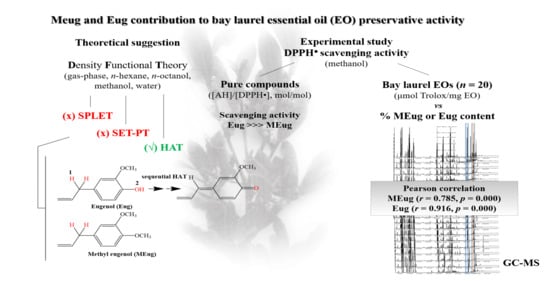
Suggestions on the Contribution of Methyl Eugenol and Eugenol to Bay Laurel (Laurus nobilis L.) Essential Oil Preservative Activity through Radical Scavenging
Abstract
:1. Introduction
2. Results and Discussion
2.1. Theoretical Evidence
2.1.1. Hydrogen Atom Donation
2.1.2. Electron Donation
2.1.3. Solvent Effect
Hydrogen Atom Transfer
Electron Donation
2.2. Experimental Evidence
3. Materials and Methods
3.1. EO Samples, Standards, and Chemicals
3.2. Theoretical Calculations
3.3. Estimation of Partition Coefficient (Log P)
3.4. DPPH• Assay
3.5. GC-FID and GC-MS Analyses of EOs
3.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. An overview of the biological effects of some Mediterranean essential oils on human health. Biomed Res. Int. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mutlu-Ingok, A.; Devecioglu, D.; Dikmetas, D.N.; Karbancioglu-Guler, F.; Capanoglu, E. Antibacterial, antifungal, antimycotoxigenic, and antioxidant activities of essential oils: An updated review. Molecules 2020, 25, 4711. [Google Scholar] [CrossRef] [PubMed]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Foti, M.C. Mode of antioxidant action of essential oils. In Essential Oils in Food Processing: Chemistry, Safety and Applications; Hashemi, S.M.B., Khaneghah, A.M., Sant’Ana, A.d.S., Eds.; Wiley Blackwell: New York, NY, USA, 2018; pp. 267–291. [Google Scholar]
- Bower, A.; Marquez, S.; de Mejia, E.G. The health benefits of selected culinary herbs and spices found in the traditional Mediterranean diet. Crit. Rev. Food Sci. Nutr. 2016, 56, 2728–2746. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Surai, P.; Deans, S.G. In vitro antioxidant activity of a number of plant essential oils and phytoconstituents. J. Essent. Oil Res. 2000, 12, 241–248. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Sanchez-Zapata, E.; Fernández-López, J.; Pérez-Álvarez, J.A. Antioxidant activity of essential oils of five spice plants widely used in a Mediterranean diet. Flavour Fragr. J. 2010, 25, 13–19. [Google Scholar] [CrossRef]
- Olszowy, M.; Dawidowicz, A.L. Essential oils as antioxidants: Their evaluation by DPPH, ABTS, FRAP, CUPRAC, and β-carotene bleaching methods. Monatshefte für Chem. Chem. Mon. 2016, 147, 2083–2091. [Google Scholar] [CrossRef]
- Ghadermazi, R.; Keramat, J.; Goli, S.A.H. Antioxidant activity of clove (Eugenia caryophyllata Thunb), oregano (Oringanum vulgare L) and sage (Salvia officinalis L) essential oils in various model systems. Int. Food Res. J. 2017, 24, 1628–1635. [Google Scholar]
- Alejo-Armijo, A.; Altarejos, J.; Salido, S. Phytochemicals and biological activities of laurel tree (Laurus nobilis). Nat. Prod. Commun. 2017, 12, 743–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chahal, K.; Kaur, M.; Bhardwaj, U.; Singla, N.; Kaur, A. A review on chemistry and biological activities of Laurus nobilis L. essential oil. J. Pharmacogn. Phytochem. 2017, 6, 1153–1161. [Google Scholar]
- Ramos, C.; Teixeira, B.; Batista, I.; Matos, O.; Serrano, C.; Neng, N.R.; Nogueira, J.M.F.; Nunes, M.L.; Marques, A. Antioxidant and antibacterial activity of essential oil and extracts of bay laurel Laurus nobilis Linnaeus (Lauraceae) from Portugal. Nat. Prod. Res. 2012, 26, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Hassiotis, C.; Dina, E. The influence of aromatic plants on microbial biomass and respiration in a natural ecosystem. Isr. J. Ecol. Evol. 2011, 56, 181–196. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Wink, M.; Setzer, W.N. Radical scavenging and antioxidant activities of essential oil components ? An experimental and computational investigation. Nat. Prod. Commun. 2015, 10, 153–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewinsohn, E.; Ziv-Raz, I.; Dudai, N.; Tadmor, Y.; Lastochkin, E.; Larkov, O.; Chaimovitsh, D.; Ravid, U.; Putievsky, E.; Pichersky, E.; et al. Biosynthesis of estragole and methyl-eugenol in sweet basil (Ocimum basilicum L). Developmental and chemotypic association of allylphenol O-methyltransferase activities. Plant Sci. 2000, 160, 27–35. [Google Scholar] [CrossRef]
- Yahyaa, M.; Berim, A.; Nawade, B.; Ibdah, M.; Dudareva, N.; Ibdah, M. Biosynthesis of methyleugenol and methylisoeugenol in Daucus carota leaves: Characterization of eugenol/isoeugenol synthase and O-Methyltransferase. Phytochemistry 2019, 159, 179–189. [Google Scholar] [CrossRef]
- Tan, K.H.; Nishida, R. Methyl eugenol: Its occurrence, distribution, and role in nature, especially in relation to insect behavior and pollination. J. Insect Sci. 2012, 12. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.; Teixeira, B.; Nunes, M.L. Bay laurel (Laurus nobilis) oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: London, UK, 2016; pp. 239–246. [Google Scholar]
- Regulation (EC) No 1334/2008 of the European Parliament and of the Council. On flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. Off. J. Eur. Union 2008, L 354/34, 34–50.
- IARC Methyl eugenol. IARC Monogr. Eval. Carcinog. Risks to Humans. 2013, pp. 407–433. Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono101-013.pdf (accessed on 16 April 2021).
- Sohilait, J.H.; Kainama, H. Free radical scavenging activity of essential oil of Eugenia caryophylata from Amboina Island and derivatives of eugenol. Open Chem. 2019, 17, 422–428. [Google Scholar] [CrossRef]
- Yi, F.; Sun, J.; Bao, X.; Ma, B.; Sun, M. Influence of molecular distillation on antioxidant and antimicrobial activities of rose essential oils. Lwt 2019, 102, 310–316. [Google Scholar] [CrossRef]
- Koroch, A.R.; Simon, J.E.; Juliani, H.R. Essential oil composition of purple basils, their reverted green varieties (Ocimum basilicum) and their associated biological activity. Ind. Crops Prod. 2017, 107, 526–530. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M.Z. On the use of DFT computations to the radical scavenging activity studies of natural phenolic compounds. In Density Functional Theory: Principles, Applications and Analysis; Morin, J., Pelletier, J.M., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2013; pp. 121–146. [Google Scholar]
- Nenadis, N.; Sigalas, M.P. A DFT study on the radical scavenging activity of maritimetin and related aurones. J. Phys. Chem. A 2008, 112, 12196–12202. [Google Scholar] [CrossRef]
- Chowdhry, B.Z.; Ryall, J.P.; Dines, T.J.; Mendham, A.P. Infrared and Raman spectroscopy of eugenol, isoeugenol, and methyl eugenol: Conformational analysis and vibrational assignments from density functional theory calculations of the anharmonic fundamentals. J. Phys. Chem. A 2015, 119, 11280–11292. [Google Scholar] [CrossRef] [PubMed]
- Bakalbassis, E.G.; Nenadis, N.; Tsimidou, M. A density functional theory study of structure-activity relationships in caffeic and dihydrocaffeic acids and related monophenols. J. Am. Oil Chem. Soc. 2003, 80, 459–466. [Google Scholar] [CrossRef]
- Frankel, E.N.; Huang, S.W.; Kanner, J.; German, J.B. Interfacial phenomena in the evaluation of antioxidants: Bulk oils vs. emulsions. J. Agric. Food Chem. 1994, 42, 1054–1059. [Google Scholar] [CrossRef]
- Boulebd, H. DFT study of the antiradical properties of some aromatic compounds derived from antioxidant essential oils: C–H bond vs. O–H bond. Free Radic. Res. 2019, 53, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Leem, H.H.; Kim, E.O.; Seo, M.J.; Choi, S.W. Antioxidant and anti-inflammatory activities of eugenol and its derivatives from clove (Eugenia caryophyllata Thunb.). J. Korean Soc. Food Sci. Nutr. 2011, 40, 1361–1370. [Google Scholar] [CrossRef]
- da Silva, F.F.M.; Monte, F.J.Q.; de Lemos, T.L.G.; do Nascimento, P.G.G.; de Medeiros Costa, A.K.; de Paiva, L.M.M. Eugenol derivatives: Synthesis, characterization, and evaluation of antibacterial and antioxidant activities. Chem. Cent. J. 2018, 12, 34. [Google Scholar] [CrossRef] [Green Version]
- Joshi, R.K. Chemical composition, in vitro antimicrobial and antioxidant activities of the essential oils of Ocimum gratissimum, O. sanctum and their major constituents. Indian J. Pharm. Sci. 2013, 75, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denisov, E.T.; Denisova, T. (Eds.) Handbook of Antioxidants: Bond Dissociation Energies, Rate Constants, Activation Energies, and Enthalpies of Reactions, 2nd ed.; CRC Press LCC: Boca Raton, FL, USA, 2000; ISBN 0-8493-9004-4. [Google Scholar]
- Marzouki, H.; Piras, A.; Salah, K.B.H.; Medini, H.; Pivetta, T.; Bouzid, S.; Marongiu, B.; Falconieri, D. Essential oil composition and variability of Laurus nobilis L. growing in Tunisia, comparison and chemometric investigation of different plant organs. Nat. Prod. Res. 2009, 23, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Marzouki, H.; Khaldi, A.; Marongiu, B.; Piras, A.; Harzallah-Skhiri, F. Chemical polymorphism of essential oils from populations of Laurus nobilis grown on Tunisia, Algeria and France. Nat. Prod. Commun. 2011, 6, 1483–1486. [Google Scholar] [CrossRef] [Green Version]
- Fiorini, C.; Fourasté, I.; David, B.; Bessière, J.M. Composition of the flower, leaf and stem essential oils from Laurus nobilis L. Flavour Fragr. J. 1997, 12, 91–93. [Google Scholar] [CrossRef]
- Fidan, H.; Stefanova, G.; Kostova, I.; Stankov, S.; Damyanova, S.; Stoyanova, A.; Zheljazkov, V.D. Chemical composition and antimicrobial activity of Laurus nobilis L. essential oils from Bulgaria. Molecules 2019, 24, 804. [Google Scholar] [CrossRef] [Green Version]
- Taban, A.; Saharkhiz, M.J.; Niakousari, M. Sweet bay (Laurus nobilis L.) essential oil and its chemical composition, antioxidant activity and leaf micromorphology under different extraction methods. Sustain. Chem. Pharm. 2018, 9, 12–18. [Google Scholar] [CrossRef]
- Ballesteros, J.L.; Tacchini, M.; Spagnoletti, A.; Grandini, A.; Paganetto, G.; Neri, L.M.; Marengo, A.; Angiolella, L.; Guerrini, A.; Sacchetti, G. Rediscovering medicinal Amazonian aromatic plants: Piper carpunya (Piperaceae) essential oil as paradigmatic study. Evid. Based Complement. Altern. Med. 2019, 2019. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Mikallou, M.; Petropoulos, S.; Tzortzakis, N. Profiling of essential oils components and polyphenols for their antioxidant activity of medicinal and aromatic plants grown in different environmental conditions. Agronomy 2020, 10, 727. [Google Scholar] [CrossRef]
- Xie, J.; Schaich, K.M. Re-evaluation of the 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH) assay for antioxidant activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef] [PubMed]
- Gaussian 09; Revision A.1; Software for quantum chemical calculations; Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Barone, V.; Cossi, M.; Tomasi, J. A new definition of cavities for the computation of solvation free energies by the polarizable continuum model. J. Chem. Phys. 1997, 107, 3210–3221. [Google Scholar] [CrossRef]
- Cancès, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Broto, P.; Moreau, G.; Vandycke, C. Molecular structures: Perception, autocorrelation descriptor and SAR studies: System of atomic contributions for the calculation of the n-octanol/water partition coefficients. Eur. J. Med. Chem. 1984, 19, 71–78. [Google Scholar]
- Sellami, I.H.; Rebey, I.B.; Sriti, J.; Rahali, F.Z.; Limam, F.; Marzouk, B. Drying sage (Salvia officinalis L.) plants and its effects on content, chemical composition, and radical scavenging activity of the essential oil. Food Bioprocess Technol. 2012, 5, 2978–2989. [Google Scholar] [CrossRef]



| BDE | ΔΒDE 1 | IP | ΔIP 1 | PA | ΔPA 1 | |
|---|---|---|---|---|---|---|
| AH | kcal/mol | |||||
| Phe | 84.3 | 0.0 | 191.8 | 0.0 | 348.0 | 0.0 |
| Anis | - | - | 185.1 | −6.7 | - | - |
| Guai | 83.4 | −0.9 | 177.8 | −14.0 | 352.7 | 4.8 |
| Ver | - | - | 170.6 | −21.2 | - | - |
| MEug | 72.7 2 | −11.6 | 166.8 | −25.0 | 360.9 2 | 12.9 |
| Eug | 82.4/72.3 2 | −1.9/−12.0 | 171.4 | −20.4 | 351.4/364.1 2 | 3.4/16.1 |
| MiEug | - | - | 158.8 | −33.0 | - | - |
| iEug | 79.2 | −5.1 | 162.4 | −29.4 | 347.5 | −0.5 |
| Est | 72.5 2 | −11.8 | 176.8 | −15.0 | 364.6 2 | 16.6 |
| Aneth | - | - | 165.4 | −26.4 | - | - |
| Solvent | n-Hexane | n-Octanol | Methanol | Water | ||||
|---|---|---|---|---|---|---|---|---|
| BDE | ΔBDE 1 | BDE | ΔBDE 1 | BDE | ΔBDE 1 | BDE | ΔBDE 1 | |
| AH | kcal/mol | |||||||
| Phe | 86.3 | 0 | 88.5 | 0 | 89.2 | 0 | 89.1 | 0 |
| Guai | 83.8 | −2.5 | 83.7 | −4.8 | 83.7 | −5.5 | 83.6 | −5.5 |
| MEug | 74.1 2 | −12.2 | 75.7 2 | −12.8 | 76.0 2 | −13.3 | 76.1 2 | −13.0 |
| Eug | 82.3/73.5 2 | −4.0/−12.8 | 81.6/74.9 2 | −6.9/−13.6 | 80.9/75.4 2 | −8.3/−13.9 | 81.3/75.2 2 | −7.8/−13.9 |
| iEug | 79.3 | −7.0 | 78.6 | −9.9 | 78.4 | −10.8 | 78.3 | −10.8 |
| Est | 73.7 2 | −12.6 | 75.2 2 | −13.3 | 75.5 2 | −13.8 | 75.6 2 | −13.5 |
| Solvent | n-Octanol | Methanol | Water | |||
|---|---|---|---|---|---|---|
| IP | ΔIP 1 | IP | ΔIP 1 | IP | ΔIP 1 | |
| AH | kcal/mol | |||||
| Phe | 139.4 | 0.0 | 134.9 | 0.0 | 133.2 | 0.0 |
| Anis | 140.1 | 0.7 | 136.6 | 1.7 | 135.7 | 2.5 |
| Guai | 130.4 | −9.0 | 126.4 | −8.5 | 125.4 | −7.8 |
| Ver | 130.2 | −9.2 | 127.1 | −7.8 | 126.3 | −6.9 |
| MEug | 127.5 | −11.9 | 124.4 | −10.5 | 123.7 | −9.5 |
| Eug | 125.6 | −13.8 | 121.6 | −13.3 | 120.6 | −12.6 |
| MiEug | 123.1 | −16.3 | 120.3 | −14.6 | 119.6 | −13.6 |
| iEug | 121.0 | −18.4 | 117.5 | −17.4 | 116.6 | −16.6 |
| Est | 133.2 | −6.2 | 129.6 | −5.3 | 128.7 | −4.5 |
| Aneth | 125.6 | −13.8 | 122.5 | −12.4 | 121.7 | −11.5 |
| Solvent | n-Octanol | Methanol | Water | |||
|---|---|---|---|---|---|---|
| PA | ΔPA 1 | PA | ΔPA 1 | PA | ΔPA 1 | |
| AH | kcal/mol | |||||
| Phe | 301.6 | 0.0 | 297.8 | 0.0 | 296.5 | 0.0 |
| Guai | 302.0 | 0.4 | 297.2 | −0.6 | 295.9 | −0.6 |
| Eug | 302.6/326.8 2 | 1.0/25.2 | 297.7/323.7 2 | −0.1/26.7 | 296.5/322.9 2 | 0/26.5 |
| MEug | 324.7 2 | 23.1 | 321.8 2 | 24.0 | 321.0 2 | 24.5 |
| iEug | 301.4 | −0.2 | 296.8 | −1.0 | 295.6 | −0.9 |
| Est | 327.4 2 | 25.8 | 324.4 2 | 26.6 | 323.5 2 | 27.0 |
| EO | Antioxidant Activity (n = 3) | Most Abundant Volatiles (GC-MS Analyses 1) | Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| μmol Trolox/mg EO (μmol α-Tocopherol/mg EO) | α-Pinene | Limonene | 1,8-Cineole | Linalool | Terpinen-4-ol | α-Terpinyl Acetate | MEug | Eug | MEug/Eug | |
| RI 2 | ||||||||||
| 1043 | 1120 | 1159 | 1456 | 1584 | 1683 | 2082 | 2338 | |||
| m/z3 | ||||||||||
| n.a. 4 | 68, 93, 136 | 43, 81, 154 | 41, 71, 153 | 71, 111, 154 | 43, 121, 181 | 147, 163, 178 | 103, 149, 164 | |||
| Percent Composition (%) | ||||||||||
| OL1 | 4.4 ± 0.0 g (4.8 ± 0.0 g) | 6.0 | 0.9 | 61.9 | 2.8 | 3.4 | 9.1 | 1.1 | 0.8 | 1.38 |
| OL2 | 5.6 ± 0.0 h (6.0 ± 0.0 h) | 7.1 | 1.6 | 51.0 | 3.0 | 2.8 | 14.0 | 1.7 | 1.2 | 1.42 |
| OL3 | 4.7 ± 0.1 g (5.0 ± 0.1 g) | 5.6 | 2.1 | 58.4 | 0.9 | 2.5 | 14.2 | 1.1 | 0.8 | 1.38 |
| AL1 | 8.1 ± 0.1 i (8.4 ± 0.1 i) | - | - | 34.1 | 10.6 | 6.1 | 28.0 | 7.6 | 2.1 | 3.62 |
| AL2 | 3.6 ± 0.0 c (4.0 ± 0.0 c) | 2.5 | 3.5 | 48.6 | 1.8 | 4.3 | 16.1 | 1.3 | 1.5 | 0.87 |
| AL3 | 3.9 ± 0.2 abd (4.3 ± 0.2 abd) | 7.1 | 2.9 | 59.5 | 3.3 | 1.6 | 8.9 | 2.5 | 0.5 | 5.00 |
| SL1 | 7.7 ± 0.2 k (8.0 ± 0.2 k) | 6.4 | 2.2 | 58.2 | 6.1 | 1.6 | 8.8 | 2.2 | 2.1 | 1.05 |
| SL2 | 3.6 ± 0.1 ac (4.0 ± 0.1 ac) | 7.5 | 2.6 | 58.0 | 3.5 | 2.2 | 8.9 | 2.2 | 0.5 | 4.40 |
| SL3 | 4.0 ± 0.1 bd (4.4 ± 0.1bd) | 7.5 | 2.7 | 59.1 | 3.5 | 1.9 | 8.2 | 2.2 | 0.5 | 4.40 |
| FL1 | 3.8 ± 0.3 abc (4.2 ± 0.3 abc) | 6.4 | 2.3 | 59.6 | 3.5 | 2.3 | 10.2 | 2.2 | 0.5 | 4.40 |
| FL2 | 4.2 ± 0.2 d (4.5 ± 0.2 d) | 8.2 | 2.7 | 54.7 | 3.6 | 1.7 | 10.4 | 2.6 | 0.6 | 4.33 |
| FL3 | 3.9 ± 0.1 abd (4.3 ± 0.1 abd) | 7.0 | 2.5 | 59.1 | 3.5 | 1.5 | 9.7 | 2.8 | 0.5 | 5.60 |
| EL1 | 3.0 ± 0.1 f (3.4 ± 0.1 f) | 6.7 | 2.5 | 63.0 | 3.8 | 1.9 | 9.3 | 1.0 | 0.4 | 2.50 |
| EL2 | 5.8 ± 0.0 h (6.1 ± 0.0 h) | 2.9 | 1.3 | 53.6 | 6.7 | 2.8 | 13.2 | 5.2 | 1.2 | 4.33 |
| VL2 | 8.1 ± 0.3 i (8.4 ± 0.3 i) | 3.6 | 2.3 | 47.7 | 12.9 | 1.3 | 14.0 | 8.0 | 2.1 | 3.81 |
| HL | 2.4 ± 0.0 e (2.8 ± 0.0 e) | 7.4 | 2.2 | 61.4 | 3.6 | 1.3 | 10.7 | 1.1 | 0.4 | 2.75 |
| DL | 3.2 ± 0.2 f (3.7 ± 0.2 f) | 8.6 | 3.1 | 57.7 | 3.5 | 1.6 | 8.7 | 2.1 | 0.4 | 5.25 |
| AmL | 2.5 ± 0.1 e (2.9 ± 0.1 e) | 3.2 | 1.4 | 53.2 | 10.7 | - | 13.2 | - | 0.3 | 0.00 |
| ML | 2.1 ± 0.3 j (2.6 ± 0.3 j) | 3.2 | 1.6 | 61.2 | 1.4 | 2.4 | 12.9 | 0.8 | 0.3 | 2.67 |
| TL | 3.7 ± 0.0 abc (4.1 ± 0.0 abc) | 7.1 | 3.0 | 58.4 | 3.3 | 1.8 | 8.6 | 2.5 | 0.5 | 5.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nenadis, N.; Papapostolou, M.; Tsimidou, M.Z. Suggestions on the Contribution of Methyl Eugenol and Eugenol to Bay Laurel (Laurus nobilis L.) Essential Oil Preservative Activity through Radical Scavenging. Molecules 2021, 26, 2342. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26082342
Nenadis N, Papapostolou M, Tsimidou MZ. Suggestions on the Contribution of Methyl Eugenol and Eugenol to Bay Laurel (Laurus nobilis L.) Essential Oil Preservative Activity through Radical Scavenging. Molecules. 2021; 26(8):2342. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26082342
Chicago/Turabian StyleNenadis, Nikolaos, Maria Papapostolou, and Maria Z. Tsimidou. 2021. "Suggestions on the Contribution of Methyl Eugenol and Eugenol to Bay Laurel (Laurus nobilis L.) Essential Oil Preservative Activity through Radical Scavenging" Molecules 26, no. 8: 2342. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26082342