu, J.; Wu, C.; Szymanski, C.; McNeill, J. Amplified energy transfer in conjugated polymer nanoparticle tags and sensors. Nanoscale 2010, 2, 1999–2011. [Google Scholar] [CrossRef]
- Wu, C.; McNeill, J. Swelling-controlled polymer phase and fluorescence properties of polyfluorene nanoparticles. Langmuir 2008, 24, 5855–5861. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Ye, F.; Zeigler, M.; Wu, C.; Chiu, D.T. Near-infrared fluorescent dye-doped semiconducting polymer dots. ACS Nano 2011, 5, 1468–1475. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Zheng, Y.; Szymanski, C.; McNeill, J. Energy Transfer in a Nanoscale Multichromophoric System: Fluorescent Dye-Doped Conjugated Polymer Nanoparticles. J. Phys. Chem. C Nanomater. Interfaces 2008, 112, 1772–1781. [Google Scholar] [CrossRef] [Green Version]
- Kuimova, M.K.; Yahioglu, G.; Ogilby, P.R. Singlet oxygen in a cell: Spatially dependent lifetimes and quenching rate constants. J. Am. Chem. Soc. 2009, 131, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Shen, X.; Xu, Q.H.; Cao, Y. Gold nanorod enhanced conjugated polymer/photosensitizer composite nanoparticles for simultaneous two-photon excitation fluorescence imaging and photodynamic therapy. Nanoscale 2019, 11, 19551–19560. [Google Scholar] [CrossRef]
- Guan, Z.; Polavarapu, L.; Xu, Q.H. Enhanced two-photon emission in coupled metal nanoparticles induced by conjugated polymers. Langmuir 2010, 26, 18020–18023. [Google Scholar] [CrossRef]
- Jiang, X.F.; Pan, Y.; Jiang, C.; Zhao, T.; Yuan, P.; Venkatesan, T.; Xu, Q.H. Excitation Nature of Two-Photon Photoluminescence of Gold Nanorods and Coupled Gold Nanoparticles Studied by Two-Pulse Emission Modulation Spectroscopy. J. Phys. Chem. Lett. 2013, 4, 1634–1638. [Google Scholar] [CrossRef]
- Guan, Z.; Gao, N.; Jiang, X.F.; Yuan, P.; Han, F.; Xu, Q.H. Huge enhancement in two-photon photoluminescence of Au nanoparticle clusters revealed by single-particle spectroscopy. J. Am. Chem. Soc. 2013, 135, 7272–7277. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Li, S.; Li, L.; Yao, S.Q.; Xu, Q.H. Highly efficient, conjugated-polymer-based nano-photosensitizers for selectively targeted two-photon photodynamic therapy and imaging of cancer cells. Chemistry 2015, 21, 2214–2221. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shen, X.; Li, L.; Yuan, P.; Guan, Z.; Yao, S.Q.; Xu, Q.H. Conjugated-polymer-based red-emitting nanoparticles for two-photon excitation cell imaging with high contrast. Langmuir 2014, 30, 7623–7627. [Google Scholar] [CrossRef] [PubMed]
- Arnbjerg, J.; Jimenez-Banzo, A.; Paterson, M.J.; Nonell, S.; Borrell, J.I.; Christiansen, O.; Ogilby, P.R. Two-photon absorption in tetraphenylporphycenes: Are porphycenes better candidates than porphyrins for providing optimal optical properties for two-photon photodynamic therapy? J. Am. Chem. Soc. 2007, 129, 5188–5199. [Google Scholar] [CrossRef] [PubMed]
- Karotki, A.; Drobizhev, M.; Kruk, M.; Spangler, C.; Nickel, E.; Mamardashvili, N.; Rebane, A. Enhancement of two-photon absorption in tetrapyrrolic compounds. J. Opt. Soc. Am. B 2003, 20. [Google Scholar] [CrossRef] [Green Version]
- Pu, K.-Y.; Liu, B. Bioimaging: Fluorescent Conjugated Polyelectrolytes for Bioimaging (Adv. Funct. Mater. 18/2011). Adv. Funct. Mater. 2011, 21, 3407. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, L.; Yang, Q.; Lv, F.; Wang, S. Water-soluble conjugated polymers for imaging, diagnosis, and therapy. Chem. Rev. 2012, 112, 4687–4735. [Google Scholar] [CrossRef]
- Wu, C.; Chiu, D.T. Highly fluorescent semiconducting polymer dots for biology and medicine. Angew. Chem. Int. Ed. Engl. 2013, 52, 3086–3109. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Neretina, S.; El-Sayed, M.A. Gold nanorods: From synthesis and properties to biological and biomedical applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Aslan, K.; Malyn, S.N.; Geddes, C.D. Metal-enhanced phosphorescence (MEP). Chem. Phys. Lett. 2006, 427, 432–437. [Google Scholar] [CrossRef]
- Zhang, Y.; Aslan, K.; Previte, M.J.; Malyn, S.N.; Geddes, C.D. Metal-enhanced phosphorescence: Interpretation in terms of triplet-coupled radiating plasmons. J. Phys. Chem. B 2006, 110, 25108–25114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Aslan, K.; Previte, M.J.; Geddes, C.D. Plasmonic engineering of singlet oxygen generation. Proc. Natl. Acad. Sci. USA 2008, 105, 1798–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, D.; Xu, Q.H. Separation distance dependent fluorescence enhancement of fluorescein isothiocyanate by silver nanoparticles. Chem. Commun. 2007, 3, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Labouret, T.; Audibert, J.F.; Pansu, R.B.; Palpant, B. Plasmon-Assisted Production of Reactive Oxygen Species by Single Gold Nanorods. Small 2015, 11, 4475–4479. [Google Scholar] [CrossRef] [PubMed]
- Keyvan Rad, J.; Mahdavian, A.R.; Khoei, S.; Shirvalilou, S. Enhanced Photogeneration of Reactive Oxygen Species and Targeted Photothermal Therapy of C6 Glioma Brain Cancer Cells by Folate-Conjugated Gold-Photoactive Polymer Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 19483–19493. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, L.; Li, S.; Jiang, X.-F.; Jiang, C.; Zhou, N.; Gao, N.; Xu, Q.-H. Gold nanorod-enhanced two-photon excitation fluorescence of conjugated oligomers for two-photon imaging guided photodynamic therapy. J. Mater. Chem. C 2019, 7, 14693–14700. [Google Scholar] [CrossRef]
- Dykman, L.A.; Khlebtsov, N.G. Gold Nanoparticles in Biology and Medicine: Recent Advances and Prospects. Acta Nat. 2011, 3, 34–55. [Google Scholar] [CrossRef] [Green Version]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed. Engl. 2010, 49, 3280–3294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stranik, O.; McEvoy, H.; McDonagh, C.; MacCraith, B. Plasmonic enhancement of fluorescence for sensor applications. Sens. Actuators B Chem. 2005, 107, 148–153. [Google Scholar] [CrossRef]
- Khatua, S.; Paulo, P.M.; Yuan, H.; Gupta, A.; Zijlstra, P.; Orrit, M. Resonant plasmonic enhancement of single-molecule fluorescence by individual gold nanorods. ACS Nano 2014, 8, 4440–4449. [Google Scholar] [CrossRef] [Green Version]
- Nooney, R.I.; Stranik, O.; McDonagh, C.; MacCraith, B.D. Optimization of plasmonic enhancement of fluorescence on plastic substrates. Langmuir 2008, 24, 11261–11267. [Google Scholar] [CrossRef]
- Erwin, W.R.; MacKenzie, R.C.; Bardhan, R. Understanding the limits of plasmonic enhancement in organic photovoltaics. J. Phys. Chem. C 2018, 122, 7859–7866. [Google Scholar] [CrossRef]
- Zeng, S.; Yu, X.; Law, W.-C.; Zhang, Y.; Hu, R.; Dinh, X.-Q.; Ho, H.-P.; Yong, K.-T. Size dependence of Au NP-enhanced surface plasmon resonance based on differential phase measurement. Sens. Actuators B Chem. 2013, 176, 1128–1133. [Google Scholar] [CrossRef]
- Law, W.-C.; Yong, K.-T.; Baev, A.; Prasad, P.N. Sensitivity improved surface plasmon resonance biosensor for cancer biomarker detection based on plasmonic enhancement. ACS Nano 2011, 5, 4858–4864. [Google Scholar] [CrossRef]
- Chance, R.; Prock, A.; Silbey, R. Molecular fluorescence and energy transfer near interfaces. Adv. Chem. Phys. 1978, 37, 1–65. [Google Scholar]
- Chen, H.; Shao, L.; Li, Q.; Wang, J. Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 2013, 42, 2679–2724. [Google Scholar] [CrossRef]
- Giannini, V.; Fernandez-Dominguez, A.I.; Heck, S.C.; Maier, S.A. Plasmonic nanoantennas: Fundamentals and their use in controlling the radiative properties of nanoemitters. Chem. Rev. 2011, 111, 3888–3912. [Google Scholar] [CrossRef] [PubMed]
- Kinkhabwala, A.; Yu, Z.; Fan, S.; Avlasevich, Y.; Müllen, K.; Moerner, W.E. Large single-molecule fluorescence enhancements produced by a bowtie nanoantenna. Nat. Photonics 2009, 3, 654–657. [Google Scholar] [CrossRef]
- Jeong, Y.; Kook, Y.M.; Lee, K.; Koh, W.G. Metal enhanced fluorescence (MEF) for biosensors: General approaches and a review of recent developments. Biosens. Bioelectron. 2018, 111, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Yu, K.; Li, L.; Zhang, T.; Guan, Z.; Gao, N.; Yuan, P.; Li, S.; Yao, S.Q.; Xu, Q.-H. Gold nanorod enhanced two-photon excitation fluorescence of photosensitizers for two-photon imaging and photodynamic therapy. ACS Appl. Mater. Interfaces 2014, 6, 2700–2708. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Radiative decay engineering 5: Metal-enhanced fluorescence and plasmon emission. Anal. Biochem. 2005, 337, 171–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fothergill, S.M.; Joyce, C.; Xie, F. Metal enhanced fluorescence biosensing: From ultra-violet towards second near-infrared window. Nanoscale 2018, 10, 20914–20929. [Google Scholar] [CrossRef] [Green Version]
- Malola, S.; Lehtovaara, L.; Enkovaara, J.; Hakkinen, H. Birth of the localized surface plasmon resonance in monolayer-protected gold nanoclusters. ACS Nano 2013, 7, 10263–10270. [Google Scholar] [CrossRef]
- McLean, A.; Wang, R.; Huo, Y.; Cooke, A.; Hopkins, T.; Potter, N.; Li, Q.; Isaac, J.; Haidar, J.; Jin, R.; et al. Synthesis and Optical Properties of Two-Photon-Absorbing Au25(Captopril)18-Embedded Polyacrylamide Nanoparticles for Cancer Therapy. ACS Appl. Nano Materials 2020, 3, 1420–1430. [Google Scholar] [CrossRef]
- Kuruppuarachchi, M.; Savoie, H.; Lowry, A.; Alonso, C.; Boyle, R.W. Polyacrylamide nanoparticles as a delivery system in photodynamic therapy. Mol. Pharm. 2011, 8, 920–931. [Google Scholar] [CrossRef]
- Tang, W.; Xu, H.; Park, E.J.; Philbert, M.A.; Kopelman, R. Encapsulation of methylene blue in polyacrylamide nanoparticle platforms protects its photodynamic effectiveness. Biochem. Biophys. Res. Commun. 2008, 369, 579–583. [Google Scholar] [CrossRef] [Green Version]
- Olesiak-Banska, J.; Waszkielewicz, M.; Matczyszyn, K.; Samoc, M. A closer look at two-photon absorption, absorption saturation and nonlinear refraction in gold nanoclusters. RSC Adv. 2016, 6, 98748–98752. [Google Scholar] [CrossRef] [Green Version]
- Reuveni, T.; Motiei, M.; Romman, Z.; Popovtzer, A.; Popovtzer, R. Targeted gold nanoparticles enable molecular CT imaging of cancer: An in vivo study. Int. J. Nanomed. 2011, 6, 2859–2864. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.D.; Luo, Z.; Chen, J.; Song, S.; Yuan, X.; Shen, X.; Wang, H.; Sun, Y.; Gao, K.; Zhang, L.; et al. Ultrasmall glutathione-protected gold nanoclusters as next generation radiotherapy sensitizers with high tumor uptake and high renal clearance. Sci. Rep. 2015, 5, 8669. [Google Scholar] [CrossRef]
- Wang, J.; Zhuo, X.; Xiao, X.; Mao, R.; Wang, Y.; Wang, J.; Liu, J. AlPcS-loaded gold nanobipyramids with high two-photon efficiency for photodynamic therapy in vivo. Nanoscale 2019, 11, 3386–3395. [Google Scholar] [CrossRef]
- Meshalkin, Y.P.; Chunosova, S.S. Two-photon absorption cross section of aluminium phthalocyanine excited by a femtosecond Ti: Sapphire laser. Quantum Electron. 2005, 35, 527. [Google Scholar] [CrossRef] [Green Version]
- Nawrot, K.C.; Wawrzynczyk, D.; Bezkrovnyi, O.; Kepinski, L.; Cichy, B.; Samoc, M.; Nyk, M. Functional CdS-Au Nanocomposite for Efficient Photocatalytic, Photosensitizing, and Two-Photon Applications. Nanomater 2020, 10, 715. [Google Scholar] [CrossRef]
- Bazylińska, U.; Zieliński, W.; Kulbacka, J.; Samoć, M.; Wilk, K.A. New diamidequat-type surfactants in fabrication of long-sustained theranostic nanocapsules: Colloidal stability, drug delivery and bioimaging. Colloids Surf. B. 2016, 137, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Drozdek, S.; Szeremeta, J.; Lamch, L.; Nyk, M.; Samoc, M.; Wilk, K.A. Two-Photon Induced Fluorescence Energy Transfer in Polymeric Nanocapsules Containing CdSe x S1–x/ZnS Core/Shell Quantum Dots and Zinc (II) Phthalocyanine. J. Phys. Chem. C 2016, 120, 15460–15470. [Google Scholar] [CrossRef]
- Wawrzyńczyk, D.; Bazylińska, U.; Lamch, Ł.; Kulbacka, J.; Szewczyk, A.; Bednarkiewicz, A.; Wilk, K.A.; Samoć, M. Förster Resonance Energy Transfer-Activated Processes in Smart Nanotheranostics Fabricated in a Sustainable Manner. ChemSusChem 2019, 12, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Del. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef]
- Maeda, H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J. Control. Release 2012, 164, 138–144. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Vijayaraghavan, P.; Chiang, W.-H.; Chen, H.-H.; Liu, T.-I.; Shen, M.-Y.; Omoto, A.; Kamimura, M.; Soga, K.; Chiu, H.-C. Targeted delivery of functionalized upconversion nanoparticles for externally triggered photothermal/photodynamic therapies of brain glioblastoma. Theranostics 2018, 8, 1435. [Google Scholar] [CrossRef]
- Hamblin, M.R. Upconversion in photodynamic therapy: Plumbing the depths. Dalton Trans. 2018, 47, 8571–8580. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, X.; Bu, W. Upconversion-based photodynamic cancer therapy. Coord. Chem. Rev. 2019, 379, 82–98. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; You, W.; Su, J.; Yu, S.; Dai, T.; Huang, Y.; Chen, X.; Song, X.; Chen, Z. Near-infrared-excited upconversion photodynamic therapy of extensively drug-resistant Acinetobacter baumannii based on lanthanide nanoparticles. Nanoscale 2020, 12, 13948–13957. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Luo, Z.; Qin, X.; Chen, Q.; Liu, X. Lanthanide-activated nanoparticles: A toolbox for bioimaging, therapeutics, and neuromodulation. Acc. Chem. Res. 2020, 53, 2692–2704. [Google Scholar] [CrossRef] [PubMed]
- González-Béjar, M.; Liras, M.; Francés-Soriano, L.; Voliani, V.; Herranz-Pérez, V.; Duran-Moreno, M.; Garcia-Verdugo, J.M.; Alarcon, E.I.; Scaiano, J.C.; Pérez-Prieto, J. NIR excitation of upconversion nanohybrids containing a surface grafted Bodipy induces oxygen-mediated cancer cell death. J. Mater. Chem. B 2014, 2, 4554–4563. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Tan, M.; Ohulchanskyy, T.Y.; Lovell, J.F.; Chen, G. Recent progress in upconversion photodynamic therapy. Nanomaterials 2018, 8, 344. [Google Scholar] [CrossRef] [Green Version]
- Buchner, M.; Calavia, P.G.; Muhr, V.; Kröninger, A.; Baeumner, A.J.; Hirsch, T.; Russell, D.A.; Marin, M.J. Photosensitiser functionalised luminescent upconverting nanoparticles for efficient photodynamic therapy of breast cancer cells. Photochem. Photobiol. Sci. 2019, 18, 98–109. [Google Scholar] [CrossRef] [Green Version]

























| Molecule Number | λex (nm) | σ2 (GM) | ΦΔ | ΦF | Page Number |
|---|---|---|---|---|---|
| 1 | 700–1000 | 400 | - | - | 7 |
| 2 | 700–1000 | 1000 | - | - | 7 |
| 3 | 800 | 200 | - | - | 8 |
| 4 | 800 | 31 | - | - | 8 |
| 5 | 710 | 270 | 0.12 | 0.12 | 10 |
| 6 | 710 | 2800 | 0.89 | 0.93 | 10 |
| 7 | - | - | - | - | 10 |
| 8 | 720 | 4857.4 | - | - | 10 |
| 9 | 760 | 522 | - | 2.0 × 10−4 | 11 |
| 10 | 760 | 492 | - | 6.0 × 10−5 | 11 |
| 11 | 800 | 245 | 0.75 | - | 12 |
| 12 | 705 | 10.58 | - | - | 12 |
| 13 | 705 | 98.9 | - | - | 12 |
| 14 | 910 | 4000 | 0.38 | - | 13 |
| 15 | 910 | 21,500 | 0.19 | - | 13 |
| 16 | 725 | 3115 | 0.47 | - | 15 |
| 17 | 775 | 3700 | 0.27 | - | 15 |
| 18 | 800 | 730 | 0.73 | 0.04 | 15 |
| 19 | 800 | 580 | 0.50 | 0.025 | 15 |
| 20 | 820 | 1000 | - | 0.59 | 17 |
| 21 | 820 | 880 | - | 0.62 | 17 |
| 22 | 820 | 1100 | - | 0.55 | 17 |
| 23 | 820 | 750 | - | 0.63 | 17 |
| 24 | 820 | 1600 | - | 0.48 | 17 |
| 25 | 820 | 1750 | - | 0.52 | 17 |
| 26 | 820 | 1100 | - | 0.36 | 17 |
| 27 | 820 | 980 | - | 0.38 | 17 |
| 28 | 808 | 25.5 | 0.39 | - | 17 |
| 29 | - | - | 0.31 | - | 17 |
| 30 | - | - | 0.33 | - | 17 |
| 31 | 740 | 112 | 0.57 | - | 19 |
| 32 | 740 | 95 | 0.66 | - | 19 |
| 33 | - | - | - | - | 20 |
| 34 | 750 | 8.6 × 106 | - | - | 20 |
| 35 | - | - | - | - | 22 |
| 36 | 820 | 1207 | 0.49 | 0.55 | 23 |
| 37 | 820 | 1293 | 0.47 | 0.54 | 23 |
| 38 | 800 | 2160 | - | - | 24 |
| 39 | 820 | 256 | - | - | 25 |
| 40 | 800 | 280 | 0.46 | 0.48 | 27 |
| 41 | 800 | 830 | 0.16 | - | 28 |
| 42 | - | - | 0.14 | - | 28 |
| 43 | 800 | 26,000 | - | - | 30 |
| 44 | 725 | 15.8 × 103 | - | - | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robbins, E.; Leroy-Lhez, S.; Villandier, N.; Samoć, M.; Matczyszyn, K. Prospects for More Efficient Multi-Photon Absorption Photosensitizers Exhibiting Both Reactive Oxygen Species Generation and Luminescence. Molecules 2021, 26, 6323. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26206323
Robbins E, Leroy-Lhez S, Villandier N, Samoć M, Matczyszyn K. Prospects for More Efficient Multi-Photon Absorption Photosensitizers Exhibiting Both Reactive Oxygen Species Generation and Luminescence. Molecules. 2021; 26(20):6323. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26206323
Chicago/Turabian StyleRobbins, Emma, Stéphanie Leroy-Lhez, Nicolas Villandier, Marek Samoć, and Katarzyna Matczyszyn. 2021. "Prospects for More Efficient Multi-Photon Absorption Photosensitizers Exhibiting Both Reactive Oxygen Species Generation and Luminescence" Molecules 26, no. 20: 6323. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26206323