Method Validation for Determination of Thallium by Inductively Coupled Plasma Mass Spectrometry and Monitoring of Various Foods in South Korea
Abstract
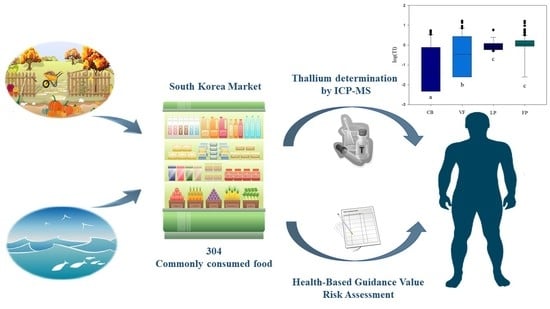
:1. Introduction
2. Results and Discussion
2.1. Method Validation and Quality Control
2.2. Concentration of Tl in Foods
2.2.1. Concentration of Tl in Agricultural, Fishery, and Livestock Products
2.2.2. Concentration of Tl in Processed Foods
2.3. Comparing the Concentrations of Tl among Food Groups
2.4. Health Risk Assessment for Thallium through Food Consumption
3. Materials and Methods
3.1. Instrumentation
3.2. Reagents and Solutions
3.3. Sample Collection and Processing
3.4. Sample Preparation
3.5. Single-Lab Validation and Quality Control
3.6. Statistical Analysis
3.7. Risk Assessment and Exposure to Thallium
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ufelle, A.C.; Barchowsky, A. Toxic Effects of Metals. In Casarett & Doull’s Toxicology: The Basic Science of Poisons, 9th ed.; Klaassen, C.D., Ed.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Schoer, J. Thallium. In Anthropogenic Compounds; Springer: Berlin/Heidelberg, Germany, 1984; pp. 143–214. [Google Scholar]
- ATSDR. Toxicological Profile for Thallium. 1992. Available online: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=309&tid=49 (accessed on 25 August 2021).
- EPA. Suspended, Cancelled and Restricted Pesticides. 1985. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/91017N0P.txt?ZyActionD=ZyDocument&Client=EPA&Index=1981%20Thru%201985&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&UseQField=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5CZYFILES%5CINDEX%20DATA%5C81THRU85%5CTXT%5C00000023%5C91017N0P.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1 (accessed on 2 August 2021).
- Britten, J.S.; Blank, M. Thallium activation of the (Na+-K+)-activated ATPase of rabbit kidney. Biochim. Biophys. Acta 1968, 159, 160–166. [Google Scholar] [CrossRef]
- Sun, T.W.; Xu, Q.Y.; Zhang, X.J.; Wu, Q.; Liu, Z.S.; Kan, Q.C.; Sun, C.Y.; Wang, L. Management of thallium poisoning in patients with delayed hospital admission. Clin. Toxicol. 2012, 50, 65–69. [Google Scholar] [CrossRef]
- Ferguson, T.J. Thallium. In Poisoning & Drug Overdose, 6th ed.; Olson, K.R., Ed.; The McGraw-Hill Companies: New York, NY, USA, 2012; Chapter 148. [Google Scholar]
- WHO. ENVIRONMENTAL HEALTH CRITERIA 182; Thallium. 1996. Available online: https://inchem.org/documents/ehc/ehc/ehc182.htm (accessed on 13 July 2021).
- USEPA. National Primary Drinking Water Regulations. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 21 October 2021).
- C.C.o.M.o.t.E. Canadian Water Quality Guidelines for the Protection of Aquatic Life. 1999. Available online: https://ccme.ca/en/res/thallium-en-canadian-water-quality-guidelines-for-the-protection-of-aquatic-life.pdf (accessed on 25 August 2021).
- C.C.o.M.o.t.E. Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health. 1999. Available online: https://ccme.ca/en/res/thallium-canadian-soil-quality-guidelines-for-the-protection-of-environmental-and-human-health-en.pdf (accessed on 25 August 2021).
- M.o.F.a.D.S. Korea Food Code. 2021. Available online: http://www.foodsafetykorea.go.kr/foodcode/index.jsp (accessed on 20 September 2021).
- Tyler, G.; Yvon, J. ICP-OES, ICP-MS and AAS Techniques Compared. In ICP Optical Emission Spectroscopy Technical Note; Jobin Yvon S.A.S, Horiba Group: Longjumeau, France, 1995; Volume 5. [Google Scholar]
- Voica, C.; Roba, C.; Iordache, A.M. Lithium Levels in Food from the Romanian Market by Inductively Coupled Plasma-Mass Spectrometry (ICP-MS): A Pilot Study. Anal. Lett. 2021, 54, 242–254. [Google Scholar] [CrossRef]
- Vera, J.B.; Bisinoti, M.C.; Amaral, C.D.B.; Gonzalez, M.H. ICP-quadrupole MS for accurate determination of chromium in environmental and food matrices. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100421. [Google Scholar] [CrossRef]
- Kilic, S.; Soylak, M. Determination of trace element contaminants in herbal teas using ICP-MS by different sample preparation method. J. Food Sci. Technol. 2020, 57, 927–933. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Wang, Y.; Tsang, D.C.; Yang, X.; Beiyuan, J.; Yin, M.; Xiao, T.; Jiang, Y.; Lin, W.; et al. Emerging risks of toxic metal(loid)s in soil-vegetables influenced by steel-making activities and isotopic source apportionment. Environ. Int. 2021, 146, 106207. [Google Scholar] [CrossRef]
- Bronzi, B.; Brilli, C.; Beone, G.M.; Fontanella, M.C.; Ballabio, D.; Todeschini, R.; Consonni, V.; Grisoni, F.; Parri, F.; Buscema, M. Geographical identification of Chianti red wine based on ICP-MS element composition. Food Chem. 2020, 315, 126248. [Google Scholar] [CrossRef] [PubMed]
- Olivares, J.A.; Houk, R.S. Suppression of Analyte Signal by Various Concomitant Salts in Inductively Coupled Plasma Mass-Spectrometry. Anal. Chem. 1986, 58, 20–25. [Google Scholar] [CrossRef]
- Tan, S.H.; Horlick, G. Matrix-Effect Observations in Inductively Coupled Plasma Mass-Spectrometry. J. Anal. At. Spectrom. 1987, 2, 745–763. [Google Scholar] [CrossRef]
- Wilschefski, S.C.; Baxter, M.R. Inductively Coupled Plasma Mass Spectrometry: Introduction to Analytical Aspects. Clin. Biochem. Rev. 2019, 40, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Duffey, K.J.; Popkin, B.M. Sodium and potassium intake patterns and trends in South Korea. J. Hum. Hypertens. 2013, 27, 298–303. [Google Scholar] [CrossRef]
- Eurachem. Setting and Using Target Uncertainty in Chemical Measurement. 2015. Available online: https://www.eurachem.org/index.php/publications/guides/gd-stmu (accessed on 15 April 2021).
- Karbowska, B.; Rebis, T.; Milczarek, G. Electrode Modified by Reduced Graphene Oxide for Monitoring of Total Thallium in Grain Products. Int. J. Environ. Res. Public Health 2018, 15, 653. [Google Scholar] [CrossRef] [Green Version]
- Esposito, M.; De Roma, A.; Cavallo, S.; Miedico, O.; Chiaravalle, E.; Soprano, V.; Baldi, L.; Gallo, P. Trace elements in vegetables and fruits cultivated in Southern Italy. J. Food Compos. Anal. 2019, 84, 103302. [Google Scholar] [CrossRef]
- Biata, N.R.; Mashile, G.P.; Ramontja, J.; Mketo, N.; Nomngongo, P.N. Application of ultrasound-assisted cloud point extraction for preconcentration of antimony, tin and thallium in food and water samples prior to ICP-OES determination. J. Food Compos. Anal. 2019, 76, 14–21. [Google Scholar] [CrossRef]
- Robinson, B.; Anderson, C. Element case studies: Thallium and noble metals. In Agromining: Farming for Metals; Springer: Cham, Switzerland, 2018; pp. 253–261. [Google Scholar]
- Karbowska, B. Presence of thallium in the environment: Sources of contaminations, distribution and monitoring methods. Environ. Monit. Assess. 2016, 188, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Fan, Y.; Yuan, F.; Cooke, D.; Zhang, X.; Li, L. A preliminary investigation and evaluation of the thallium environmental impacts of the unmined Xiangquan thallium-only deposit in Hexian, China. Environ. Geol. 2008, 54, 131–145. [Google Scholar] [CrossRef]
- Van Overmeire, I.; Pussemier, L.; Hanot, V.; De Temmerman, L.; Hoenig, M.; Goeyens, L. Chemical contamination of free-range eggs from Belgium. Food Addit. Contam. 2006, 23, 1109–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grace, E.J.; MacFarlane, G.R. Assessment of the bioaccumulation of metals to chicken eggs from residential backyards. Sci. Total Environ. 2016, 563, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Franke, B.M.; Hadorn, R.; Bosset, J.O.; Gremaud, G.; Kreuzer, M. Is authentication of the geographic origin of poultry meat and dried beef improved by combining multiple trace element and oxygen isotope analysis? Meat Sci. 2008, 80, 944–947. [Google Scholar] [CrossRef]
- Ikemoto, T.; Nguyen, P.C.T.; Okuda, N.; Iwata, A.; Omori, K.; Tanabe, S.; Tuyen, B.C.; Takeuchi, I. Biomagnification of trace elements in the aquatic food web in the Mekong Delta, South Vietnam using stable carbon and nitrogen isotope analysis. Arch. Environ. Contam. Toxicol. 2008, 54, 504–515. [Google Scholar] [CrossRef]
- Tendler, B.; Ohiozebau, E.; Codling, G.; Giesy, J.P.; Jones, P.D. Concentrations of Metals in Fishes from the Athabasca and Slave Rivers of Northern Canada. Environ. Toxicol. Chem. 2020, 39, 2180–2195. [Google Scholar] [CrossRef]
- Agah, H.; Leermakers, M.; Elskens, M.; Fatemi, S.M.R.; Baeyens, W. Accumulation of trace metals in the muscle and liver tissues of five fish species from the Persian Gulf. Environ. Monit. Assess. 2009, 157, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Migaszewski, Z.M.; Gałuszka, A. Abundance and fate of thallium and its stable isotopes in the environment. Rev. Environ. Sci. Bio/Technol. 2021, 20, 5–30. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.; Liu, J.; Wang, J.; Li, X.; Zhang, Y.; Liu, Y. Health risks of thallium in contaminated arable soils and food crops irrigated with wastewater from a sulfuric acid plant in western Guangdong province, China. Ecotoxicol. Environ. Saf. 2013, 90, 76–81. [Google Scholar] [CrossRef]
- Liu, J.; Luo, X.; Wang, J.; Xiao, T.; Chen, D.; Sheng, G.; Yin, M.; Lippold, H.; Wang, C.; Chen, Y. Thallium contamination in arable soils and vegetables around a steel plant—A newly-found significant source of Tl pollution in South China. Environ. Pollut. 2017, 224, 445–453. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Xiao, T.; Bao, Z.; Lippold, H.; Luo, X.; Yin, M.; Ren, J.; Chen, Y.; Linghu, W. Geochemical dispersal of thallium and accompanying metals in sediment profiles from a smelter-impacted area in South China. Appl. Geochem. 2018, 88, 239–246. [Google Scholar] [CrossRef]
- Jiang, Y.; Wei, X.; He, H.; She, J.; Liu, J.; Fang, F.; Zhang, W.; Liu, Y.; Wang, J.; Xiao, T.; et al. Transformation and fate of thallium and accompanying metal(loid)s in paddy soils and rice: A case study from a large-scale industrial area in China. J. Hazard. Mater. 2022, 423, 126997. [Google Scholar] [CrossRef]
- Ning, Z.; Liu, E.; Yao, D.; Xiao, T.; Ma, L.; Liu, Y.; Li, H.; Liu, C. Contamination, oral bioaccessibility and human health risk assessment of thallium and other metal(loid)s in farmland soils around a historic Tl–Hg mining area. Sci. Total Environ. 2021, 758, 143577. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, X.; Sun, Y.; Tsang, D.C.W.; Qi, J.; Zhang, W.; Li, N.; Yin, M.; Wang, J.; Lippold, H.; et al. Thallium pollution in China and removal technologies for waters: A review. Environ. Int. 2019, 126, 771–790. [Google Scholar] [CrossRef]
- Peter, A.L.J.; Viraraghavan, T. Thallium: A review of public health and environmental concerns. Environ. Int. 2005, 31, 493–501. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, D.-J.; Ahn, B.-K. Distributions and concentrations of thallium in Korean soils determined by single and sequential extraction procedures. Bull. Environ. Contam. Toxicol. 2015, 94, 756–763. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Wei, X.; Ren, S.; Yang, X.; Beiyuan, J.; Wei, L.; Liu, J.; She, J.; Zhang, W.; et al. Escalating health risk of thallium and arsenic from farmland contamination fueled by cement-making activities: A hidden but significant source. Sci. Total Environ. 2021, 782, 146603. [Google Scholar] [CrossRef]
- Schunk, P.F.T.; Kalil, I.C.; Pimentel-Schmitt, E.F.; Lenz, D.; de Andrade, T.U.; Ribeiro, J.S.; Endringer, D.C. ICP-OES and Micronucleus Test to Evaluate Heavy Metal Contamination in Commercially Available Brazilian Herbal Teas. Biol. Trace Elemt. Res. 2016, 172, 258–265. [Google Scholar] [CrossRef]
- Pearson, A.J.; Ashmore, E. Risk assessment of antimony, barium, beryllium, boron, bromine, lithium, nickel, strontium, thallium and uranium concentrations in the New Zealand diet. Food Addit. Contam. Part A 2020, 37, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Drewnowska, M.; Falandysz, J.; Chudzinska, M.; Hanc, A.; Saba, M.; Baraikiewicz, D. Leaching of arsenic and sixteen metallic elements from Amanita fulva mushrooms after food processing. LWT Food Sci. Technol. 2017, 84, 861–866. [Google Scholar] [CrossRef]
- Loula, M.; Kana, A.; Vosmanska, M.; Koplik, R.; Mestek, O. Transfer of thallium from rape seed to rape oil is negligible and oil is fit for human consumption. Food Addit. Contam. Part A 2016, 33, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.N. Effects of Processing on Heavy Metal Content of Foods. In Impact of Processing on Food Safety; Jackson, L.S., Knize, M.G., Morgan, J.N., Eds.; Springer: Boston, MA, USA, 1999; pp. 195–211. [Google Scholar]
- Liu, J.; Wei, X.; Zhou, Y.; Tsang, D.C.W.; Bao, Z.; Yin, M.; Lippold, H.; Yuan, W.; Wang, J.; Feng, Y.; et al. Thallium contamination, health risk assessment and source apportionment in common vegetables. Sci. Total Environ. 2020, 703, 135547. [Google Scholar] [CrossRef]
- Notten, M.J.; Oosthoek, A.J.P.; Rozema, J.; Aerts, R. Heavy metal concentrations in a soil–plant–snail food chain along a terrestrial soil pollution gradient. Environ. Pollut. 2005, 138, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Dudka, S.; Miller, W.P. Accumulation of potentially toxic elements in plants and their transfer to human food chain. J. Environ. Sci. Health Part B 1999, 34, 681–708. [Google Scholar] [CrossRef]
- K.D.C.A.P.A. Korea National Health and Nutrition Examination Survey. 2020. Available online: https://knhanes.kdca.go.kr/knhanes/eng/index.do (accessed on 2 January 2021).
- RIVM. Maximum Permissible Risk Levels for Human Intake of Soil Contaminants. 1998. Available online: https://www.rivm.nl/bibliotheek/rapporten/711701004.pdf (accessed on 25 August 2021).
- MFDS. Report on Reassessment of Heavy Metals Criteria of South Korean Food. 2017. Available online: https://www.mfds.go.kr/brd/m_511/view.do?seq=27035&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=2 (accessed on 20 June 2021).
- Mindak, W.R.; Cheng, J.; Capar, S.G. Elemental Analysis Manual for Food and Related Products. 2014. Available online: https://www.fda.gov/media/89333/download (accessed on 15 June 2020).
- MFDS. Guideline for Analysis of Heavy Metals in Food. 2019. Available online: http://nifds.go.kr/brd/m_18/view.do?seq=12471&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=12 (accessed on 15 June 2020).
- MFDS. Food Nutrient Component Data Base. 2021. Available online: https://www.foodsafetykorea.go.kr/fcdb/ (accessed on 10 January 2021).

| Classification | Representative Food | Regression Equation | R2 | MLOD a (μg kg−1) | MLOQ b (μg kg−1) |
|---|---|---|---|---|---|
| Non-fatty Solid | Rice | y = 117366x − 208.52 | 1 | 0.0092 | 0.0293 |
| Hydrated Solid | Apple | y = 38252x + 774.86 | 0.9999 | 0.0498 | 0.1585 |
| Fatty Solid | Beef | y = 85346x − 6.9005 | 0.9997 | 0.0070 | 0.0222 |
| Salty Solid | Sea Salt | y = 67663x + 165.49 | 0.9999 | 0.0207 | 0.0661 |
| Non-Fatty Liquid | Orange Juice | y = 88399x − 441.29 | 1 | 0.0126 | 0.0401 |
| Fatty Liquid | Sesame Oil | y = 111833x − 120.50 | 1 | 0.0225 | 0.0715 |
| Category | Subcategory (n) a | Frequency of Detection b (%) | Min–Max c (μg kg−1) | Median (μg kg−1) | Mean ± SD c (μg kg−1) |
|---|---|---|---|---|---|
| Agricultural Products | Cereals (32) | 50 | 0.00–5.27 | 0.20 | 0.83 ± 1.47 |
| Beans (10) | 50 | 0.00–2.31 | 0.00 | 0.49 ± 0.80 | |
| Tuber crops (6) | 100 | 3.78–13.21 | 5.13 | 6.67 ± 3.70 | |
| Vegetables (50) | 66 | 0.02–15.93 | 0.34 | 1.59 ± 3.21 | |
| Mushrooms (6) | 0 | 0.02–0.02 | 0.02 | 0.02 ± 0.00 | |
| Fruits (9) | 56 | 0.02–7.00 | 0.34 | 1.48 ± 2.39 | |
| Livestock Products | Eggs (3) | 100 | 0.65–5.72 | 1.03 | 2.46 ± 2.31 |
| Meats (9) | 100 | 0.51–1.63 | 1.00 | 0.79 ± 0.38 | |
| Fishery Products | Fishes (21) | 100 | 0.41–1.87 | 1.03 | 1.06 ± 0.37 |
| Cephalopoda (6) | 100 | 1.04–1.22 | 1.07 | 1.10 ± 0.06 | |
| Shellfishes (10) | 100 | 1.20–6.02 | 1.88 | 2.55 ± 1.46 | |
| Crustaceans (4) | 100 | 1.07–1.79 | 1.14 | 1.28 ± 0.29 | |
| Sea Algae (9) | 22 | 0.02–12.72 | 0.02 | 1.21 ± 1.40 |
| Category | Subcategory (n) a | Frequency of Detection b (%) | Min–Max c (μg kg −1) | Median (μg kg −1) | Mean ± SD c (μg kg −1) |
|---|---|---|---|---|---|
| Beverages | Infusion Teas (18) | 39 | 0.01–1.99 | 0.52 | 0.61 ± 0.63 |
| Other Beverages (21) | 33 | 0.01–4.12 | 0.01 | 0.49 ± 0.97 | |
| Other Processed Foods | Snacks (6) | 100 | 0.01–0.01 | 1.10 | 0.01 ± 0.00 |
| Salted Seafood processing Products (12) | 25 | 0.01–1.41 | 0.01 | 0.30 ± 0.52 | |
| Pickled Foods (12) | 8 | 0.01–19.46 | 0.01 | 1.63 ± 5.38 | |
| Sauces (18) Oil Products (27) | 0 | 0.01–0.01 | 0.01 | 0.01 ± 0.00 | |
| 0 | 0.01–0.01 | 0.01 | 0.01 ± 0.00 | ||
| Dairy Products (6) | 0 | 0.01–0.01 | 0.01 | 0.01 ± 0.00 | |
| Cereal Products (3) | 0 | 0.00–0.00 | 0.00 | 0.00 ± 0.00 | |
| Livestock Processing Products (3) | 0 | 0.01–0.01 | 0.01 | 0.01 ± 0.00 | |
| Noodles (3) | 0 | 0.01–0.01 | 0.01 | 0.01 ± 0.00 |
| Food Group | Concentration (μg kg−1) | Exposure Dose (μg kg−1 bw day−1) | % HBGV | |
|---|---|---|---|---|
| Agricultural Products | Cereals | 0.01–5.27 | 0.0000–0.0001 | 0.0000–0.0345 |
| Fruits | 0.01–7.00 | 0.0000–0.0011 | 0.0000–0.5625 | |
| Beans | 0.01–2.32 | 0.0000–0.0001 | 0.0000–0.0549 | |
| Mushrooms | 0.01 | 0.0000–0.0000 | 0.0001–0.0002 | |
| Tuber Crops | 3.78–13.21 | 0.0012–0.0029 | 0.6050–1.4682 | |
| Vegetables | 0.01–15.93 | 0.0000–0.0032 | 0.0001–1.5807 | |
| Livestock Products | Eggs | 0.65–5.72 | 0.0003–0.0028 | 0.1604-1.4109 |
| Meats | 0.51–1.63 | 0.0001–0.0009 | 0.0502-0.4326 | |
| Fishery Products | Crustaceans | 1.07–1.79 | 0.0000–0.0000 | 0.0130-0.0216 |
| Fishes | 0.41–1.87 | 0.0000–0.0001 | 0.0027-0.0440 | |
| Cephalopoda | 1.04–1.22 | 0.0000–0.0001 | 0.0143-0.0445 | |
| Shellfishes | 1.20–6.02 | 0.0000–0.0001 | 0.0106-0.0606 | |
| Seaweeds | 0.01–12.72 | 0.0000–0.0002 | 0.0000-0.0900 | |
| Processed Products | Beverages | 0.01–4.12 | 0.0000–0.0003 | 0.0001-0.1549 |
| Other Processes Products | 0.00–19.46 | 0.0000–0.0014 | 0.0000-0.6881 | |
| Classification | Sample Weight (g) | Food Group |
|---|---|---|
| Non-Fatty Solid | 0.5 | Cereals, Tubers, Beans, Snacks, Cereal Products |
| Hydrated Solid | 0.5 | Fruits, Vegetables, Mushrooms, Sea Algae |
| Fatty Solid | 0.5 | Meats, Eggs, Fishes, Cephalopods, Shellfishes, Crustaceans |
| Salty Solid | 0.1 | Sauces, Pickled Foods, Salt-Added Products, Noodles |
| Non-Fatty Liquid | 0.5 | Infusion Teas, Other Beverages |
| Fatty Liquid | 0.3 | Oil Products, Dairy Products |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-h.; Ra, W.-j.; Cho, S.; Choi, S.; Soh, B.; Joo, Y.; Lee, K.-W. Method Validation for Determination of Thallium by Inductively Coupled Plasma Mass Spectrometry and Monitoring of Various Foods in South Korea. Molecules 2021, 26, 6729. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26216729
Kim Y-h, Ra W-j, Cho S, Choi S, Soh B, Joo Y, Lee K-W. Method Validation for Determination of Thallium by Inductively Coupled Plasma Mass Spectrometry and Monitoring of Various Foods in South Korea. Molecules. 2021; 26(21):6729. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26216729
Chicago/Turabian StyleKim, Yeon-hee, Wook-jin Ra, Solyi Cho, Shinai Choi, Bokyung Soh, Yongsung Joo, and Kwang-Won Lee. 2021. "Method Validation for Determination of Thallium by Inductively Coupled Plasma Mass Spectrometry and Monitoring of Various Foods in South Korea" Molecules 26, no. 21: 6729. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26216729