Hydrophilic and Functionalized Nanographene Oxide Incorporated Faster Dissolving Megestrol Acetate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Nanographene Oxide Study
2.2. Drug Composites Surface Morphology Analysis
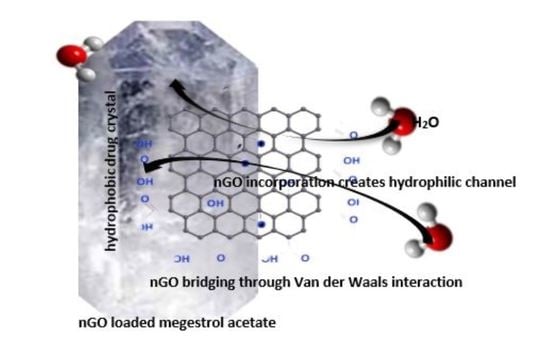
2.3. nGO-Drug Composite
2.4. Properties of MA-nGO Composites
2.5. In Vitro Dissolution Study
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Nanographene Oxide (nGO) Suspension
3.3. Incorporation of (nGO) into MA via Antisolvent Precipitation
3.4. Physical and Chemical Characterization of Prepared Drug Composites
3.5. In Vitro Dissolution Testing Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability:
References
- Viswanathan, P.; Muralidaran, Y.; Ragavan, G. Chapter 7—Challenges in oral drug delivery: A nano-based strategy to overcome. Nanostructures for oral medicine. J. Micro Nano Technol. 2017, 173–201. [Google Scholar] [CrossRef]
- Goke, K.; Lorenz, T.; Repanas, A.; Schneider, F.; Steiner, D.; Baumann, K.; Bunjes, H.; Dietzel, A.; Finke, J.H.; Glasmacher, B.; et al. Novel strategies for the formulation and processing of poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2018, 126, 40–56. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, H.; Zhao, Y.; Ma, Z. Pharmaceutical Dispersion Techniques for Dissolution and Bioavailability Enhancement of Poorly Water-Soluble Drugs. Pharmaceutics 2018, 10, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leleux, J.; Williams, R.O. Recent advancements in mechanical reduction methods: Particulate systems. J. Drug Dev. Ind. Pharm. 2014, 40, 289–300. [Google Scholar] [CrossRef]
- Scholz, P.; Keck, C.M. Nanocrystals: From raw material to the final formulated oral dosage form a review. Curr. Pharm. Des. 2015, 21, 4217–4228. [Google Scholar] [CrossRef]
- He, Y.; Ho, C.; Yang, D.; Chen, J.; Orton, E. Measurement and accurate interpretation of the solubility of pharmaceutical salts. J. Pharm. Sci. 2017, 106, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, T.; Lan, Y.; Wu, B.; Shi, Z. Nanosuspensions containing oridonin/hp-beta-cyclodextrin inclusion complexes for oral bioavailability enhancement via improved dissolution and permeability. J. AAPS Pharm. Sci. Technol. 2016, 17, 400–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadade, D.D.; Pekamwar, S.S. Pharmaceutical cocrystals: Regulatory and strategic aspects, design and development. Adv. Pharm. Bull. 2016, 6, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Cagel, M.; Tesan, F.C.; Bernabeu, E.; Salgueiro, M.J.; Zubillaga, M.B.; Moretton, M.A.; Chiappetta, D.A. Polymeric mixed micelles as nanomedicines: Achievements and perspectives. Eur. J. Pharm. Biopharm. 2017, 113, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Vo, C.L.; Park, C.; Lee, B.J. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2013, 85, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Vranikova, B.; Gajdziok, J. Liqui-solid systems and aspects influencing their research and development. J. Acta Pharm. 2013, 63, 447–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalepu, S.; Nekkanti, V. Improved delivery of poorly soluble compounds using nanoparticle technology: A review. Drug Deliv. Transl. Res. 2016, 6, 319–332. [Google Scholar] [CrossRef]
- Chen, K.; Mitra, S. Incorporation of functionalized carbon nanotubes into hydrophobic drug crystals for enhancing aqueous dissolution. J. Colloids Surf. B Biointerfaces 2019, 173, 386–391. [Google Scholar] [CrossRef]
- Thakkar, M.; Islam, M.S.; Railkar, A.; Mitra, S. Antisolvent precipitative immobilization of micro and nanostructured griseofulvin on laboratory cultured diatom frustules for enhanced aqueous dissolution. Colloids Surf. B Biointerfaces 2020, 196, 111308. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi. Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Michele, A.D.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bamrungsap, S.; Zhao, Z.; Chen, T.; Wang, L.; Li, C.; Fu, T.; Tan, W. Nanotechnology in Therapeutics. A Focus on Nanoparticles as a Drug Delivery System. Discl. J. Nanomed. 2012, 7, 1253–1271. [Google Scholar] [CrossRef]
- Wang, J.; Hu, X.; Xiang, D. Nanoparticle drug delivery systems: An excellent carrier for tumor peptide vaccines. J. Drug Del. 2018, 25, 1319–1327. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. J. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Dockery, L.; Daniel, M.C. Dendronized Systems for the Delivery of Chemotherapeutics. J. Adv. Cancer Res. 2018, 139, 85–120. [Google Scholar] [CrossRef]
- Daniyal, M.; Liu, B.; Wang, W. Comprehensive Review on Graphene Oxide for Use in Drug Delivery System. J. Curr. Med. Chem. 2020, 27, 3665–3685. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.C.; Jana, N.R. Chapter 5—Application of Carbon-Based Nanomaterials as Drug and Gene Delivery Carrier. 5.3.3.2 Graphene Oxide in Drug Delivery. In Carbon Nanomaterials for Biological and Medical Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 163–203. [Google Scholar] [CrossRef]
- Pei, X.; Zhu, Z.; Gan, Z.; Chen, J.; Zhang, X.; Cheng, X.; Wan, Q.; Wang, J. PEGylated nano-graphene oxide as a nanocarrier for delivering mixed anticancer drugs to improve anticancer activity. J. Sci. Rep. 2020, 10, 2717. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, H.N.; Khan, M.S.; Wu, H.F. Graphene oxide as a nanocarrier for gramicidin (GOGD) for high antibacterial performance. J. RSC Adv. 2014, 4, 50035–50046. [Google Scholar] [CrossRef]
- Wei, Y.; Zhou, F.; Zhang, D.; Chen, Q.; Xing, D. Graphene Oxide Based Smart Drug Delivery System for Tumor Mitochondria-Targeting Photodynamic Therapy. Nanoscale 2016, 8, 3530–3538. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Ding, R.; Zhao, X.; Li, Y.; Qu, L.; Pei, H.; Yildirimer, L.; Wu, Z.; Zhang, W. Graphene-based nanomaterials for drug and/or gene delivery, bioimaging, and tissue engineering. Rev. J. Drug Discov. Today 2017, 22, 1302–1317. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Hu, K.; Chen, Y.; Yu, M.; Wang, D.; Wang, Q.; Yong, K.T.; Lu, F.; Liang, Y.; Li, Z. SiRNA delivery with pegylated graphene oxide nanosheets for combined photothermal and gene therapy for pancreatic cancer. J. Theranostics. 2017, 7, 1133–1148. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Shen, H.; Song, S.; Zhang, L.; Chen, W.; Dai, J.; Zhang, Z. Graphene Oxide Incorporated PLGA Nanofibrous Scaffold for Solid Phase Gene Delivery into Mesenchymal Stem Cells. J. Nanosci. Nanotechnol. 2018, 18, 2286–2293. [Google Scholar] [CrossRef]
- Wu, L.; Xie, J.; Li, T.; Mai, Z.; Wang, L.; Wang, X.; Chen, T. Gene delivery ability of polyethylene imine and polyethylene glycol dual-functionalized nanographene oxide in 11 different cell lines. J. R. Soc. Open Sci. 2017, 4, 170822. [Google Scholar] [CrossRef] [Green Version]
- Han, X.M.; Zheng, K.W.; Wang, R.L.; Yue, S.F.; Chen, J.; Zhao, Z.W.; Song, F.; Su, Y.; Ma, Q. Functionalization and optimization-strategy of graphene oxide-based nanomaterials for gene and drug delivery. J. Am. Transl. Res. 2020, 12, 1515–1534. [Google Scholar]
- Khim Chng, E.L.; Pumera, M. Toxicity of graphene related materials and transition metal dichalcogenides. J. RSC Adv. 2015, 5, 3074–3080. [Google Scholar] [CrossRef]
- Wu, B.; Wu, J.; Liu, S.; Shen, Z.; Chen, L.; Zhang, X.X.; Ren, H.Q. Combined effects of graphene oxide and zinc oxide nanoparticle on human A549 cells: Bioavailability, toxicity and mechanisms. J. Environ. Sci. Nano 2019, 6, 635. [Google Scholar] [CrossRef]
- Lalwani, G.; D’Agati, M.; Khan, A.M.; Sitharaman, B. Toxicology of graphene-based nanomaterials. J. Adv. Drug Deliv. Rev. 2016, 105 Pt B, 109–144. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Yang, S.T.; Liu, J.H.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y.; Wang, H. In vitro toxicity evaluation of graphene oxide on A549 cells. J. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.T.; Liu, Z. Graphene in mice: Ultrahigh in vivo tumor uptake and efficient photothermal therapy. J. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef]
- Duch, M.C.; Budinger, G.S.; Liang, Y.T.; Soberanes, S.; Urich, D.; Chiarella, S.E.; Campochiaro, L.A.; Gonzalez, A.; Chandel, N.S.; Hersam, M.C.; et al. Minimizing oxidation and stable nanoscale dispersion improves the biocompatibility of graphene in the lung. J. Nano Lett. 2011, 11, 5201–5207. [Google Scholar] [CrossRef] [Green Version]
- Santhosh, K.K.; Modak, M.D.; Paik, P. Graphene Oxide for Biomedical Applications. J. Nanomed. Res. 2017, 5, 00136. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, P.; Parandhaman, T.; Ramalingam, B.; Duraipandy, N.; Kiran, M.S.; Das, S.K. Fabrication of Nontoxic Reduced Graphene Oxide Protein Nano framework as Sustained Antimicrobial Coating for Biomedical Application. J. ACS Appl. Mater. Interfaces 2017, 9, 38255–38269. [Google Scholar] [CrossRef]
- Shen, S.C.; Ng, W.K.; Letchmanan, K.; Yee Lim, R.T.; Hee Tan, R.B. Graphene nanosheets encapsulated poorly soluble drugs with an enhanced dissolution rate. J. Carbon Lett. 2018, 27, 18–25. [Google Scholar] [CrossRef]
- Hu, H.; Wang, X.; Lee, K.I.; Ma, K.; Hu, H.; Xin, J.H. Graphene oxide-enhanced sol-gel transition sensitivity and drug release performance of an amphiphilic copolymer-based nanocomposite. Sci. Rep. 2016, 6, 31815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, S.S.; Wu, S.Y.; Lee, T.P.; Olson, J.S.; Stevens, M.R.; Dixon, T.; Schuster, M.W. Improvement in quality-of-life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: Results of a double-blind, placebo-controlled study. J. Am. Geriatr. Soc. 2000, 48, 485–492. [Google Scholar] [CrossRef]
- Jang, K.; Yoon, S.; Kim, S.E.; Cho, J.Y.; Yoon, S.H.; Lim, K.S.; Yu, K.S.; Jang, I.J.; Lee, H. Novel nanocrystal formulation of megestrol acetate has improved bioavailability compared with the conventional micronized formulation in the fasting state. J. Drug Des. Devel. Ther. 2014, 8, 851–858. [Google Scholar] [CrossRef] [Green Version]
- Geller, J.; Albert, J.; Geller, S.; Lopez, D.; Cantor, T.; Yen, S. Effect of megestrol acetate (Megace) on steroid metabolism and steroid-protein binding in the human prostate. J. Clin. Endocrinol. Metab. 1976, 43, 1000–1008. [Google Scholar] [CrossRef]
- Femia, R.A.; Goyette, R.E. The Science of Megestrol Acetate Delivery. Potential to Improve Outcomes in Cachexia. J. BioDrugs 2005, 19, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Zhao, R.; Dong, L.C.; Wong, G. Characterization of nanoparticles for drug delivery applications. Microsc. Microanal. 2005, 11, 1934–1935. [Google Scholar] [CrossRef] [Green Version]
- Ha, E.S.; Kim, J.S.; Baek, I.H.; Yoo, J.W.; Jung, Y.; Moon, H.R.; Kim, M.S. Development of megestrol acetate solid dispersion nanoparticles for enhanced oral delivery by using a supercritical antisolvent process. Drug Design Dev. Ther. 2015, 9, 4269–4277. [Google Scholar]
- Sylvestre, J.P.; Tang, M.C.; Furtos, A.; Leclair, G.; Meunier, M.; Leroux, J.C. Nanosization of megestrol acetate by laser fragmentation in aqueous milieu. J. Control Release 2011, 149, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Cho, E.; Cho, W.; Cha, K.H.; Park, J.; Kim, M.S.; Kim, J.S.; Park, H.J.; Hwang, S.J. Enhanced dissolution of megestrol acetate microcrystals prepared by antisolvent precipitation process using hydrophilic additives. Int. J. Pharm. 2010, 396, 91–98. [Google Scholar] [CrossRef]
- Hong, S.W.; Lee, B.S.; Park, S.J.; Jeon, H.R.; Moon, K.Y.; Kang, M.H.; Park, S.H.; Choi, S.U.; Song, W.H.; Lee, J.; et al. Solid dispersion formulations of megestrol acetate with copovidone for enhanced dissolution and oral bioavailability. J. Arch. Pharm. Res. 2011, 34, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Shekunov, B.Y.; Chattopadhyay, P.; Seitzinger, J.; Huff, R. Nanoparticles of poorly water-soluble drugs prepared by supercritical fluid extraction of emulsions. J. Pharm. Res. 2006, 23, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.B.; Shen, Z.G.; Wang, J.X.; Zhao, H.; Chen, J.F.; Yun, J. Nanosization of megestrol acetate by liquid precipitation. J. Ind. Eng. Chem. Res. 2009, 48, 8493–8499. [Google Scholar] [CrossRef]
- Islam, M.S.; Renner, F.; Azizighannad, S.; Mitra, S. Direct incorporation of nano graphene oxide (nGO) into hydrophobic drug crystals for enhanced aqueous dissolution. J. Colloids Surf. B Biointerfaces 2020, 189, 110827. [Google Scholar] [CrossRef] [PubMed]
- Neklyudov, V.V.; Khafizov, N.R.; Sedov, I.A.; Dimiev, A.M. New insights into the solubility of graphene oxide in water and alcohols. J. Phys. Chem. Chem. Phys. 2017, 19, 17000–17008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadfar, E.; Shafiei, F.; Isfahani, T.M. Structural Relationship Study of Octanol-Water Partition Coefficient of Some Sulfa Drugs Using GA-MLR and GA-ANN Methods. J. Curr. Comp. Aid. Drug Des. 2019, 15. [Google Scholar] [CrossRef]










| Nano Graphene Oxide EDS (Energy Dispersive X-ray Spectroscopy) Quantitative Results | |
|---|---|
| Carbon (Wt. %) | 52.58 |
| Oxygen (Wt. %) | 47.42 |
| Drug | 50% Dissolution Time (T50) | 80% Dissolution Time (T80) | Initial Dissolution Rate (µg/min) | Melting Points (°C) |
|---|---|---|---|---|
| MA | 138 | ** (not dissolved) | 14.41 | 219.8 |
| MA-nGO-1.05 | 42 | 130 | 28.54 | 219.1 |
| MA-nGO-1.09 | 49 | 112.5 | 33.05 | 219.2 |
| MA-nGO-1.04 | 27 | 97 | 60.62 | 218.8 |
| Drug | 50% Dissolution Time (T50) | 80% Dissolution Time (T80) | Initial Dissolution Rate (µg/min) |
|---|---|---|---|
| MA | ** (not dissolved) | ** (not dissolved) | 84.1 |
| MA-nGO-1.05 | 93 | ** (not dissolved) | 128.2 |
| MA-nGO-1.09 | 97 | ** (not dissolved) | 154.6 |
| MA-nGO-1.04 | 92 | ** (not dissolved) | 232.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.S.; Renner, F.; Foster, K.; Oderinde, M.S.; Stefanski, K.; Mitra, S. Hydrophilic and Functionalized Nanographene Oxide Incorporated Faster Dissolving Megestrol Acetate. Molecules 2021, 26, 1972. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26071972
Islam MS, Renner F, Foster K, Oderinde MS, Stefanski K, Mitra S. Hydrophilic and Functionalized Nanographene Oxide Incorporated Faster Dissolving Megestrol Acetate. Molecules. 2021; 26(7):1972. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26071972
Chicago/Turabian StyleIslam, Mohammad Saiful, Faradae Renner, Kimberly Foster, Martin S. Oderinde, Kevin Stefanski, and Somenath Mitra. 2021. "Hydrophilic and Functionalized Nanographene Oxide Incorporated Faster Dissolving Megestrol Acetate" Molecules 26, no. 7: 1972. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26071972