Guayule (Parthenium argentatum A. Gray), a Renewable Resource for Natural Polyisoprene and Resin: Composition, Processes and Applications
Abstract
:1. Introduction
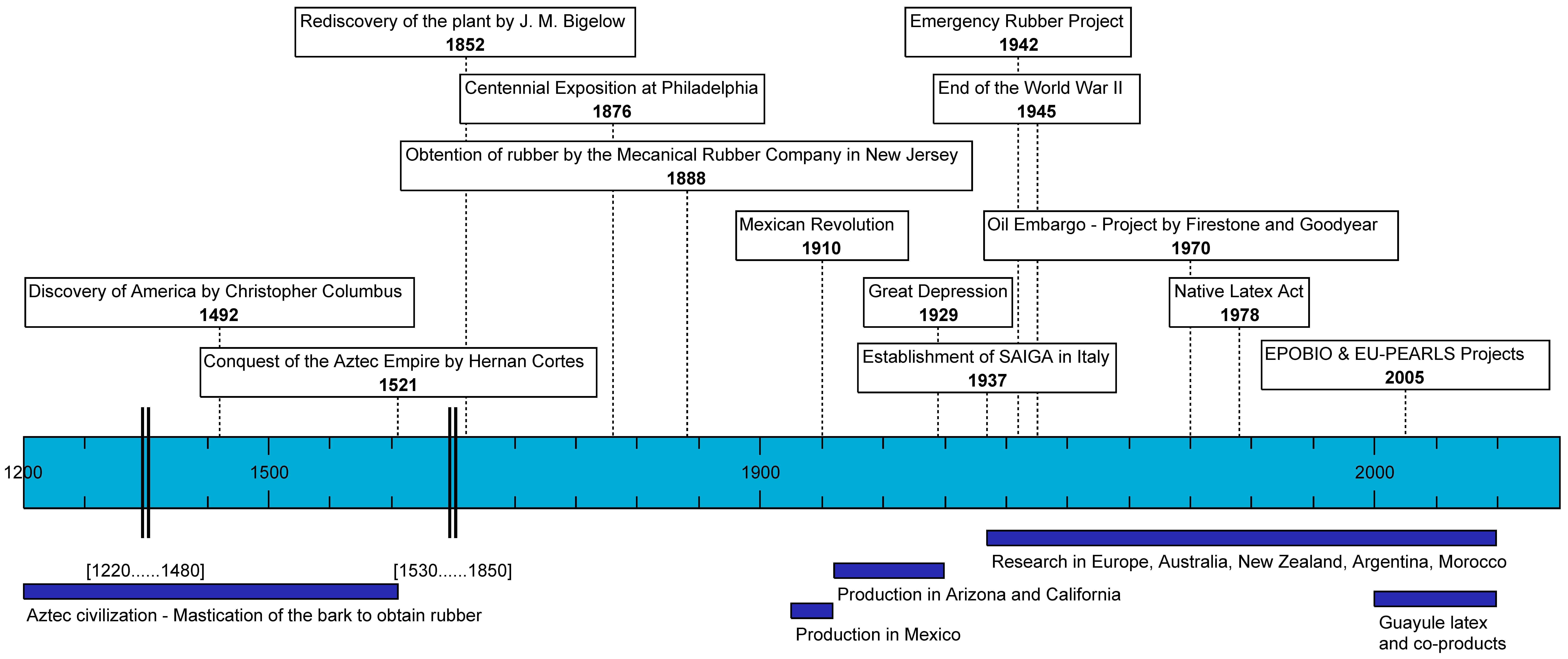
2. History of Guayule
3. Botanical Description, Geographical Distribution and Cultivation Practices
3.1. Botanical Description
3.2. Geographical Distribution
3.3. Cultivation
4. Distribution and Biosynthesis of Polyisoprene and Resin
4.1. Distribution of Polyisoprene and Resin in the Shrub
4.1.1. IPP as Central Precursor Unit
4.1.2. Biosynthesis of Polyisoprene
5. Chemical Composition of Extractables
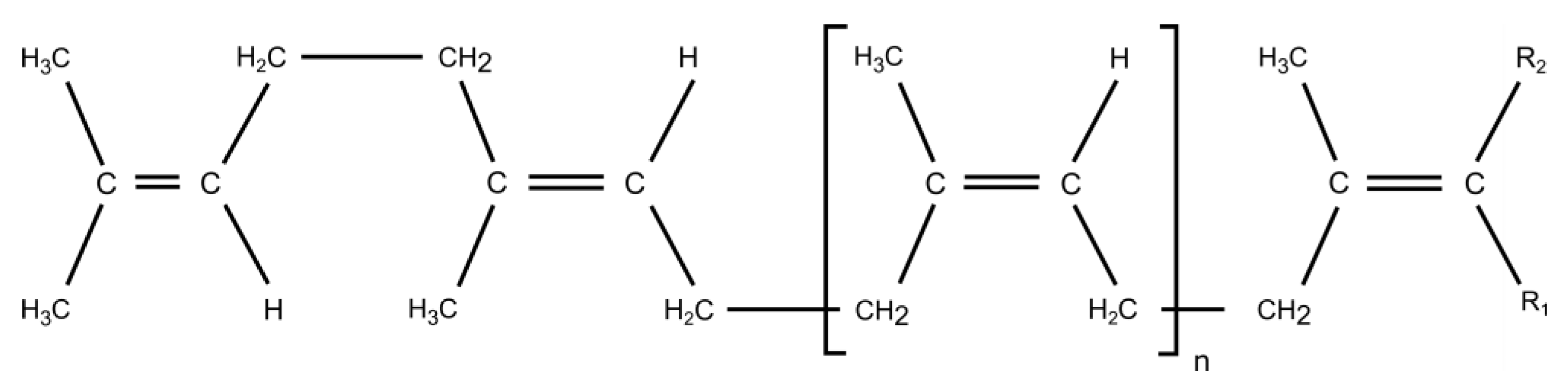
5.1. Polyisoprene
5.2. Resin
5.3. Solid Residues
6. Processes to Extract Dry Rubber and Latex
6.1. Industrial Processes
6.1.1. Flotation
- The usual method of disposing of this bagasse is to burn it as fuel but, because of the residual rubber, it is an expensive fuel and it implies a great loss to the manufacturer [72].
- A lot of rubber is still trapped in the bagasse, but a prolonged grinding in view of increasing the yield reduces the tensile-elongation properties of the rubber and makes it softer and thus more liable to retain fiber and other foreign matter [75].
- Improvement to clean the raw material: extraction of the rubber with a mixture of acetone, amyl oxyhydrate, methyl oxyhydrate, and alcohol while heating. The resin, oil, and wax can be then separated from solvents by distillation [79]. Another option is to distillate water and essential oil from the obtained rubber [80].
- Improvement of the grinding method: replace the rubs or grinds by a suitable comminuting cut. The main advantage of the process is that it makes possible the use of continuously operating machines.
- Elimination of the resin with a solvent before the original process. The crude woody material can be treated preliminarily with a volatile solvent in which the resin is soluble while the rubber is insoluble (acetone, ethyl alcohol, methyl alcohol), made a much-shortened grinding operation possible, and the particles of rubber display a greater tendency to cohere together than to adhere to other materials [70,81,82].
- Improvement of the separation of the rubber stuck in bagasse: decreasing the specific gravity of the rubber particles, by making them lighter and thus increasing their buoyancy in separating fluid, by adding petroleum-distillate.
- To avoid rubber deterioration, the shrub can be treated with a preservative or stabilizing agent which will stabilize or preserve the rubber: immersion in a tank containing a solution of 1% of dimethyl-paraphenylenediamine [71].
- Improvement of the liberation of the rubber by exposing the shrub to suitable gaseous agent, which penetrates the cells and raises enough pressure to expand instantaneously, like an explosion [74].
6.1.2. Sequential Extraction
6.1.3. Simultaneous Extraction
- Fractionating rubber by molecular weight using solvents [85].
- Use of different solvents: Bridgestone/Firestone developed a continuous processing using simultaneous extraction with pentane-acetone azeotrope, known as “Simultaneous Extraction and Rubber Fractionation” (SERF) [86].
- Texas A&M University has developed a process based on a batch-mixing screw pressing extraction [87].
6.1.4. Supercritical CO2 Process
6.1.5. Latex Process
- Improvement of centrifugation: a continuous process using a centrifugal separator to concentrate dilute rubber dispersion in such a way that little or no pasty or firmly coagulated rubber is formed in the bowl to avoid interfering with the efficiency of separation [86].
- Improvement of the cleaning of the product: by cleaning the latex with polar and non-polar solvent (simultaneously or sequentially) or by freezing the latex, or drying the latex to obtain dry rubber [87].
- Improvement of grinding shrub: grinding plants by rotary shearing [50].
- Improvement of latex stabilization: in order to stabilize the latex, grinding of the plants can be made in a buffer containing ammonium hydroxide (or potassium hydroxide, sodium colehydroxide, sodium bicarbonate) and an antioxidant, such as sodium sulfite (or butylated hydroxytoluene, butylated hydroxyanisole) [90].
6.2. Analytical Methods
7. Proposed Applications for Guayule-Derived Products
7.1. Polyisoprene
7.2. Resin
7.3. Leaves
7.4. Bagasse
8. Economic Considerations
- Some of them try to reduce production costs by investing in the agronomical practices and extraction process in order to increase the yields. Using varietal selection in order to increase field yield for example.
- Others try to sell manufactured products (like gloves or tyres) rather than raw-materials (latex or rubber) because the last make generally a small part of the end-product price. This is vertical integration.
- Another route is to focus on high-value market, here the latex market rather than the rubber one, for producing non-allergenic latex for high value medical gloves, for example.
- The last way is a kind of horizontal integration by valorizing all coproducts: latex, rubber, resin, bagasse… The bio-refinery path: relying on different products and markets, in order to cover the production cost.
9. Nagoya Protocol
10. Future Trends
Author Contributions
Funding
Conflicts of Interest
References
- Minh-Thu, D.-A. La Chimie du Végétal, Pour du Biosourcé au Quotidien. Available online: http://www.lactualitechimique.org/Ressources/AC-Decouverte/La-chimie-du-vegetal-pour-du-biosource-au-quotidien (accessed on 11 May 2020).
- Seidel, A.; Kirk, R.E.; Othmer, D.F. Kirk-Othmer Encyclopedia of Chemical Technology; Wiley-Interscience: Hoboken, NJ, USA, 2007; pp. 1–26. ISBN 978-0-471-48496-7. [Google Scholar]
- Pioch, D.; Palu, S. Du Caoutchouc Naturel en Europe. Available online: https://www.pourlascience.fr/sd/biologie-vegetale/du-caoutchouc-naturel-en-europe-6119.php (accessed on 22 November 2019).
- Cornish, K.; Brichta, J.L.; Yu, P.; Wood, D.F.; Mcglothlin, M.W.; Martin, J.A. Guayule Latex Provides a Solution for the Critical Demands of the Non-Allergenic Medical Products Market. Agro Food Ind. Hi Tech 2001, 12, 27–32. [Google Scholar]
- Bajaj, Y.P.S. Medicinal and Aromatic Plants IX; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-662-08618-6. [Google Scholar]
- Bowers, J.E. Natural Rubber-Producing Plants for the United States; U.S. Department of Agriculture, National Agricultural Library: Beltsville, MD, USA, 1990.
- Venkatachalam, P.; Geetha, N.; Sangeetha, P.; Thulaseedharan, A. Natural Rubber Producing Plants: An Overview. Afr. J. Biotechnol. 2013, 12, 1297–1310. [Google Scholar]
- Van Beilen, J.B.; Poirier, Y. Guayule and Russian Dandelion as Alternative Sources of Natural Rubber. Crit. Rev. Biotechnol. 2007, 27, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Clarivate Analytics Web of Science. Available online: Webofknowledge.com (accessed on 5 May 2020).
- United Nations Sustainable Development Goals: Knowledge Platform. Available online: https://sustainabledevelopment.un.org/ (accessed on 2 July 2020).
- Nakayama, F.S. Guayule Future Development. Ind. Crops Prod. 2005, 22, 3–13. [Google Scholar] [CrossRef]
- Mooibroek, H.; Cornish, K. Alternative Sources of Natural Rubber. Appl. Microbiol. Biotechnol. 2000, 53, 355–365. [Google Scholar] [CrossRef]
- Lloyd, F.E. Guayule (Parthenium argentatum Gray); A Rubber-Plant of the Chihuahuan Desert; Cargenie Institution of Washington: Washington, DC, USA, 1911. [Google Scholar]
- Wayne Whitworth, J.; Whitehead, E. Guayule, Natural Rubber: A Technical Publication with Emphasis on Recent Findings; Guayule Administration Management Comitee and USDA Cooperative State Research Service: Tucson, AZ, USA, 1991.
- Hammond, B.; Polhamus, L. Research on Guayule (Parthenium argentatum): 1942–1959; Technical Bulletin N°1327; Agricultural Research Service, USDA: Washington, DC, USA, 1965.
- National Academy of Sciences. Guayule, An. Alternative Source of Natural Rubber; National Academy of Sciences: Washington, DC, USA, 1977. [Google Scholar]
- Campos-Lopez, E. Natural Resources and Development in Arid Regions; Routledge: Abingdon, UK, 2019; ISBN 978-0-429-72507-4. [Google Scholar]
- Zanetti, F. Guayule Rubber a Strategic Raw Material for Europe with a Long-Term History within the AAIC (Association for the Advancement of Industrial Crops). In Proceedings of the AAIC Congress, Montpellier, France; 2019. [Google Scholar]
- Monti, A. The Future of Guayule in Europe. In Proceedings of the AAIC Congress, Montpellier, France; 2014. [Google Scholar]
- Domenici, P.V. All Info-S.1816-95th Congress (1977–1978): Native Latex Commercialization and Economic Development Act. Available online: https://www.congress.gov/bill/95th-congress/senate-bill/1816/all-info (accessed on 22 April 2020).
- Wood, M.A. Guayule Latex Process Is Licensed. Available online: https://www.ars.usda.gov/news-events/news/research-news/1997/guayule-latex-process-is-licensed/ (accessed on 21 August 2020).
- Final Report Summary-EPOBIO. Available online: https://cordis.europa.eu/project/id/22681/reporting (accessed on 22 April 2020).
- Final Report Summary-EU-PEARLS (EU-Based Production and Exploitation of Alternative Rubber and Latex Sources). Available online: https://cordis.europa.eu/project/id/212827/reporting (accessed on 1 May 2020).
- From Seed to Tread: Bridgestone Reveals First Tires Made Entirely of Natural Rubber Components from Company’s Guayule Research Operations. Available online: https://www.bridgestoneamericas.com/content/bscorpcomm-sites/americas/en/newsroom/press-releases/2015/-from-seed-to-tread--bridgestone-reveals-first-tires-made-entire.html (accessed on 22 April 2020).
- Rehbein, M. Coopertires. Available online: http://coopertire.com/ (accessed on 8 June 2020).
- Parthenium argentatum, A. Gray. Available online: https://www.itis.gov/ (accessed on 19 June 2020).
- Rasutis, D.; Soratana, K.; McMahan, C.; Landis, A.E. A Sustainability Review of Domestic Rubber from the Guayule Plant. Ind. Crops Prod. 2015, 70, 383–394. [Google Scholar] [CrossRef]
- Julien, M.; McFadyen, R.; Cullen, J. Biological Control. of Weeds in Australia; CSIRO: Canberra, Australia, 2012. [Google Scholar]
- Hunsaker, D.J.; Elshikha, D.M.; Bronson, K.F. High Guayule Rubber Production with Subsurface Drip Irrigation in the US Desert Southwest. Agric. Water Manag. 2019, 220, 1–12. [Google Scholar] [CrossRef]
- Doe, J. EUropean NAtural Rubber Substitute from Guayule. Available online: https://ec.europa.eu/growth/content/european-natural-rubber-substitute-guayule_en (accessed on 8 April 2020).
- Coates, W.; Ayerza, R.; Ravetta, D. Guayule Rubber and Latex Content-Seasonal Variations over Time in Argentina. Ind. Crops Prod. 2001, 14, 85–91. [Google Scholar] [CrossRef]
- Dissanayake, P.; George, D.L.; Gupta, M.L. Improved Guayule Lines Outperform Old Lines in South-East Queensland. Ind. Crops Prod. 2007, 25, 178–189. [Google Scholar] [CrossRef]
- Bekaardt, C.R. Establishment of Guayule (Parthenium argentatum Gray); University of Stellenbosch: Stellenbosch, South Africa, 2002. [Google Scholar]
- Ray, D.T. Guayule: A Source of Natural Rubber. New Crop. 1993, 338–343. [Google Scholar]
- Jasso de Rodríguez, D.; Angulo-Sánchez, J.L.; Rodríguez-García, R. An Overview of Guayule Research and Development in Mexico. Ind. Crops Prod. 2006, 24, 269–273. [Google Scholar] [CrossRef]
- Angulo-Sánchez, J.L.; Neira-Velázquez, G.; de Rodriguez, D.J. Multimodal Molecular Weight Distributions of Guayule Rubber from High Rubber Yielding Lines. Ind. Crops Prod. 1995, 4, 113–120. [Google Scholar] [CrossRef]
- Sidhu, O.P.; Ratti, N.; Behl, H.M. Quantitative and Qualitative Variations in Resin Content and Guayulins (A and B) among Different Guayule Cultivars. J. Agric. Food Chem. 1995, 43, 2012–2015. [Google Scholar] [CrossRef]
- Kajiura, H.; Suzuki, N.; Mouri, H.; Watanabe, N.; Nakazawa, Y. Elucidation of Rubber Biosynthesis and Accumulation in the Rubber Producing Shrub, Guayule (Parthenium argentatum Gray). Planta 2018, 247, 513–526. [Google Scholar] [CrossRef]
- Amor, A.; Sanier, C.; Verdeil, J.-L.; Lartaud, M.; Punvichai, T.; Tardan, E.; Palu, S.; Pioch, D. Biomass Imaging as a Tool for Addressing the Challenge of Multiple-Product Guayule Biorefinery. In Proceedings of the Association for the Advancement of Industrial Crops International Conference, Rochester, NY, USA, 24 September 2016. [Google Scholar]
- Estilai, A. Biomass, Rubber, and Resin Yield Potentials of New Guayule Germplasm. Bioresour. Technol. 1991, 35, 119–125. [Google Scholar] [CrossRef]
- Cantú, D.J.; Angulo-Sánchez, J.L.; Rodríguez-García, R.; Kuruvadi, S. Seasonal Growth, Rubber and Resin Yield Characteristics of Guayule under Natural Environmental Conditions. Ind. Crops Prod. 1997, 6, 131–137. [Google Scholar] [CrossRef]
- Kush, A. Isoprenoid Biosynthesis: The Hevea Factory. Plant Physiol. Biochem. 1994, 32, 761–767. [Google Scholar]
- Men, X.; Wang, F.; Chen, G.-Q.; Zhang, H.-B.; Xian, M. Biosynthesis of Natural Rubber: Current State and Perspectives. IJMS 2018, 20, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonner, J.; Galston, A.W. The Physiology and Biochemistry of Rubber Formation in Plants. Bot. Rev. 1947, 13, 543–596. [Google Scholar] [CrossRef]
- Swanson, K.M.; Hohl, R.J. Anti-Cancer Therapy: Targeting the Mevalonate Pathway. Curr. Cancer Drug Targets 2006, 6, 15–37. [Google Scholar] [CrossRef]
- Niu, F.-X.; Lu, Q.; Bu, Y.-F.; Liu, J.-Z. Metabolic Engineering for the Microbial Production of Isoprenoids: Carotenoids and Isoprenoid-Based Biofuels. Synth. Syst. Biotechnol. 2017, 2, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, R.A.; Walsh, S. The Ontogeny of Rubber Formation in Guayule, Parthenium argentatum Gray. Bot. Gaz. 1983, 144, 391–400. [Google Scholar] [CrossRef]
- Cornish, K.; Wood, D.F.; Windle, J.J. Rubber Particles from Four Different Species, Examined by Transmission Electron Microscopy and Electron-Paramagnetic-Resonance Spin Labeling, Are Found to Consist of a Homogeneous Rubber Core Enclosed by a Contiguous, Monolayer Biomembrane. Planta 1999, 210, 85–96. [Google Scholar] [CrossRef]
- Bhowmick, A.K.; Stephens, H.L. Handbook of Elastomers, 2nd ed.; Marcel Dekker Inc.: New York, NY, USA, 2020; ISBN 0-8247-0383-9. [Google Scholar]
- Amor, A.; Palu, S.; Pioch, D.; Dorget, M. Extraction of Polyisoprene with High Molar Mass. U.S. Patent 2018086854A1, 4 April 2016. [Google Scholar]
- Junkong, P.; Cornish, K.; Ikeda, Y. Characteristics of Mechanical Properties of Sulphur Cross-Linked Guayule and Dandelion Natural Rubbers. RSC Adv. 2017, 7, 50739–50752. [Google Scholar] [CrossRef] [Green Version]
- Banigan, T.F.; Verbiscar, A.J.; Weber, C.W. Composition of Guayule Leaves, Seed and Wood. J. Agric. Food Chem. 1982, 30, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Belmares, H.; Jimenez, L.L.; Ortega, M. New Rubber Peptizers and Coatings Derived from Guayule Resin (Parthenium argentatum Gray). Ind. Eng. Chem. Prod. Res. Dev. 1980, 19, 107–111. [Google Scholar] [CrossRef]
- Dehghanizadeh, M.; Cheng, F.; Jarvis, J.M.; Holguin, F.O.; Brewer, C.E. Characterization of Resin Extracted from Guayule (Parthenium argentatum): A Dataset Including GC–MS and FT-ICR MS. Data Brief. 2020, 31, 105989. [Google Scholar] [CrossRef]
- Schloman, W.W.; Hively, R.A.; Krishen, A.; Andrews, A.M. Guayule Byproduct Evaluation: Extract Characterization. J. Agric. Food Chem. 1983, 31, 873–876. [Google Scholar] [CrossRef]
- de Vivar, A.R.; Martinez-Vasquez, M.; Matsubara, C.; Perez-Sanchez, G.; Joseph-Nathan, P. Triperpenes in Parthenium argentatum, Structures of Argentatins C and D. Phytochemistry 1990, 19, 915–918. [Google Scholar] [CrossRef]
- Maatooq, G.T.; Hoffmann, J.J. Pyridine Alkaloids from A Parthenium Hybrid. Z. Für Nat. C 2002, 57, 211–215. [Google Scholar] [CrossRef] [Green Version]
- Costa, E.; Aguado, J.; Ovejero, G.; Cañizares, P. Conversion of Guayule Resin to C1-C10 Hydrocarbons on Zeolite Based Catalysts. Fuel 1992, 71, 109–113. [Google Scholar] [CrossRef]
- Mears, J.A. The Flavonoids of Parthenium, L. J. Nat. Prod. 1980, 43, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Querci, C.; Del Prete, D.; Caldararo, M.; Girotti, G. Method for the Separation of the Isoprenic Constituents of Guayule-World Intellectual Property Organization. WO 2017021852, 2 September 2017. [Google Scholar]
- Haagen-Smit, A.J.; Siu, R. Chemical Investigations in Guayule. I. Essential Oil of Guayule, Parthenium argentatum, Gray. J. Am. Chem. Soc. 1944, 66, 2068–2074. [Google Scholar] [CrossRef]
- Scora, R.W.; Kumamoto, J. Essential Leaf Oils of Parthenium argentatum, A. Gray. J. Agric. Food Chem. 1979, 27, 642–643. [Google Scholar] [CrossRef]
- Estilai, A. Oil Content and Fatty Acid Composition of Seed Oil from Guayule Plants with Different Chromosome Numbers. J. Am. Oil Chem. Soc. 1993, 70, 547–549. [Google Scholar] [CrossRef]
- Earle, F.R.; Jones, Q. Analyses of Seed Samples from 113 Plant Families. Econ. Bot. 1962, 16, 221–250. [Google Scholar] [CrossRef]
- Chow, P.; Nakayama, F.S.; Blahnik, B.; Youngquist, J.A.; Coffelt, T.A. Chemical Constituents and Physical Properties of Guayule Wood and Bark. Ind. Crops Prod. 2008, 28, 303–308. [Google Scholar] [CrossRef]
- Traub, H.P.; Slattery, M.C. Analysis of Levulins, Inulin, and Monosaccharides in Guayule. Bot. Gaz. 1946, 108, 295–299. [Google Scholar] [CrossRef]
- Fangmeier, D.D.; Rubis, D.D.; Taylor, B.B.; Foster, K.E. Guayule for Rubber Production in Arizona; College of Agriculture, University of Arizona: Tucson, AZ, USA, 1984; Volume Technical Bulletin 252. [Google Scholar]
- Lawrence, W.A. Art of Extracting Rubber without Solvents-United States Patent Office. U.S. Patent 741258A, 13 October 1903. [Google Scholar]
- Delafond, E. Mechanical Separation of Rubber from Rubber-Bearing Plants-United States Patent Office. U.S. Patent 24236505A, 11 December 1935. [Google Scholar]
- Chute, H.O.; Randel, F.L. Process of Producing Rubber-United States Patent Office. U.S. Patent 957495A, 5 October 1910. [Google Scholar]
- Spence, D. Rubber-United States Patent Office. U.S. Patent 1918671, 18 July 1933. [Google Scholar]
- Van der Linde, H.T.G. Recovery of Rubber-United States Patent Office. U.S. Patent 979902A, 27 December 1910. [Google Scholar]
- Spence, D. Extraction of Rubber from Guayule-United States Patent Office. U.S. Patent 1753185A, 1 April 1930. [Google Scholar]
- Yeandle, W.H. Recovery of Rubber-United States Patent Office. U.S. Patent 1695676A, 12 August 1928. [Google Scholar]
- Tint, H.; Murray, C.W. Method of Extracting Rubber from Plants-United States Patent Office. U.S. Patent 245969A, 23 August 1949. [Google Scholar]
- Von Stechow, K. Method of Extraction of Pure Raw Rubber from Rubber-Plants-United States Patent Office. U.S. Patent 814407A, 6 March 1906. [Google Scholar]
- Carnahan, G.H. Method of and Apparatus for Treating Gumlike Sustances-United States Patent Office. U.S. Patent 1671570A, 29 May 1928. [Google Scholar]
- Bradshaw, G.B. Process of Extracting Rubber-United States Patent Office. U.S. Patent 848567A, 26 March 1907. [Google Scholar]
- Kay, E.L.; Fulton, C. Process for Extracting Rubber and By-Products from Guayule and Guayule-like Shrubs-United States Patent Office. U.S. Patent 4435337A, 6 March 1984. [Google Scholar]
- Buchanan, R.A. Extraction of Rubber or Rubberlike Substances from Fibrous Plant Materials-United States Patent Office. U.S. Patent 4136131A, 23 January 1979. [Google Scholar]
- Kay, E.L.; Gutierrez, R. Extraction of Rubber and/or Resin from Rubber Containing Plants with a Monophase Solvent Mixture-United States Patent Office. U.S. Patent 468715A, 9 February 1892. [Google Scholar]
- Beinor, R.T.; Cole, W.M. Solvent Fractionation of Guayule Rubber-United States Patent Office. U.S. Patent 4623713A, 18 November 1986. [Google Scholar]
- Cole, W.M.; Fenske, S.L.; Serbin, D.J.; Malani, S.R.; Clark, F.J.; Beattie, J.L. Process for Extracting Resin from Guayule Plants-United Kingdom Patent Application. U.S. Patent 2174403A, 18 November 1986. [Google Scholar]
- Martentis, R.A.; Martin, J.A.; Plamthottam, S.; Cornish, K. Extraction and Fractionation of Biopolymers and Resins from Plant Materials-Australian Patent Office. AU Patent 2006302814B2, 21 April 2006. [Google Scholar]
- Spence, D. Preparation of Latex from Rubber-Producing Plants-United States Patent Office. U.S. Patent 2119030A, 31 May 1938. [Google Scholar]
- Jones, E.P. Process of Concentrating Aqueous Rubber Dispersion by Centrifuging-United States Patent Office. U.S. Patent 2475141A, 5 July 1949. [Google Scholar]
- Jones, E.; Cole, W.M.; Huang, Y.; Tomaszewski, W. Processes for Recovering Rubber from Natural Rubber Latex-World Intellectual Property Organization. U.S. Patent 2009129249A2, 21 May 2009. [Google Scholar]
- Cornish, K.; Marentis, R.T. Rapid Expanded Solvent Extraction-World Intellectual Property Organization. U.S. Patent 2009051606A1, 26 February 2009. [Google Scholar]
- Punvichai, T.; Amor, A.; Tardan, E.; Palu, S.; Pioch, D. SC-CO2 Extraction of Guayule Biomass (Parthenium argentatum)-Yield and Selectivity towards Valuable Co-Products, Lipids and Terpenics. Biointerface Res. Appl. Chem. 2016, 6, 1777–1787. [Google Scholar]
- Cornish, K.; McCoy, R.G.; Martin, J.A.; Jali, W.; Nocera, A. Biopolymer Extraction from Plant Material-United States Patent Office. U.S. Patent 20120063969A1, 15 March 2012. [Google Scholar]
- Cornish, K. Bioprocessing of Harvested Plant Materials for Extraction of Biopolymers and Related Materials and Methods-United States Patent Office. U.S. Patent 20150376434A1, 31 December 2015. [Google Scholar]
- Coffelt, T.A.; Williams, C.F. Characterization and Recycling of Waste Water from Guayule Latex Extraction. Ind. Crops Prod. 2009, 29, 648–653. [Google Scholar] [CrossRef]
- Punvichai, T. Etude de l’extraction Sélective de Composants Non-Polyisoprèniques de Parthenium argentatum Avec Des Fluides Sous Pression, Montpellier SUPAGRO; Ecole doctorale GAIA: Montpellier, France, 2016. [Google Scholar]
- Coffelt, T.A.; Nakayama, F.S. A Six Step Harvesting Procedure for Guayule Small Plots for Laboratory Analyses. Issues New Crops New Uses 2007, 66–71. [Google Scholar]
- Pearson, C.H.; Cornish, K.; Rath, D.J. Extraction of Natural Rubber and Resin from Guayule Using an Accelerated Solvent Extractor. Ind. Crops Prod. 2013, 43, 506–510. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Coffelt, T.A.; Cornish, K. Improved Methods for Extraction and Quantification of Resin and Rubber from Guayule. Ind. Crops Prod. 2009, 30, 9–16. [Google Scholar] [CrossRef]
- Kroeger, K.D.; Stumpf, D.K.; LaGrandeur, L.M.H.; Hoffmann, J.J. Determination of Latex Content in Guayule. Ind. Crops Prod. 1996, 5, 213–216. [Google Scholar] [CrossRef]
- Suchat, S.; Pioch, D.; Palu, S.; Tardan, E.; van Loo, E.N.; Davrieux, F. Fast Determination of the Resin and Rubber Content in Parthenium argentatum Biomass Using near Infrared Spectroscopy. Ind. Crops Prod. 2013, 45, 44–51. [Google Scholar] [CrossRef]
- Cornish, K.; Chapman, M.H.; Nakayama, F.S.; Vinyard, S.H.; Whitehand, L.C. Latex Quantification in Guayule Shrub and Homogenate. Ind. Crops Prod. 1999, 10, 121–136. [Google Scholar] [CrossRef]
- Black, L.T.; Hamerstrand, G.E.; Kwolek, W.F. Analysis of Rubber, Resin, and Moisture Content of Guayule by Near Infrared Reflectance Spectroscopy. Rubber Chem. Technol. 1985, 58, 304–313. [Google Scholar] [CrossRef]
- Kleine, L.G.; Foster, M.A. Chemometric NIR Calibration for Guayule Analysis. In Proceedings of the First International Conference on New Ind. Crops Prod, Riverside, CA, USA; 1990. [Google Scholar]
- Taurines, M.; Brancheriau, L.; Palu, S.; Pioch, D.; Tardan, E.; Boutahar, N.; Sartre, P.; Meunier, F. Determination of Natural Rubber and Resin Content of Guayule Fresh Biomass by near Infrared Spectroscopy. Ind. Crops Prod. 2019, 177–184. [Google Scholar] [CrossRef]
- De Jong, W.H.; Geertsma, R.E.; Tinkler, J.J.B. Medical Devices Manufactured from Latex: European Regulatory Initiatives. Methods 2002, 27, 93–98. [Google Scholar] [CrossRef]
- Medical Rubber Products & Medical Device Product Development. Available online: https://www.precisiondippings.co.uk/medical/ (accessed on 11 May 2020).
- Tullo, A.H. Chemical & Engineering News; A.C.S.: Washington, DC, USA, 2015. [Google Scholar]
- Niroomand, A.; Schuman, T.P.; Thames, S.F. Hydroxylated guayule rubber in powder coatings. JctJ. Coat. Technol. 1996, 68, 15–20. [Google Scholar]
- Bultman, J.D.; Gilbertson, R.L.; Adaskaveg, J.; Amburgey, T.L.; Parikh, S.V.; Bailey, C.A. The Efficacy of Guayule Resin as a Pesticide. Bioresour. Technol. 1991, 35, 197–201. [Google Scholar] [CrossRef]
- Nakayama, F.S.; Vinyard, S.H.; Chow, P.; Bajwa, D.S.; Youngquist, J.A.; Muehl, J.H.; Krzysik, A.M. Guayule as a Wood Preservative. Ind. Crops Prod. 2001, 14, 105–111. [Google Scholar] [CrossRef]
- Kang, A.; Lee, T.S. Secondary Metabolism for Isoprenoid-based Biofuels. In Biotechnology for Biofuel Production and Optimization; Elsevier: New York, NY, USA, 2016; pp. 35–71. ISBN 978-0-444-63475-7. [Google Scholar]
- Martínez-Vázquez, M.; Martinez, R.; Perez, G.E.; Diaz, M.; Sanchez, M.H. Antimicrobial Properties of Argentatine A, Isolated from Parthenium argentatum. Fitoterapia 1994, 65, 371–372. [Google Scholar]
- Lopez Propiedades Anticancerígenas y Antinflamatorias del Guayule. Available online: https://www.gaceta.unam.mx/universitarios-usan-resina-del-guayule-como-antiinflamatorio-y-anticancerigeno/ (accessed on 10 June 2020).
- Parra-Delgado, H.; García-Pillado, F.; Sordo, M.; Ramírez-Apan, T.; Martínez-Vázquez, M.; Ostrosky-Wegman, P. Evaluation of the Cytotoxicity, Cytostaticity and Genotoxicity of Argentatins A and B from Parthenium argentatum (Gray). Life Sci. 2005, 77, 2855–2865. [Google Scholar] [CrossRef] [PubMed]
- Spano, N.; Meloni, P.; Idda, I.; Mariani, A.; Pilo, M.; Nurchi, V.; Lachowicz, J.; Rivera, E.; Orona-Espino, A.; Sanna, G. Assessment, Validation and Application to Real Samples of an RP-HPLC Method for the Determination of Guayulins A, B, C and D in Guayule Shrub. Separations 2018, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, E.; Reynolds, G.; Thompson, J. Potent Contact Allergen in the Rubber Plant Guayule (Parthenium argentatum). Science 1981, 211, 1444–1445. [Google Scholar] [CrossRef]
- Marwah, N.; Masohan, A.; Bangwal, D.; Bhatia, V.K. Chemical Composition of Guayule (Parthenium argentatum Gray) Wax. J. Wood Chem. Technol. 1994, 14, 563–576. [Google Scholar] [CrossRef]
- Piluzza, G.; Campesi, G.; Molinu, M.G.; Re, G.A.; Sulas, L. Bioactive Compounds from Leaves and Twigs of Guayule Grown in a Mediterranean Environment. Plants 2020, 9, 442. [Google Scholar] [CrossRef] [Green Version]
- Kuester, J.L. Conversion of Guayule Residues into Fuel Energy Products. Bioresour. Technol. 1991, 35, 217–222. [Google Scholar] [CrossRef]
- Boateng, A.A.; Mullen, C.A.; Goldberg, N.M.; Hicks, K.B.; McMahan, C.M.; Whalen, M.C.; Cornish, K. Energy-Dense Liquid Fuel Intermediates by Pyrolysis of Guayule (Parthenium argentatum) Shrub and Bagasse. Fuel 2009, 88, 2207–2215. [Google Scholar] [CrossRef]
- La Production|Société Internationale de Plantations d’Hévéas. Available online: http://siph.wipiv.com/repartition-production-mondiale (accessed on 9 December 2019).
- Nicod, T. Etude sur les stratégies d’adaptation des petits planteurs d’hévéa Thaïlandais, face à la chute du prix du caoutchouc naturel. Agritrop 2017, 99. [Google Scholar]
- Sfeir, N.; Chapuset, T.; Palu, S.; Lançon, F.; Amor, A.; García García, J.; Snoeck, D. Technical and Economic Feasibility of a Guayule Commodity Chain in Mediterranean Europe. Ind. Crops Prod. 2014, 59, 55–62. [Google Scholar] [CrossRef]
- The Outbreak of Pestalotiopsis Disease Affecting Huge Hectarage of Rubber Plantations in Indonesia-IRCo. Available online: https://ircorubber.com/2019/pestalotiopsis-endanger-national-rubber-production/ (accessed on 22 April 2020).
- Rubber. Available online: https://tradingeconomics.com/commodity/rubber (accessed on 10 June 2020).
- Daily Prices, Association of Natural Rubber Producing Countries. Available online: http://www.anrpc.org/html/daily-prices.aspx?ID1=26&ID=27&PID=35 (accessed on 10 June 2020).
- Dorget, M.; Palu, S. Pioch Industrial Crops: Promoting Sustainability. In Proceedings of the AAIC 2016 Association for the Advancement of Industrial Crops, Montpellier, France, 24–28 September 2016. [Google Scholar]
- About the Nagoya Protocol. Available online: https://www.cbd.int/abs/about/ (accessed on 10 June 2020).





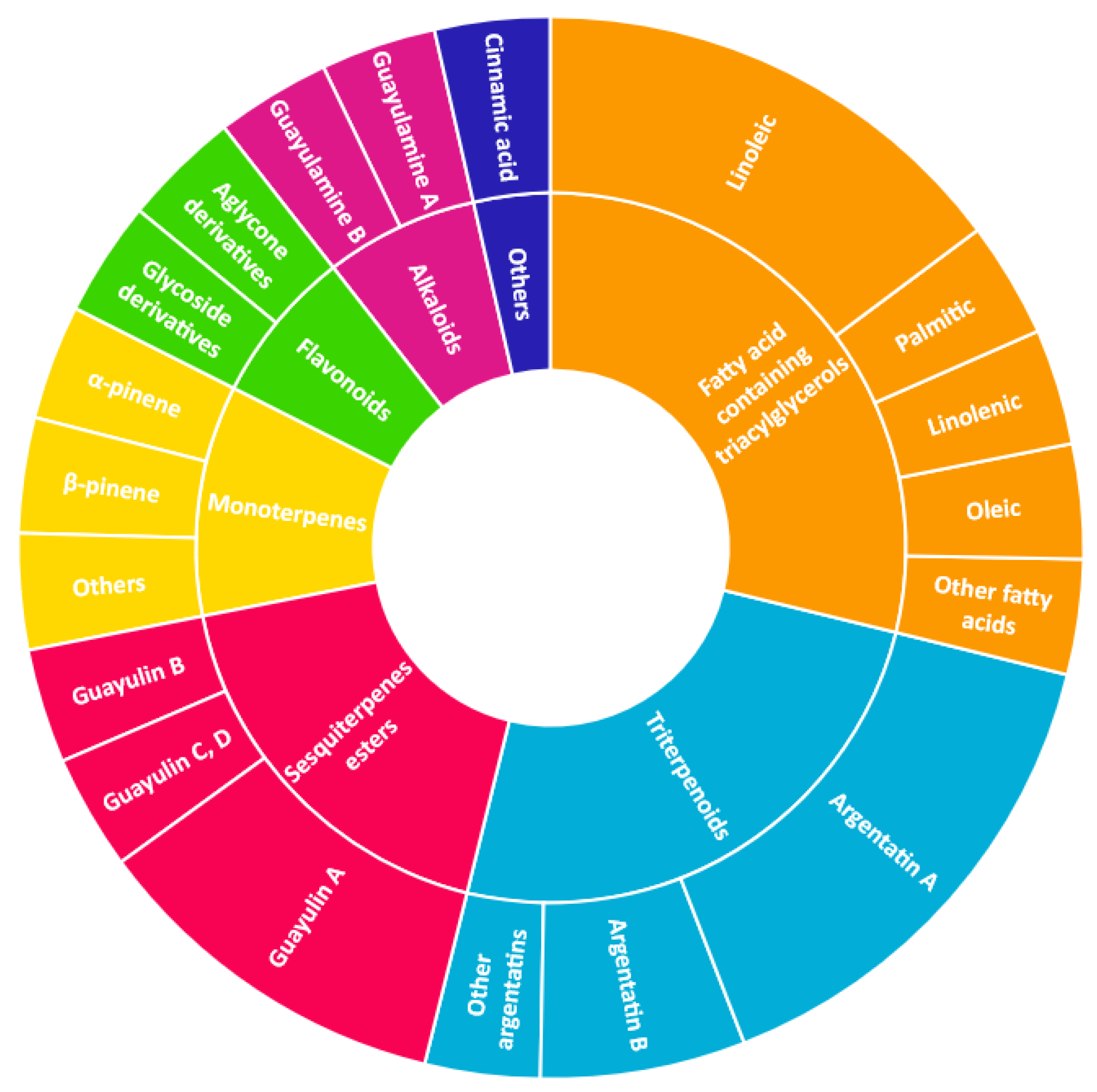




| Monoterpenes (3–5%) Analysis: GC |  |
| Sesquiterpenes (7–10%) Analysis: HPLC, GC |  |
| Triterpenes (20–50%) Analysis: HPLC |  |
| Alkloids Analysis: HPLC, NMR |  |
| Organic acids Analysis: HPLC |  |
| Fatty acid of the triacylglyerols (15–25%) Analysis: GPC, GC |  |
| Process | Advantages | Disadvantages |
|---|---|---|
| Flotation Process | Simple, first process Water-based process | Bad quality rubber/lot of resin Important losses |
| Sequential Extraction | Semi-batch mode Good extraction yield Resin removed by preliminary step | Petrochemical solvents in large quantities |
| Simultaneous Extraction | One pot extraction Good quality rubber | Petrochemical solvents in large quantities |
| Supercritical CO2 process | Selective extraction of PI One pot extraction Little quantity of co-solvent | Expensive process Special processing conditions |
| Latex process | PI with high molecular mass Water-based process Continuous process | Difficult to extract in the latex form |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rousset, A.; Amor, A.; Punvichai, T.; Perino, S.; Palu, S.; Dorget, M.; Pioch, D.; Chemat, F. Guayule (Parthenium argentatum A. Gray), a Renewable Resource for Natural Polyisoprene and Resin: Composition, Processes and Applications. Molecules 2021, 26, 664. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26030664
Rousset A, Amor A, Punvichai T, Perino S, Palu S, Dorget M, Pioch D, Chemat F. Guayule (Parthenium argentatum A. Gray), a Renewable Resource for Natural Polyisoprene and Resin: Composition, Processes and Applications. Molecules. 2021; 26(3):664. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26030664
Chicago/Turabian StyleRousset, Amandine, Ali Amor, Teerasak Punvichai, Sandrine Perino, Serge Palu, Michel Dorget, Daniel Pioch, and Farid Chemat. 2021. "Guayule (Parthenium argentatum A. Gray), a Renewable Resource for Natural Polyisoprene and Resin: Composition, Processes and Applications" Molecules 26, no. 3: 664. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26030664








