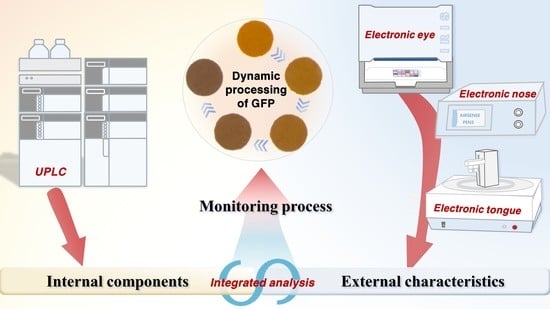
A Novel Method for Quality Evaluation of Gardeniae fructus Praeparatus during Heat Processing Based on Sensory Characteristics and Chemical Compositions
Abstract
:1. Introduction
2. Results
2.1. Determination of Content in Five GFP Processed Decoction Pieces
2.2. Color Analysis of Five GFP Processed Decoction Pieces
2.3. Olfactory Analysis of Five GFP Processed Decoction Pieces
2.4. Gustatory Analysis of Five GFP Processed Decoction Pieces
2.5. Integration Analysis of External Characteristics and Internal Components
2.5.1. Correlation Analysis
2.5.2. Cluster Heatmap Analysis
2.5.3. Artificial Neural Network Analysis
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Preparation of Samples and Reference Substance
4.3. UPLC Analysis
4.4. Electronic Eye Analysis
4.5. Electronic Nose Analysis
4.6. Electronic Tongue Analysis
4.7. Multi-Source Data Integration
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhao, Z.; Liang, Z.; Chan, K.; Lu, G.; Lai Mei Lee, E.; Chen, H.; Li, L. A unique issue in the standardization of Chinese materia medica: Processing. Planta Med. 2010, 76, 1975–1986. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Cui, Q.; Wu, H.; Qiao, Y.; Zheng, Y.; Wu, Z. New sensor technologies in quality evaluation of Chinese materia medica: 2010–2015. Acta Pharm. Sin. B 2017, 7, 137–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wei, X.; Wan, G.; Yang, X.; Shi, J. Historical evolution and modern research progress of quality evaluation based on character identification of traditional Chinese medicinal materials. Chin. J. Exp. Tradit. Med. Formulae 2021, 27, 189–196. [Google Scholar] [CrossRef]
- Chen, Q.; Shi, Z.; Wang, Y.; Zhang, R.; Hu, H. Study on effects of sweating treatment and different drying methods on the quality of Gentianae macrophyllae Radix. Sci. Rep. 2021, 11, 9717. [Google Scholar] [CrossRef]
- Fei, C.; Ren, C.; Wang, Y.; Li, L.; Li, W.; Yin, F.; Lu, T.; Yin, W. Identification of the raw and processed Crataegi Fructus based on the electronic nose coupled with chemometric methods. Sci. Rep. 2021, 11, 1849. [Google Scholar] [CrossRef]
- Yang, L.; Gong, Y.; Xu, M.; Yang, L.; Chen, J.; Dong, L. Research on relationship between color and composition changes in processing of rhubarb charcoal based on “external and internal correlation”. Chin. Tradit. Herb. Drugs 2020, 51, 5705–5713. [Google Scholar] [CrossRef]
- Liang, Z.; Huang, G.; Cui, T.; Zhang, Z.; Sun, D. Analysis on the correlation of index composition and powder color during microwave processing of Cibotium baronetz. Chin. Tradit. Patent Med. 2020, 42, 1548–1552. [Google Scholar] [CrossRef]
- Xiong, Y.; Xiao, X.; Yang, X.; Yan, D.; Zhang, C.; Zou, H.; Lin, H.; Peng, L.; Xiao, X.; Yan, Y. Quality control of Lonicera japonica stored for different months by electronic nose. J. Pharmaceut. Biomed. 2014, 91, 68–72. [Google Scholar] [CrossRef]
- Eckert, C.; Lutz, C.; Breitkreutz, J.; Woertz, K. Quality control of oral herbal products by an electronic tongue—Case study on sage lozenges. Sens. Actuat. B-Chem. 2011, 156, 204–212. [Google Scholar] [CrossRef]
- Li, J.; Shao, Y.; Yao, Y.; Yu, Y.; Cao, G.; Zou, H.; Yan, Y. A novel quality evaluation method for magnolia bark using electronic nose and colorimeter data with multiple statistical algorithms. J. Tradit. Chin. Med. Sci. 2020, 7, 221–227. [Google Scholar] [CrossRef]
- Xie, D.; Peng, W.; Chen, J.; Li, L.; Zhao, C.-B.; Yang, S.; Xu, M.; Wu, C.; Ai, L. A novel method for the discrimination of Hawthorn and its processed products using an intelligent sensory system and artificial neural networks. Food Sci. Biotechnol. 2016, 25, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, H.; Xue, X.; Liu, X.; Tian, F.; Poh, Y.; Cai, H.; Lee, Y.H.; Yu, H.; Ong, S.P.; et al. A metabolomics approach to study the dual modulation by characterization of chemical alteration during processing of Gardeniae Fructus using UPLC-ESI-QTOF. Anal. Methods 2016, 8, 3629–3635. [Google Scholar] [CrossRef]
- Liu, L.; Gan, L.; Zeng, Z.; Li, G.; Li, Y. Optimization of processing technology and establishment of quality standard of Gardenia jasminoides. J. Chin. Med. Mater. 2019, 42, 1527–1532. [Google Scholar] [CrossRef]
- Xia, M.; Wang, Y.; Zheng, Y.; Song, Y.; Liu, T.; Zhang, C. Comparising the quality changes of different fried forms of Gardeniae Fructus Praeparatus during processing based on color-component correlation analysis. China J. Chin. Mater. Med. 2021, 46, 10. [Google Scholar] [CrossRef]
- Xu, M.; Yang, S.; Peng, W.; Liu, Y.; Xie, D.; Li, X.; Wu, C. A novel method for the discrimination of Semen Arecae and its processed products by using computer vision, electronic nose, and electronic tongue. Evid.-Based Complentary Altern. Med. 2015, 2015, 753942. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, D.; Deng, X.; Zhao, B.; Xue, X.; Wang, S.; Zhang, Y.; Meng, J. Quality assessment of crude and processed Leonuri Fructus by chemical and color analysis combined with chemometric method. Chin. Herb. Med. 2018, 10, 388–395. [Google Scholar] [CrossRef]
- Fu, X.; Yang, C.; Wu, Z.; Liu, J.; Wu, J.; Pei, J.; Huang, X.; Wang, F. Correlation between color and content of eight components of Gardeniae Fructus at different harvest time. China J. Chin. Mater. Med. 2020, 45, 3191–3202. [Google Scholar] [CrossRef]
- Huang, Y.; Li, C.; Hu, H.; Wang, Y.; Shen, M.; Nie, S.; Chen, J.; Zeng, M.; Xie, M. Simultaneous determination of acrylamide and 5-hydroxymethylfurfural in heat-processed foods employing enhanced matrix removal-lipid as a new dispersive solid-phase extraction sorbent followed by liquid chromatography-tandem mass spectrometry. J. Agr. Food Chem. 2019, 67, 5017–5025. [Google Scholar] [CrossRef]
- Woo, K.S.; Hwang, I.G.; Kim, H.Y.; Jang, K.I.; Lee, J.; Kang, T.S.; Jeong, H.S. Thermal degradation characteristics and antioxidant activity of fructose solution with heating temperature and time. J. Med. Food. 2011, 14, 167. [Google Scholar] [CrossRef]
- Hsieh, H.J.; Lin, J.A.; Chen, K.T.; Cheng, K.C.; Hsieh, C.W. Thermal treatment enhances the α-glucosidase inhibitory activity of bitter melon (Momordica charantia) by increasing the free form of phenolic compounds and the contents of Maillard reaction products. J. Food Sci. 2021, 86, 3109–3121. [Google Scholar] [CrossRef]
- Guan, D.; Fang, Z.; Pan, Z.; Lu, W.; Liu, X.; Liang, C.; Wu, Z.; Gou, W. Establishing a standard verification method for Five Flavors of Chinese medicine. Chin. J. Basic Med. Tradit. Chin. Med. 2011, 17, 1333–1335. [Google Scholar]
- Li, H.; Zhang, W.; Tang, X.; Wu, C.; Yu, S.; Zhao, Z. Identification of bitter-taste compounds in class-III caramel colours. Flavour Fragr. J. 2021, 36, 404–411. [Google Scholar] [CrossRef]
- Summerson, V.; Gonzalez Viejo, C.; Szeto, C.; Wilkinson, K.L.; Torrico, D.D.; Pang, A.; De Bei, R.; Fuentes, S. Classification of smoke contaminated Cabernet Sauvignon berries and leaves based on chemical fingerprinting and machine learning algorithms. Sensors 2020, 20, 5099. [Google Scholar] [CrossRef] [PubMed]
- Commission, C.P. Pharmacopeia of the People’s Republic of China, Volume 1; China Medical Science and Technology Press: Beijing, China, 2020; p. 260. [Google Scholar]
- Jiang, H.; Huang, Y.; You, Z. SAEROF: An ensemble approach for large-scale drug-disease association prediction by incorporating rotation forest and sparse autoencoder deep neural network. Sci. Rep. 2020, 10, 4972. [Google Scholar] [CrossRef]
- Yeo, M.T.Y.; Yeo, P.L.Q.; Bi, X.; Henry, C.J. Energy density and nutrient contents of selective Chinese new year snacks. Foods 2020, 9, 1137. [Google Scholar] [CrossRef]
- Lee, H.H.; Lee, A.J.; Park, W.S.; Lee, J.; Park, J.; Park, B.; Joung, J.Y.; Lee, K.H.; Hong, D.; Kim, S.H. Epithelial splicing regulatory protein (ESPR1) expression in an unfavorable prognostic factor in prostate cancer patients. Front. Oncol. 2020, 10, 556650. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Wang, Y.; Zhang, Q.; Liu, W.; Li, K.; Xia, M.; Jia, Z.; Zhang, C. A Novel Method for Quality Evaluation of Gardeniae fructus Praeparatus during Heat Processing Based on Sensory Characteristics and Chemical Compositions. Molecules 2022, 27, 3369. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27113369
Zheng Y, Wang Y, Zhang Q, Liu W, Li K, Xia M, Jia Z, Zhang C. A Novel Method for Quality Evaluation of Gardeniae fructus Praeparatus during Heat Processing Based on Sensory Characteristics and Chemical Compositions. Molecules. 2022; 27(11):3369. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27113369
Chicago/Turabian StyleZheng, Yinghao, Yun Wang, Qing Zhang, Weihong Liu, Kai Li, Mengyu Xia, Zhe Jia, and Cun Zhang. 2022. "A Novel Method for Quality Evaluation of Gardeniae fructus Praeparatus during Heat Processing Based on Sensory Characteristics and Chemical Compositions" Molecules 27, no. 11: 3369. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27113369