Nano-Silica Modified with Diamine for Capturing Azo Dye from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Amine-Modified Nano-Silica
2.3. Adsorption of Methyl Orange Dye
2.3.1. Adsorption Equilibrium
2.3.2. Adsorption Kinetics
2.4. Characterization
3. Results and Discussion
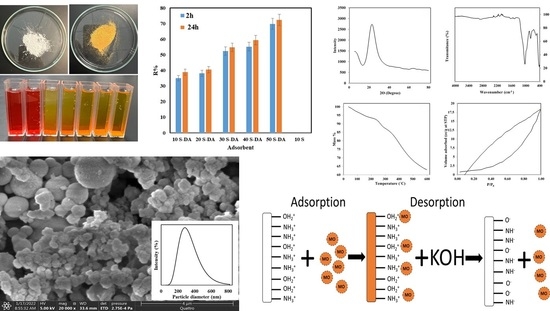
3.1. Characterization of the Adsorbent
3.2. Adsorption of MO
3.3. Regeneration of the Adsorbent
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saha, T.K.; Bhoumik, N.C.; Karmaker, S.; Ahmed, M.G.; Ichikawa, H.; Fukumori, Y. Adsorption of Methyl Orange onto Chitosan from Aqueous Solution. J. Water Resour. Prot. 2010, 2, 898–906. [Google Scholar] [CrossRef] [Green Version]
- Egwuonwu, P. Adsorption of Methyl Red and Methyl Orange Using. Acad. Res. Int. 2013, 4, 330–338. [Google Scholar]
- Hanoon, M.A.; Ahmed, M.J. Adsorption of Methyl Orange from Wastewater by using Biochar. Iraqi J. Chem. Pet. Eng. 2019, 20, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Iwuozor, K.O.; Ighalo, J.O.; Emenike, E.C.; Ogunfowora, L.A.; Igwegbe, C.A. Adsorption of methyl orange: A review on adsorbent performance. Curr. Res. Green Sustain. Chem. 2021, 4, 100179. [Google Scholar] [CrossRef]
- Ali, M.; Sarkar, A.; Pandey, M.D.; Pandey, S. Efficient Precipitation of Dyes from Dilute Aqueous Solutions of Ionic Liquids. Anal. Sci. 2006, 22, 1051–1053. [Google Scholar] [CrossRef] [Green Version]
- Junior, O.M.C.; Barros, M.A.S.D.; Pereira, N.C. Study on coagulation and flocculation for treating effluents of textile industry. Acta Sci. Technol. 2013, 35, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Javaid, R.; Qazi, U.Y. Catalytic Oxidation Process for the Degradation of Synthetic Dyes: An Overview. Int. J. Environ. Res. Public Health 2019, 16, 2066. [Google Scholar] [CrossRef] [Green Version]
- Rane, A.; Joshi, S.J. Biodecolorization and Biodegradation of Dyes: A Review. Open Biotechnol. J. 2021, 15, 97–108. [Google Scholar] [CrossRef]
- Taha, A.; Da’Na, E.; Hessien, M. Evaluation of catalytic and adsorption activity of iron nanoparticles greenly prepared under different conditions: Box–Behnken design. Mol. Simul. 2020, 48, 8–18. [Google Scholar] [CrossRef]
- Da’Na, E.; Taha, A.; Afkar, E. Green Synthesis of Iron Nanoparticles by Acacia nilotica Pods Extract and Its Catalytic, Adsorption, and Antibacterial Activities. Appl. Sci. 2018, 8, 1922. [Google Scholar] [CrossRef] [Green Version]
- Al-Arjan, W.S.; Al-Saeed, S.; Nazir, S.; Da’Na, E. Synthesis of porous chlorophyll coated SiO2/Fe3O4 nanocomposites for the photocatalytic degradation of organic pollutants. React. Kinet. Mech. Catal. 2022, 135, 555–570. [Google Scholar] [CrossRef]
- Da’Na, E.; Taha, A.; Hessien, M. Application of ZnO–NiO greenly synthesized nanocomposite adsorbent on the elimination of organic dye from aqueous solutions: Kinetics and equilibrium. Ceram. Int. 2020, 47, 4531–4542. [Google Scholar] [CrossRef]
- Khalaf, M.M.; Da’Na, E.; Al-Amer, K.; Hessien, M. Experimental Design Modeling of the Effect of Hexagonal Wurtzite—ZnO Synthesis Conditions on Its Characteristics and Performance as a Cationic and Anionic Adsorbent. Molecules 2019, 24, 3884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rovani, S.; Santos, J.J.; Corio, P.; Fungaro, D.A. Highly Pure Silica Nanoparticles with High Adsorption Capacity Obtained from Sugarcane Waste Ash. ACS Omega 2018, 3, 2618–2627. [Google Scholar] [CrossRef] [PubMed]
- Menezes, J.M.C.; Bento, A.M.D.S.; da Silva, J.H.; Filho, F.J.D.P.; da Costa, J.G.M.; Coutinho, H.D.M.; Teixeira, R.N.P. Equilibrium, kinetics and thermodynamics of lead (II) adsorption in bioadsorvent composed by Caryocar coriaceum Wittm barks. Chemosphere 2020, 261, 128144. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Kaware, J. Regeneration and Recovery in Adsorption—A Review. Int. J. Innov. Sci. Eng. Technol. 2014, 1, 61–64. [Google Scholar]
- Huang, R.; Liu, Q.; Huo, J.; Yang, B. Adsorption of methyl orange onto protonated cross-linked chitosan. Arab. J. Chem. 2017, 10, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Umpuch, C.; Sakaew, S. Removal of methyl orange from aqueous solutions by adsorption using chitosan intercalated montmorillonite. Songklanakarin J. Sci. Technol. 2013, 35, 451–459. [Google Scholar]
- El Maguana, Y.; Elhadiri, N.; Benchanaa, M.; Chikri, R. Adsorption Thermodynamic and Kinetic Studies of Methyl Orange onto Sugar Scum Powder as a Low-Cost Inorganic Adsorbent. J. Chem. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Rosanti, A.D.; Kusumawati, Y.; Hidayat, F.; Fadlan, A.; Wardani, A.R.; Anggraeni, H.A. Adsorption of Methylene Blue and Methyl Orange from Aqueous Solution using Orange Peel and CTAB-Modified Orange Peel. J. Turk. Chem. Soc. Sect. A Chem. 2022, 9, 237–246. [Google Scholar] [CrossRef]
- Moghaddasi, F.; Heravi, M.M.; Bozorgmehr, M.R.; Ardalan, P.; Ardalan, T. Kinetic and Thermodynamic Study on the Removal of Methyl Orange From Aqueous Solution by Adsorption onto Camel Thorn Plant. Asian J. Chem. 2010, 22, 5093–5100. [Google Scholar]
- Lu, Y.; Chen, J.; Bai, Y.; Gao, J.; Peng, M. Adsorption Properties of Methyl Orange in Water by Sheep Manure Biochar. Pol. J. Environ. Stud. 2019, 28, 3791–3797. [Google Scholar] [CrossRef]
- Aroke, U.O.; Momoh, R.O.; Hamidu, L.A.J.; Buhari, U. Removal of Azo Dye Methyl Orange in Aqueous Solution by Kaolinite Clay: Equilibrium Isotherms, Kinetics and Error Analyses. Saudi J. Eng. Technol. 2020, 5, 422–433. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Chen, X.D.; Zhuang, J.T.; Zhou, Y.P.; Huang, Y.; Liu, Z.L. Adsorption Removal of Methyl Orange from Aqueous Solution by Mesoporous Al2O3. Adv. Mater. Res. 2012, 554–556, 498–501. [Google Scholar] [CrossRef]
- Li, Y.; Sui, K.; Liu, R.; Zhao, X.; Zhang, Y.; Liang, H.; Xia, Y. Removal of Methyl Orange from Aqueous Solution by Calcium Alginate/Multi-walled Carbon Nanotubes Composite Fibers. Energy Procedia 2012, 16, 863–868. [Google Scholar] [CrossRef] [Green Version]
- Turov, V.V.; Chuiko Institute of Surface Chemistry of National Academy of Sciences of Ukraine; Gun’Ko, V.M.; Krupska, T.V.; Protsak, I.S.; Pakhlov, E.M. Structural and adsorption features of amorphous nanosilica modified by various addition of polymethylsiloxane. Him. Fiz. Teh. Poverhni 2019, 10, 203–218. [Google Scholar] [CrossRef] [Green Version]
- Gizli, N.; Arabacı, M. Improvement of the Sorption Performance of Nanosilica and Polymeric Solid Supports by Impregnation with Ionic Liquid for the Removal of Cr (VI) Ions from Aqueous Solutions. J. Turk. Chem. Soc. Sect. B Chem. Eng. 2017, 1, 49–70. [Google Scholar]
- Zhang, G.; Zhou, Y.; Ding, Z.; Fu, L.; Wang, S. Nanosilica-supported thiosemicarbazide–glutaraldehyde polymer for selective Au(iii) removal from aqueous solution. RSC Adv. 2017, 7, 55215–55223. [Google Scholar] [CrossRef] [Green Version]
- Rita, S.; Eti, R.; Tetty, K. Aminopropyltrimethoxysilane (APTMS) modified nano silica as heavy metal iron (Fe) adsorbents in peat water. AIP Conf. Proc. 2018, 2014, 020163. [Google Scholar] [CrossRef]
- Duan, Y.; Song, Y.; Zhou, L. Facile synthesis of polyamidoamine dendrimer gel with multiple amine groups as a super adsorbent for highly efficient and selective removal of anionic dyes. J. Colloid Interface Sci. 2019, 546, 351–360. [Google Scholar] [CrossRef]
- Song, Y.; Tan, J.; Wang, G.; Zhou, L. Superior amine-rich gel adsorbent from peach gum polysaccharide for highly efficient removal of anionic dyes. Carbohydr. Polym. 2018, 199, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Da’Na, E.; Al-Arjan, W.S.; Al-Saeed, S.; El-Aassar, M.R. One-Pot Synthesis of Amine-Functionalized Nano-Silica via Sol-Gel Assisted by Reverse Micelle Microemulsion for Environmental Application. Nanomaterials 2022, 12, 947. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Plaza, P.; Navas, M.J.; Wybraniec, S.; Michałowski, T.; Asuero, A.G. An Overview of the Kjeldahl Method of Nitrogen Determination. Part II. Sample Preparation, Working Scale, Instrumental Finish, and Quality Control. Crit. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
- Pham, T.; Bui, T.T.; Nguyen, V.T.; Van Bui, T.K.; Tran, T.T.; Phan, Q.C.; Hoang, T.H. Adsorption of Polyelectrolyte onto Nanosilica Synthesized from Rice Husk: Characteristics, Mechanisms, and Application for Antibiotic Removal. Polymers 2018, 10, 220. [Google Scholar] [CrossRef] [Green Version]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Nawaz, T.; Zulfiqar, S.; Sarwar, M.I.; Iqbal, M. Synthesis of diglycolic acid functionalized core-shell silica coated Fe3O4 nanomaterials for magnetic extraction of Pb(II) and Cr(VI) ions. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- He, X.; Mahtabani, A.; Rytoluoto, I.; Saarimaki, E.; Lahti, K.; Paajanen, M.; Anyszka, R.; Dierkes, W.; Blume, A. Surface Modification of Fumed Silica by Dry Silanization for PP-based Dielectric Nanocomposites. In Proceedings of the ICEMPE 2019—2nd International Conference on Electrical Materials and Power Equipment, Guangzhou, China, 7–10 April 2019; pp. 254–259. [Google Scholar] [CrossRef] [Green Version]
- Kesmez, Ö.; Kiraz, N.; Burunkaya, E.; Çamurlu, H.E.; Asiltürk, M.; Arpaç, E. Effect of amine catalysts on preparation of nanometric SiO2 particles and antireflective films via sol-gel method. J. Sol-Gel Sci. Technol. 2010, 56, 167–176. [Google Scholar] [CrossRef]
- Erdem, A.; Shahwan, T.; Çağır, A.; Eroğlu, A.E. Synthesis of aminopropyl triethoxysilane-functionalized silica and its application in speciation studies of vanadium(IV) and vanadium(V). Chem. Eng. J. 2011, 174, 76–85. [Google Scholar] [CrossRef]
- Da Silva, R.J.; Mojica-Sánchez, L.C.; Gorza, F.D.; Pedro, G.C.; Maciel, B.G.; Ratkovski, G.P.; da Rocha, H.D.; Nascimento, K.T.D.; Medina-Llamas, J.C.; Chávez-Guajardo, A.E.; et al. Kinetics and thermodynamic studies of Methyl Orange removal by polyvinylidene fluoride-PEDOT mats. J. Environ. Sci. 2020, 100, 62–73. [Google Scholar] [CrossRef]
- Tsai, F.-C.; Ma, N.; Chiang, T.; Tsai, L.-C.; Shi, J.-J.; Xia, Y.; Jiang, T.; Su, S.-K.; Chuang, F.-S. Adsorptive removal of methyl orange from aqueous solution with crosslinking chitosan microspheres. J. Water Process Eng. 2014, 1, 2–7. [Google Scholar] [CrossRef]
- Lacuesta, A.C.; Herrera, M.U.; Manalo, R.; Balela, M.D.L. Fabrication of kapok paper-zinc oxide-polyaniline hybrid nanocomposite for methyl orange removal. Surf. Coat. Technol. 2018, 350, 971–976. [Google Scholar] [CrossRef]
- Asuha, S.; Gao, Y.W.; Deligeer, W.; Yu, M.; Suyala, B.; Zhao, S. Adsorptive removal of methyl orange using mesoporous maghemite. J. Porous Mater. 2010, 18, 581–587. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, R.; Ma, D.; Zhou, B.; Zhu, L.; Yang, J. Removal of Methyl Orange from Aqueous Solution by Adsorption onto a Hydrogel Composite. Polym. Polym. Compos. 2018, 26, 161–168. [Google Scholar] [CrossRef]
- Wu, S.-C.; Yu, L.-L.; Xiao, F.-F.; You, X.; Yang, C.; Cheng, J.-H. Synthesis of aluminum-based MOF/graphite oxide composite and enhanced removal of methyl orange. J. Alloys Compd. 2017, 724, 625–632. [Google Scholar] [CrossRef]
- Azam, K.; Raza, R.; Shezad, N.; Shabir, M.; Yang, W.; Ahmad, N.; Shafiq, I.; Akhter, P.; Razzaq, A.; Hussain, M. Development of recoverable magnetic mesoporous carbon adsorbent for removal of methyl blue and methyl orange from wastewater. J. Environ. Chem. Eng. 2020, 8, 104220. [Google Scholar] [CrossRef]
- Sarvari, H.; Goharshadi, E.K.; Samiee, S.; Ashraf, N. Removal of Methyl Orange from Aqueous Solutions by Ferromagnetic Fe/Ni Nanoparticles. Phys. Chem. Res. 2018, 6, 433–444. [Google Scholar] [CrossRef]
- Zhuang, M.; Zheng, Y.; Liu, Z.; Huang, W.; Hu, X. Shape-dependent performance of TiO2 nanocrystals as adsorbents for methyl orange removal. RSC Adv. 2015, 5, 13200–13207. [Google Scholar] [CrossRef]














| Temperature (K) | Langmuir parameters | ||
| KL (L·mg−1) | qm (mg·g−1) | R2 | |
| 293 | 0.577 | 5.40 | 0.9848 |
| 313 | - | - | - |
| 333 | - | - | - |
| Mass (mg) | Pseudo-second-order parameters | ||
| Kp2 (g·mg−1·min−1) | qe (mg·g−1) | R2 | |
| 10 | 0.048 | 12.987 | 0.9998 |
| 20 | 0.041 | 6.716 | 0.9982 |
| 30 | 0.035 | 4.801 | 0.9998 |
| 40 | 0.078 | 2.927 | 0.9992 |
| 50 | 0.210 | 2.132 | 0.9999 |
| Adsorbent | Mass/Volume (g/L) | MO Concentration (mgL−1) | Removal Efficiency (%) | Adsorption Capacity (mg/g) | Notes | Ref. |
|---|---|---|---|---|---|---|
| Chitosan intercalated montmorillonite | 1/1 | 200 | 100 | pH = 2 T = 45 °C | [18] | |
| Protonated cross-linked chitosan | 100 | 100 | pH = 4.5 T = 40 °C | [17] | ||
| Sheep Manure Biochar | 4/5 | 20 | 100 | 50 | pH = 4 T = 25 °C | [22] |
| Kaolinite Clay | 50/1 | 200 | 70 | 3.5 | pH = 4 T = 25 °C | [23] |
| Camel Thorn Plant | 1/2 | 20 | 80 | 21 | pH = 4 T = 20 °C | [21] |
| Biochar | 1/2 | 50 | 137 | T = 25 °C pH = 2 | [3] | |
| CTAB-Modified Orange Peel | 4/5 | 50 | 89 | 14 | T = 25 °C | [20] |
| Chitosan | 2/1 | 33 | 10 | pH = 4 T = 33 °C | [1] | |
| Polyvinylidene fluoride-PEDOT mats | 300 | 293 | pH = 3 T = 50 °C | [40] | ||
| Crosslinking chitosan microspheres | 4/10 | 30 | 91 | 50 | T = 25 °C | [41] |
| Kapok paper-zinc oxide-polyaniline hybrid nanocomposite | 2/5 | 25 | 50 | 97 | T = 25 °C | [42] |
| Mesoporous maghemite | 5/2 | 50 | 93 | 385 | pH = 3 T = 20 °C | [43] |
| Hydrogel Composite | 1/1 | 1000 | 500 | pH = 7 T = 15 °C | [44] | |
| Aluminum-based MOF/graphite oxide composite | 0.2/1 | 40 | 399 | T = 25 °C pH = 8 | [45] | |
| Magnetic mesoporous carbon | 2/1 | 30 | 98.5 | pH = 7 T = 25 °C | [46] | |
| Ferromagnetic Fe/Ni Nanoparticles | 1.5/1 | 50 | 35 | 99.5 | pH = 1 T = 25 °C | [47] |
| TiO2 nanocrystals | 1/50 | 16 | 95 | 303 | pH = 3 T = 25 °C | [48] |
| ZnO–NiO nanocomposite | 2/1 | 6 | 3 | 100 | pH = 4 T = 20 °C | [13] |
| Amine-modified nano-silica | 10/3 | 10 | 5.4 | 100 | pH = 3.5 T = 20 °C | Current work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da’na, E. Nano-Silica Modified with Diamine for Capturing Azo Dye from Aqueous Solutions. Molecules 2022, 27, 3366. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27113366
Da’na E. Nano-Silica Modified with Diamine for Capturing Azo Dye from Aqueous Solutions. Molecules. 2022; 27(11):3366. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27113366
Chicago/Turabian StyleDa’na, Enshirah. 2022. "Nano-Silica Modified with Diamine for Capturing Azo Dye from Aqueous Solutions" Molecules 27, no. 11: 3366. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27113366