Novel Oxime Synthesized from a Natural Product of Senecio nutans SCh. Bip. (Asteraceae) Enhances Vascular Relaxation in Rats by an Endothelium-Independent Mechanism
Abstract
:1. Introduction
2. Results
2.1. Isolation of Natural Products from S. nutans and Oxime Synthesis
2.2. Effect of Metabolite–1 and Oxime–1 on Vascular Relaxation: Role of the Endothelium
2.3. Effect of Metabolite–1 and Oxime–1 on the Contractile Response to KCl: Role of External Ca2+
2.4. Effect of Metabolite–1 and Oxime–1 on the Contractile Response to PE and Extracellular Ca2+ Influx
2.5. Effect of Metabolite–1 and Oxime–1 on the Contractile Response to KCl and Bay K8644
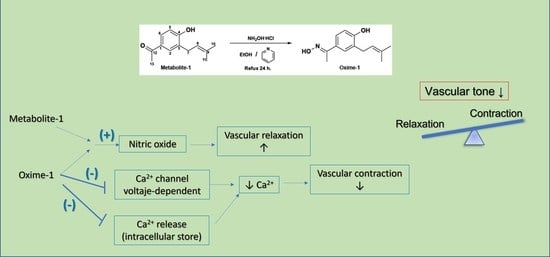
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Isolation of Natural Products from S. nutans
4.3. Synthesis of Oxime
4.4. Animals
4.5. Isolation of Rat Aorta and Vascular Reactivity Assays
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macía, M.J.; García, E.; Vidaurre, P.J. An Ethnobotanical Survey of Medicinal Plants Commercialized in the Markets of La Paz and El Alto, Bolivia. J. Ethnopharmacol. 2005, 97, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Lizarraga, E.; Castro, F.; Fernández, F.; de Lampasona, M.P.; Catalán, C.A. Antioxidant, Hemolytic and Cytotoxic Activities of Senecio Species Used in Traditional Medicine of Northwestern Argentina. Nat. Prod. Commun. 2012, 7, 607–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giberti, G.C. Herbal Folk Medicine in Northwestern Argentina: Compositae. J. Ethnopharmacol. 1983, 7, 321–341. [Google Scholar] [CrossRef]
- Villagrán, C.; Castro, V.; Sánchez, G.; Romo, M.; Latorre, C.; Hinojosa, L.F. La Tradición Surandina Del Desierto: Etnobotánica Del Área Del Salar de Atacama (Provincia de El Loa, Región de Antofagasta, Chile). Estud. Atacameños 1998, 16, 8–105. [Google Scholar] [CrossRef] [Green Version]
- Villagrán, C.; Romo, M.; Castro, V. Etnobotánica Del Sur de Los Andes de La Primera Región de Chile: Un Enlace Entre Las Culturas Altiplánicas y Las de Quebradas Altas Del Loa Superior. Chungará (Arica) 2003, 35, 73–124. [Google Scholar] [CrossRef] [Green Version]
- Ticona, L.A.; Cano-Adamuz, N.; Serban, A.M.; Sánchez, Á.R. Inhibition of HIF-1α through Suppression of NF-ΚB Activation by Compounds Isolated from Senecio Graveolens. Planta Med. Int. Open 2020, 7, e1–e11. [Google Scholar] [CrossRef] [Green Version]
- Parra, C.; Soto, E.; León, G.; Salas, C.O.; Heinrich, M.; Echiburú-Chau, C. Nutritional Composition, Antioxidant Activity and Isolation of Scopoletin from Senecio Nutans: Support of Ancestral and New Uses. Nat. Prod. Res. 2018, 32, 719–722. [Google Scholar] [CrossRef]
- Paredes, A.; Palacios, J.; Quispe, C.; Nwokocha, C.R.; Morales, G.; Kuzmicic, J.; Cifuentes, F. Hydroalcoholic Extract and Pure Compounds from Senecio Nutans Sch. Bip (Compositae) Induce Vasodilation in Rat Aorta through Endothelium-Dependent and Independent Mechanisms. J. Ethnopharmacol. 2016, 192, 99–107. [Google Scholar] [CrossRef]
- Cifuentes, F.; Paredes, A.; Palacios, J.; Muñoz, F.; Carvajal, L.; Nwokocha, C.R.; Morales, G. Hypotensive and Antihypertensive Effects of a Hydroalcoholic Extract from Senecio Nutans Sch. Bip. (Compositae) in Mice: Chronotropic and Negative Inotropic Effect, a Nifedipine-like Action. J. Ethnopharmacol. 2016, 179, 367–374. [Google Scholar] [CrossRef]
- Loyola, L.A.; Pedreros, S.; Morales, G. Para-Hydroxyacetophenone Derivatives from Senecio-Graveolens. Phytochemistry 1985, 24, 1600–1602. [Google Scholar] [CrossRef]
- Morales, G.; Borquez, J.; Loyola, L.A. A Monoterpenoid and P-Hydroxyacetophenone Derivatives from Senecio Species of the North of Chile. Bol. De La Soc. Chil. De Quim. 1996, 41, 159–166. [Google Scholar]
- Dupre, S.; Grenz, M.; Jakupovic, J.; Bohlmann, F.; Niemeyer, H.M. Eremophilane, Germacrane and Shikimic Acid-Derivatives from Chilean Senecio Species. Phytochemistry 1991, 30, 1211–1220. [Google Scholar] [CrossRef]
- Echiburú-Chau, C.; Alfaro-Lira, S.; Brown, N.; Salas, C.O.; Cuellar, M.; Santander, J.; Ogalde, J.P.; Rothhammer, F. The Selective Cytotoxicity Elicited by Phytochemical Extract from Senecio Graveolens (Asteraceae) on Breast Cancer Cells Is Enhanced by Hypoxia. Int. J. Oncol. 2014, 44, 1357–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santander, J.; Otto, C.; Lowry, D.; Cuellar, M.; Mellado, M.; Salas, C.; Rothhammer, F. Specific Gram-Positive Antibacterial Activity of 4-Hydroxy-3-(3-Methyl-2-Butenyl) Acetophenone Isolated from Senecio Graveolens. Br. Microbiol. Res. J. 2015, 5, 94–106. [Google Scholar] [CrossRef]
- Takasugi, M.; Masuda, T. Three 4’-Hydroxyacetophenone-Related Phytoalexins from Polymnia Sonchifolia. Phytochemistry 1996, 43, 1019–1021. [Google Scholar] [CrossRef]
- Kant, S.; Sellke, F.; Feng, J. Metabolic Regulation and Dysregulation of Endothelial Small Conductance Calcium Activated Potassium Channels. Eur. J. Cell Biol. 2022, 101, 151208. [Google Scholar] [CrossRef]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular Smooth Muscle Contraction in Hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef] [Green Version]
- Gallo, G.; Volpe, M.; Savoia, C. Endothelial Dysfunction in Hypertension: Current Concepts and Clinical Implications. Front. Med. 2022, 8, 798958. [Google Scholar] [CrossRef]
- Sabe, S.A.; Feng, J.; Sellke, F.W.; Abid, M.R. Mechanisms and Clinical Implications of Endothelium-Dependent Vasomotor Dysfunction in Coronary Microvasculature. Am. J. Physiol. -Heart Circ. Physiol. 2022, 322, H819–H841. [Google Scholar] [CrossRef]
- Lopes, K.S.; Marques, A.A.M.; Moreno, K.G.T.; Lorençone, B.R.; Leite, P.R.T.; da Silva, G.P.; dos Santos, A.C.; Souza, R.I.C.; Gasparotto, F.M.; Cassemiro, N.S.; et al. Small Conductance Calcium-Activated Potassium Channels and Nitric Oxide/CGMP Pathway Mediate Cardioprotective Effects of Croton Urucurana Baill. In Hypertensive Rats. J. Ethnopharmacol. 2022, 293, 115255. [Google Scholar] [CrossRef]
- Chalupsky, K.; Lobysheva, I.; Nepveu, F.; Gadea, I.; Beranova, P.; Entlicher, G.; Stoclet, J.C.; Muller, B. Relaxant Effect of Oxime Derivatives in Isolated Rat Aorta: Role of Nitric Oxide (NO) Formation in Smooth Muscle. Biochem. Pharmacol. 2004, 67, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Bolotin, D.S.; Bokach, N.A.; Demakova, M.Y.; Kukushkin, V.Y. Metal-Involving Synthesis and Reactions of Oximes. Chem. Rev. 2017, 117, 13039–13122. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Plotnikov, M.B.; Khlebnikov, A.I.; Plotnikova, T.M.; Quinn, M.T. Oximes: Novel Therapeutics with Anticancer and Anti-Inflammatory Potential. Biomolecules 2021, 11, 777. [Google Scholar] [CrossRef] [PubMed]
- Sahyoun, T.; Arrault, A.; Schneider, R. Amidoximes and Oximes: Synthesis, Structure, and Their Key Role as No Donors. Molecules 2019, 24, 2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghabbour, H.A.; El-Bendary, E.R.; El-Ashmawy, M.B.; El-Kerdawy, M.M. Synthesis, Docking Study and Beta-Adrenoceptor Activity of Some New Oxime Ether Derivatives. Molecules 2014, 19, 3417–3435. [Google Scholar] [CrossRef] [Green Version]
- Suksawat, M.; Techasen, A.; Namwat, N.; Boonsong, T.; Titapun, A.; Ungarreevittaya, P.; Yongvanit, P.; Loilome, W. Inhibition of Endothelial Nitric Oxide Synthase in Cholangiocarcinoma Cell Lines–A New Strategy for Therapy. FEBS Open Bio 2018, 8, 513–522. [Google Scholar] [CrossRef] [Green Version]
- Konukoglu, D.; Uzun, H. Endothelial Dysfunction and Hypertension. Adv. Exp. Med. Biol. 2016, 956, 511–540. [Google Scholar]
- Surcel, A.; Ng, W.P.; West-Foyle, H.; Zhu, Q.; Ren, Y.; Avery, L.B.; Krenc, A.K.; Meyers, D.J.; Rock, R.S.; Anders, R.A.; et al. Pharmacological Activation of Myosin Ii Paralogs to Correct Cell Mechanics Defects. Proc. Natl. Acad. Sci. USA 2015, 112, 1428–1433. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.H.; Lee, Y.J.; Kim, J.S.; Kang, D.G.; Lee, H.S. Cynanchum Wilfordii Ameliorates Hypertension and Endothelial Dysfunction in Rats Fed with High Fat/Cholesterol Diets. Immunopharmacol. Immunotoxicol. 2012, 34, 4–11. [Google Scholar] [CrossRef]
- Jaiswal, G.; Kumar, P. Neuroprotective Role of Apocynin against Pentylenetetrazole Kindling Epilepsy and Associated Comorbidities in Mice by Suppression of ROS/RNS. Behav. Brain Res. 2022, 419, 113699. [Google Scholar] [CrossRef]
- Nakagawa, K.; Itoya, M.; Takemoto, N.; Matsuura, Y.; Tawa, M.; Matsumura, Y.; Ohkita, M. Indoxyl Sulfate Induces ROS Production via the Aryl Hydrocarbon Receptor-NADPH Oxidase Pathway and Inactivates NO in Vascular Tissues. Life Sci. 2021, 265, 118807. [Google Scholar] [CrossRef] [PubMed]
- Ureña, J.; del Valle-Rodríguez, A.; López-Barneo, J. Metabotropic Ca2+ Channel-Induced Calcium Release in Vascular Smooth Muscle. Cell Calcium 2007, 42, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.V.; Kanagarajah, A.; Toemoe, S.; Bertrand, P.P.; Grayson, T.H.; Britton, F.C.; Leader, L.; Senadheera, S.; Sandow, S.L. TRPV3 Expression and Vasodilator Function in Isolated Uterine Radial Arteries from Non-Pregnant and Pregnant Rats. Vasc. Pharmacol. 2016, 83, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.W.; Sullivan, M.N.; Pritchard, H.A.T.; Robinson, J.J.; Earley, S. Unitary TRPV3 Channel Ca2+ Influx Events Elicit Endothelium-Dependent Dilation of Cerebral Parenchymal Arterioles. Am. J. Physiol.-Heart Circ. Physiol. 2015, 309, H2031–H2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, T.X.; Ton, H.T.; Gulyás, H.; Pórszász, R.; Tóth, A.; Russo, R.; Kay, M.W.; Sahibzada, N.; Ahern, G.P. TRPV1 Expressed throughout the Arterial Circulation Regulates Vasoconstriction and Blood Pressure. J. Physiol. 2020, 598, 5639–5659. [Google Scholar] [CrossRef]
- Gelen, V.; Çelebi, F. In Vitro Investigation Effects of 4-Hydroxyacetophenone on Rat Thoracic Aorta’s Vasomotor Activity. Pharmacogn. Res. 2018, 10, 319. [Google Scholar] [CrossRef]
- Hart, D.J.; Magomedov, N.A. Synthesis of Ent-Alantrypinone. J. Am. Chem. Soc. 2001, 123, 5892–5899. [Google Scholar] [CrossRef]
- Cifuentes, F.; Palacios, J.; Kuzmicic, J.; Carvajal, L.; Muñoz, F.; Quispe, C.; Nwokocha, C.R.; Morales, G.; Norambuena-Soto, I.; Chiong, M.; et al. Vasodilator and Hypotensive Effects of Pure Compounds and Hydroalcoholic Extract of Xenophyllum Poposum (Phil) V.A Funk (Compositae) on Rats. Phytomedicine 2018, 50, 99–108. [Google Scholar] [CrossRef]
- Ringvold, H.C.; Khalil, R.A. Protein Kinase C as Regulator of Vascular Smooth Muscle Function and Potential Target in Vascular Disorders. Adv. Pharmacol. 2017, 78, 203–301. [Google Scholar]
- Jackson, W.F. Potassium Channels in Regulation of Vascular Smooth Muscle Contraction and Growth. Adv. Pharmacol. 2017, 78, 89–144. [Google Scholar] [CrossRef] [Green Version]










| Position | Metabolite–1 | Oxime–1 |
|---|---|---|
| δH (ppm) J in Hz | δH (ppm) J in Hz | |
| 1 | ---- | ---- |
| 2 | 7.79 (2.2) | 7.40 |
| 3 | ---- | ---- |
| 4 | ---- | ---- |
| 5 | 6.91 (d), (8.3) | 6.79 (d), (8.3) |
| 6 | 7.80 (dd), (8.4–2.4) | 7.37 |
| 7 | 3.44 (d), (8.2) | 3.37 (d), (7.1) |
| 8 | 5.36 | 5.31 (t), (6.2) |
| 9 | ---- | ---- |
| 10 | 1.82 (d), (1.2) | 1.78 (s) |
| 11 | 1.82 (d), (1.2) | ---- |
| 12 | ---- | ---- |
| 13 | 2.60 (s) | 2.26 (s) |
| OH | 6.57 (s) | ---- |
| Position | Metabolite–1 | Oxime–1 |
|---|---|---|
| δH (ppm) | δH (ppm) | |
| C1 | 159.71 | 115.47 |
| C2 | 130.91 | 127.790 |
| C3 | 127.79 | 126.93 |
| C4 | 129.72 | 129.14 |
| C5 | 115.40 | 115.72 |
| C6 | 128.95 | 125.52 |
| C7 | 29.11 | 29.87 |
| C8 | 121.33 | 121.45 |
| C9 | 134.64 | 135.15 |
| C10 | 25.80 | 25.77 |
| C11 | 17.91 | 17.90 |
| C12 | 198.56 | 155.91 |
| C13 | 26.29 | 12.12 |
| Molecule | Endothelium | Endothelium-Denuded | L-NAME |
|---|---|---|---|
| Metabolite–1 | 24.44 ± 7.98 | 53.34 ± 4.56 | 66.32 ± 7.22 * |
| Oxime–1 | 10.14 ± 8.46 ### | 81.88 ± 7.79 *** | 26.49 ± 7.97 **,$$ |
| Molecule | KCl (mM) | CaCl2 (mM) |
|---|---|---|
| Control | 22.37 ± 1.40 | 0.39 ± 0.03 |
| Metabolite–1 | 21.98 ± 1.20 | 0.41 ± 0.06 |
| Oxime–1 | 37.72 ± 2.10 *** | 0.33 ± 0.07 |
| Molecule | PE (nM) | CaCl2 (mM) |
|---|---|---|
| Control | 34.04 ± 8.49 | 0.32 ± 0.03 |
| Metabolite–1 | 49.77 ± 8.44 | 0.33 ± 0.04 |
| Oxime–1 | 67.28 ± 8.67 * | 0.33 ± 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacios, J.; Paredes, A.; Catalán, M.A.; Nwokocha, C.R.; Cifuentes, F. Novel Oxime Synthesized from a Natural Product of Senecio nutans SCh. Bip. (Asteraceae) Enhances Vascular Relaxation in Rats by an Endothelium-Independent Mechanism. Molecules 2022, 27, 3333. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27103333
Palacios J, Paredes A, Catalán MA, Nwokocha CR, Cifuentes F. Novel Oxime Synthesized from a Natural Product of Senecio nutans SCh. Bip. (Asteraceae) Enhances Vascular Relaxation in Rats by an Endothelium-Independent Mechanism. Molecules. 2022; 27(10):3333. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27103333
Chicago/Turabian StylePalacios, Javier, Adrián Paredes, Marcelo A. Catalán, Chukwuemeka R. Nwokocha, and Fredi Cifuentes. 2022. "Novel Oxime Synthesized from a Natural Product of Senecio nutans SCh. Bip. (Asteraceae) Enhances Vascular Relaxation in Rats by an Endothelium-Independent Mechanism" Molecules 27, no. 10: 3333. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27103333