Catechol-Containing Schiff Bases on Thiacalixarene: Synthesis, Copper (II) Recognition, and Formation of Organic-Inorganic Copper-Based Materials
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of thiacalix[4]arene-Based Schiff Bases
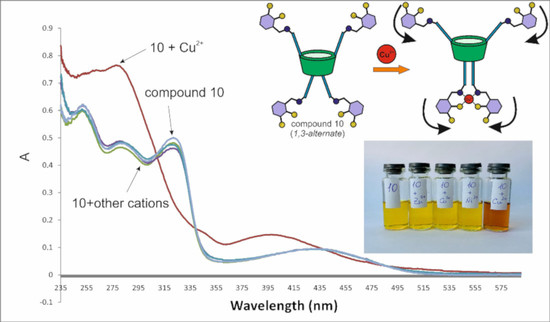
2.2. The Study of Complexing Properties of the Obtained thiacalix[4]arene-Based Schiff Bases
2.3. Synthesis and Characterization of Thiacalixarene-Copper (II) Materials
3. Materials and Methods
3.1. General
3.2. General Procedure for the Synthesis of the Compounds 8–10
3.2.1. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(4,6-di-tert-butyl-2,3-dihydroxybenzylideneamino)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene in cone Conformation (8)
3.2.2. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(4,6-di-tert-butyl-2,3-dihydroxybenzylideneamino)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene in partial cone Conformation (9)
3.2.3. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(4,6-di-tert-butyl-2,3-dihydroxybenzylideneamino)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene in 1,3-alterate Conformation (10)
3.3. Procedure for the Synthesis of Compound 11 (Monomer)
2-(4-tert-Butylphenoxy)-N-(6-(4,6-di-tert-butyl-2,3-dihydroxybenzylideneamino)hexyl)acetamide (11)
3.4. Job’s Plots
3.5. Determination of the Stability Constant and Stoichiometry of the Complex by Spectrophotometric Titration
3.6. Procedure for the Synthesis of Thiacalixarene 10—Copper (II) Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Faustini, M.; Nicole, L.; Ruiz-Hitzky, E.; Sanchez, C. History of organic–inorganic hybrid materials: Prehistory, art, science, and advanced applications. Adv. Funct. Mater. 2018, 28, 1704158. [Google Scholar] [CrossRef]
- Choudhary, N.; Islam, M.A.; Kim, J.H.; Ko, T.J.; Schropp, A.; Hurtado, L.; Weitzman, D.; Zhai, L.; Jung, Y. Two-dimensional transition metal dichalcogenide hybrid materials for energy applications. Nano Today 2018, 19, 16–40. [Google Scholar] [CrossRef]
- Mir, S.H.; Nagahara, L.A.; Thundat, T.; Mokarian-Tabari, P.; Furukawa, H.; Khosla, A. Organic-inorganic hybrid functional materials: An integrated platform for applied technologies. J. Electrochem. Soc. 2018, 165, B3137. [Google Scholar] [CrossRef]
- Rumyantseva, M.; Nasriddinov, A.; Vladimirova, S.; Tokarev, S.; Fedorova, O.; Krylov, I.; Drozdov, K.; Baranchikov, A.; Gaskov, A. Photosensitive organic-inorganic hybrid materials for room temperature gas sensor applications. Nanomaterials 2018, 8, 671. [Google Scholar] [CrossRef] [Green Version]
- Díaz, U.; Corma, A. Organic-Inorganic Hybrid Materials: Multi-Functional Solids for Multi-Step Reaction Processes. Chem. A Eur. J. 2018, 24, 3944–3958. [Google Scholar] [CrossRef]
- Parola, S.; Julián-López, B.; Carlos, L.D.; Sanchez, C. Optical properties of hybrid organic-inorganic materials and their applications. Adv. Funct. Mater. 2016, 26, 6506–6544. [Google Scholar] [CrossRef]
- Jin, H.; Li, J.; Iocozzia, J.; Zeng, X.; Wei, P.C.; Yang, C.; Li, N.; Liu, Z.; He, J.H.; Zhu, T.; et al. Hybrid organic–inorganic thermoelectric materials and devices. Angew. Chem. Int. Ed. 2019, 58, 15206–15226. [Google Scholar] [CrossRef]
- Kalaj, M.; Bentz, K.C.; Ayala, S., Jr.; Palomba, J.M.; Barcus, K.S.; Katayama, Y.; Cohen, S.M. MOF-polymer hybrid materials: From simple composites to tailored architectures. Chem. Rev. 2020, 120, 8267–8302. [Google Scholar] [CrossRef]
- Nakhaei, M.; Akhbari, K.; Phuruangrat, A. Synthesis and characterization of silver and copper metal–organic hybrid nanomaterials and their biological application. Colloid Polym. Sci. 2021, 1–9. [Google Scholar] [CrossRef]
- Li, X.; Zhong, X.; Hu, Y.; Li, B.; Sheng, Y.; Zhang, Y.; Weng, C.; Feng, M.; Han, H.; Wang, J. Organic–inorganic copper (II)-based material: A low-toxic, highly stable light absorber for photovoltaic application. J. Phys. Chem. Lett. 2017, 8, 1804–1809. [Google Scholar] [CrossRef]
- Wang, L.; Sun, H.; Sun, C.; Xu, D.; Tao, J.; Wei, T.; Zhang, Z.; Zhang, Y.; Wang, Z.; Bi, W. Lead-free, stable orange-red-emitting hybrid copper based organic–inorganic compounds. Dalton Trans. 2021, 50, 2766–2773. [Google Scholar] [CrossRef]
- Feng, Y.; Zhu, J. Copper nanomaterials and assemblies for soft electronics. Sci. China Mater. 2019, 62, 1679–1708. [Google Scholar] [CrossRef] [Green Version]
- Ingle, A.P.; Duran, N.; Rai, M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: A review. Appl. Microbiol. Biotechnol. 2014, 98, 1001–1009. [Google Scholar] [CrossRef]
- Liu, X.; Manzur, C.; Novoa, N.; Celedón, S.; Carrillo, D.; Hamon, J.R. Multidentate unsymmetrically-substituted Schiff bases and their metal complexes: Synthesis, functional materials properties, and applications to catalysis. Coord. Chem. Rev. 2018, 357, 144–172. [Google Scholar] [CrossRef]
- Shurpik, D.N.; Aleksandrova, Y.I.; Rodionov, A.A.; Razina, E.A.; Gafurov, M.R.; Vakhitov, I.R.; Evtugyn, V.G.; Gerasimov, A.V.; Kuzin, Y.I.; Evtugyn, G.A.; et al. Metallo-Supramolecular Coordination Polymers Based on Amidopyridine Derivatives of Pillar[5]arene and Cu (II) and Pd (II) Cations: Synthesis and Recognition of Nitroaromatic Compounds. Langmuir 2021, 37, 2942–2953. [Google Scholar] [CrossRef]
- More, M.S.; Joshi, P.G.; Mishra, Y.K.; Khanna, P.K. Metal complexes driven from Schiff bases and semicarbazones for biomedical and allied applications: A review. Mater. Today Chem. 2019, 14, 100195. [Google Scholar] [CrossRef]
- Liu, X.; Hamon, J.R. Recent developments in penta-, hexa-and heptadentate Schiff base ligands and their metal complexes. Coord. Chem. Rev. 2019, 389, 94–118. [Google Scholar] [CrossRef]
- Zoubi, W.A.; Al-Hamdani, A.A.S.; Ko, Y.G. Schiff bases and their complexes: Recent progress in thermal analysis. Sep. Sci. Technol. 2017, 52, 1052–1069. [Google Scholar] [CrossRef]
- Li, Z. Two organic–inorganic hybrid Schiff base copper materials: Synthesis and their catalytic properties for constructing C–N bonds. Integr. Ferroelectr. 2019, 200, 26–35. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Hsu, S.H. Hydrogels based on Schiff base linkages for biomedical applications. Molecules 2019, 24, 3005. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xu, L.; Wong, W.Y. Energy materials based on metal Schiff base complexes. Coord. Chem. Rev. 2018, 355, 180–198. [Google Scholar] [CrossRef]
- Berhanu, A.L.; Mohiuddin, I.; Malik, A.K.; Aulakh, J.S.; Kumar, V.; Kim, K.H. A review of the applications of Schiff bases as optical chemical sensors. TrAC Trends Anal. Chem. 2019, 116, 74–91. [Google Scholar] [CrossRef]
- Nikoorazm, M.; Rezaei, Z.; Tahmasbi, B. Two Schiff-base complexes of copper and zirconium oxide supported on mesoporous MCM-41 as an organic–inorganic hybrid catalysts in the chemo and homoselective oxidation of sulfides and synthesis of tetrazoles. J. Porous Mater. 2020, 27, 671–689. [Google Scholar] [CrossRef]
- Patil, D.Y.; Patil, A.A.; Khadke, N.B.; Borhade, A.V. Highly selective and sensitive colorimetric probe for Al3+ and Fe3+ metal ions based on 2-aminoquinolin-3-yl phenyl hydrazone Schiff base. Inorg. Chim. Acta 2019, 492, 167–176. [Google Scholar] [CrossRef]
- Fuller, R.O.; Koutsantonis, G.A.; Ogden, M.I. Magnetic properties of calixarene-supported metal coordination clusters. Coord. Chem. Rev. 2020, 402, 213066. [Google Scholar] [CrossRef]
- Wang, J.; Ding, X.; Guo, X. Assembly behaviors of calixarene-based amphiphile and supra-amphiphile and the applications in drug delivery and protein recognition. Adv. Colloid Interface Sci. 2019, 269, 187–202. [Google Scholar] [CrossRef]
- Li, H.; Quan, K.; Yang, X.; Li, Z.; Zhao, L.; Qiu, H. Recent developments for the investigation of chiral properties and applications of pillar[5]arenes in analytical chemistry. TrAC Trends Anal. Chem. 2020, 116026. [Google Scholar] [CrossRef]
- Español, E.S.; Villamil, M.M. Calixarenes: Generalities and their role in improving the solubility, biocompatibility, stability, bioavailability, detection, and transport of biomolecules. Biomolecules 2019, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Liu, Z.; Xin, F.; Zhao, Y. Metal-ligated pillararene materials: From chemosensors to multidimensional self-assembled architectures. Coord. Chem. Rev. 2020, 420, 213425. [Google Scholar] [CrossRef]
- Mostovaya, O.A.; Padnya, P.L.; Vavilova, A.A.; Shurpik, D.N.; Khairutdinov, B.I.; Mukhametzyanov, T.A.; Khannanov, A.A.; Kutyreva, M.P.; Stoikov, I.I. Tetracarboxylic acids on a thiacalixarene scaffold: Synthesis and binding of dopamine hydrochloride. New J. Chem. 2018, 42, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Nazarova, A.; Shurpik, D.; Padnya, P.; Mukhametzyanov, T.; Cragg, P.; Stoikov, I. Self-Assembly of Supramolecular Architectures by the Effect of Amino Acid Residues of Quaternary Ammonium Pillar[5]arenes. Int. J. Mol. Sci. 2020, 21, 7206. [Google Scholar] [CrossRef]
- Massi, M.; Ogden, M.I. Luminescent lanthanoid calixarene complexes and materials. Materials 2017, 10, 1369. [Google Scholar] [CrossRef] [Green Version]
- Nazarova, A.A.; Yakimova, L.S.; Padnya, P.L.; Evtugyn, V.G.; Osin, Y.N.; Cragg, P.J.; Stoikov, I.I. Monosubstituted pillar[5]arene functionalized with (amino) phosphonate fragments are “smart” building blocks for constructing nanosized structures with some s-and p-metal cations in the organic phase. New J. Chem. 2019, 43, 14450–14458. [Google Scholar] [CrossRef]
- Bahojb Noruzi, E.; Shaabani, B.; Geremia, S.; Hickey, N.; Nitti, P.; Kafil, H.S. Synthesis, crystal structure, and biological activity of a multidentate calix[4]arene ligand doubly functionalized by 2-hydroxybenzeledene-thiosemicarbazone. Molecules 2020, 25, 370. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.G.; Li, L.; Liu, W.L. Metallic macrocycle with a 1, 3-alternate calix[4]arene salicylideneamine ligand. J. Coord. Chem. 2009, 62, 2118–2124. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, J.; Yan, C.G. Synthesis and crystal structures of p-tert-butyldihomooxacalix[4]arene mono-Schiff bases. J. Incl. Phenom. Macrocycl. Chem. 2017, 87, 157–166. [Google Scholar] [CrossRef]
- Noamane, M.H.; Ferlay, S.; Abidi, R.; Kyritsakas, N.; Hosseini, M.W. Formation of binuclear neutral Copper (II) complexes based on p-tert-butyl-calix[4]arene and thiacalix[4]arene in 1, 3-A conformation bearing four catechols at their lower rim. Inorg. Chim. Acta 2017, 468, 260–269. [Google Scholar] [CrossRef]
- Vavilova, A.A.; Nosov, R.V.; Mostovaya, O.A.; Stoikov, I.I. Synthesis of three stereoisomers of p-tert-butylthiacalix[4]arene substituted with (ethoxycarbonyl) methoxy and fluorescent 1-amidoanthraquinone fragments. Macroheterocycles 2016, 9, 294–300. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Chen, Y.; Liu, Y. Calixarene/pillararene-based supramolecular selective binding and molecular assembly. Chin. Chem. Lett. 2019, 30, 1190–1197. [Google Scholar] [CrossRef]
- Razuvayeva, Y.; Kashapov, R.; Zakharova, L. Calixarene-based pure and mixed assemblies for biomedical applications. Supramol. Chem. 2020, 32, 178–206. [Google Scholar] [CrossRef]
- Tian, H.W.; Liu, Y.C.; Guo, D.S. Assembling features of calixarene-based amphiphiles and supra-amphiphiles. Mater. Chem. Front. 2020, 4, 46–98. [Google Scholar] [CrossRef]
- Mostovaya, O.A.; Gorbachuk, V.V.; Padnya, P.L.; Vavilova, A.A.; Evtugyn, G.A.; Stoikov, I.I. Modification of Oligo-and Polylactides With Macrocyclic Fragments: Synthesis and Properties. Front. Chem. 2019, 7, 554. [Google Scholar] [CrossRef]
- Padnya, P.; Gorbachuk, V.; Stoikov, I. The Role of Calix[n]arenes and Pillar[n]arenes in the Design of Silver Nanoparticles: Self-Assembly and Application. Int. J. Mol. Sci. 2020, 21, 1425. [Google Scholar] [CrossRef] [Green Version]
- Mostovaya, O.A.; Padnya, P.L.; Shurpik, D.N.; Shiabiev, I.E.; Stoikov, I.I. Novel lactide derivatives of p-tert-butylthiacalix[4]arene: Directed synthesis and molecular recognition of catecholamines. J. Mol. Liq. 2021, 327, 114806. [Google Scholar] [CrossRef]
- Do, T.H.; Brown, S.N. Mono- and bimetallic pentacoordinate silicon complexes of a chelating bis(catecholimine) ligand. Dalton Trans. 2019, 48, 11565–11574. [Google Scholar] [CrossRef]
- Do, T.H.; Brown, S.N. Synthesis, dynamics and redox properties of eight-coordinate zirconium catecholate complexes. Dalton Trans. 2020, 49, 11648–11656. [Google Scholar] [CrossRef]
- Arsen’ev, M.V.; Okhlopkova, L.S.; Poddel’skii, A.I.; Fukin, G.K. Binuclear Triphenylantimony(V) Catecholate Based on Redox-Active Bis-o-Benzoquinone, a Bis-Catechol-Aldimine Derivative. Russ. J. Coord. Chem. 2018, 44, 162–168. [Google Scholar] [CrossRef]
- Astaf’eva, T.V.; Arsenyev, M.V.; Rumyantcev, R.V.; Fukin, G.K.; Cherkasov, V.K.; Poddel’sky, A.I. Imine-Based Catechols and o-Benzoquinones: Synthesis, Structure, and Features of Redox Behavior. ACS Omega 2020, 5, 22179–22191. [Google Scholar] [CrossRef]
- Bindfit. Available online: http://supramolecular.org (accessed on 10 February 2021).
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 5922–5923. [Google Scholar] [CrossRef]
- Pandey, R.; Kumar, A.; Xu, Q.; Pandey, D.S. Zinc (II), copper (II) and cadmium (II) complexes as fluorescent chemosensors for cations. Dalton Trans. 2020, 49, 542–568. [Google Scholar] [CrossRef]
- Arsenyev, M.; Baranov, E.; Fedorov, A.; Chesnokov, S.; Abakumov, G. New bis-o-quinone with azine spacer. Synthesis, structure and intramolecular cyclization. Mendeleev Commun. 2015, 25, 312–314. [Google Scholar] [CrossRef]








| Ligand | 8 (cone) | 9 (partial cone) | 10 (1,3-alternate) | 11 (Monomer) |
|---|---|---|---|---|
| M/L | 1/1 | 1/1 | 1/1 | 1/2 |
| Log Kb | 5.04 ± 0.11 | 5.36 ± 0.62 | 5.46 ± 0.51 | 8.52 ± 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padnya, P.; Shibaeva, K.; Arsenyev, M.; Baryshnikova, S.; Terenteva, O.; Shiabiev, I.; Khannanov, A.; Boldyrev, A.; Gerasimov, A.; Grishaev, D.; et al. Catechol-Containing Schiff Bases on Thiacalixarene: Synthesis, Copper (II) Recognition, and Formation of Organic-Inorganic Copper-Based Materials. Molecules 2021, 26, 2334. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26082334
Padnya P, Shibaeva K, Arsenyev M, Baryshnikova S, Terenteva O, Shiabiev I, Khannanov A, Boldyrev A, Gerasimov A, Grishaev D, et al. Catechol-Containing Schiff Bases on Thiacalixarene: Synthesis, Copper (II) Recognition, and Formation of Organic-Inorganic Copper-Based Materials. Molecules. 2021; 26(8):2334. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26082334
Chicago/Turabian StylePadnya, Pavel, Ksenia Shibaeva, Maxim Arsenyev, Svetlana Baryshnikova, Olga Terenteva, Igor Shiabiev, Artur Khannanov, Artur Boldyrev, Alexander Gerasimov, Denis Grishaev, and et al. 2021. "Catechol-Containing Schiff Bases on Thiacalixarene: Synthesis, Copper (II) Recognition, and Formation of Organic-Inorganic Copper-Based Materials" Molecules 26, no. 8: 2334. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26082334