1. Introduction
Rare earth elements (REEs) are most often used in nuclear technology, ferrous and non-ferrous metallurgy, electronics and radio engineering, the chemical and silicate industry, medicine, and agriculture. The REE group contains 17 elements, including scandium (Sc), yttrium (Y), lanthanum (La), and the lanthanides (Ln): cerium (Ce), praseodymium (Pr), neodymium (Nd), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tu), ytterbium (Yb), and lutetium (Lu).
In medicine, REEs are applied in the fabrication process of medication for the clinical management of various tumors, tuberculosis, leprosy, eczema, gout, rheumatism, and gastric diseases. REEs help against marine disease and chronic vomiting syndrome (e.g., cerium salts) and are also components of embalming compositions. Organic salts of REEs have antiseptic effects (e.g., salicylates of neodymium and praseodymium) and are also used as anticoagulants. Gadolinium chloride is used to block Kupffer cells in the treatment of the liver. Some REE salts prevent blood clotting, making them valuable for blood preservation. Erbium and cerium salts increase the content of hemoglobin and red blood cells. In medical equipment, dysprosium is applied in medical lasers. Cerium and scandium are used for the manufacture of dentures [
1].
In nuclear and radiation medicine, isotopes 177Lu, 46Sc are used for the treatment of cancer tumors, isotope 153Gd for diagnosis of osteoporosis, and isotope 170Tm for the manufacture of portable X-ray medical installations.
The use of REE compounds in agriculture as microfertilizers and insectofungicides is increasing. REEs can also increase the yield of crops (e.g., rice, wheat, corn, sugarcane, sugar beets, tobacco, tea, cotton, peanuts, fruits, flowers, and others) [
1].
It is necessary to develop new, or to modernize current, schemes and processes, particularly new efficient and safer solvent extraction (SE) systems for the separation of multicomponent solutions, to improve reprocessing technology for mineral and technogenic REE-containing deposits. SE separation of REEs from nitrate or chloride aqueous solutions is widely used in global practice to produce pure individual metals and compounds [
2]. As initial raw materials, collective REE concentrates obtained from various ores are used. Usually, the separation of collective concentrates is carried out into three groups, such as light (La, Ce, Pr, Nd), medium (Sm, Eu, Gd), and heavy (Tb, Dy, Ho, Er, Tu, Yb, Lu), followed by the recovery of individual REEs. Generally, all SE separation processes operate using selective solvents of various classes or their synergic mixtures [
3].
Tri-n-butyl phosphate (TBP) is widely used in practical applications to separate light REEs from middle and heavy REEs and to separate these into individual elements. Organophosphorus and carbonic acids, such as di-(2-ethylhexyl)phosphoric acid (DEHPA or HDEHP), isododecylphosphetanic acid (IDPA), di-(2,2,4-trimethylpenthyl)phosphinic acid (Cyanex 272), 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester (PC-88A), and α-branched tertiary carboxylic acids (C
8–C
16 higher isomeric acids and neodecanoic—Versatic 10 acid) became widely used in industrial practice for separation of middle and heavy REEs [
3]. For yttrium recovery from heavy REE concentrates, the use of tertiary and quaternary ammonium compounds (QACs), in particular, tri-n-alkylammonium nitrate (TAA), methyltri-n-alkyl(octyl)ammonium nitrate (TAMA(TOMA)NO
3) and analogues including Aliquat 336, N
263(methyltri-n-octylammonium chloride), Adogen 464 (methyltrialkyl(C
8–C
10)ammonium chloride) [
4] has been proposed.
Among various solvent mixtures, including synergic ones, solutions of TAMANO
3-TBP mixtures in hydrocarbon diluents for separation of middle REEs, including the pair Sm(III)/Gd(III), following chemical recovery of europium from nitrate solutions, became widely used in the industry [
5]. TAMANO
3 (TOMANO
3)-TBP mixtures have a significant synergistic effect during SE of various REEs from nitrate solutions which increases both the distribution ratios of individual elements (D
Ln), and the separation factors of REE pairs (β
Ln1/Ln2).
The synergistic properties of TAMANO
3 (TOMANO
3)-TBP mixtures are due to the formation of mixed complex compounds in the organic phase between the extracted metal and two solvents. The composition of the extracted REE compounds in systems with TAMANO
3 (TOMANO
3)-TBP differs for different individual elements and depends on the Ln(III)-TAMANO
3-TBP ratios, the concentration of lanthanide, and each of the solvents of the mixture. In early work on the chemistry of extraction of lanthanides of the light group, as a rule, methods were used to determine the composition of the extracted compound allowing identification of only one of the extracted compounds, due to the limitations of the techniques used.
Table 1 presents generalized data on the compositions of the extracted compounds. These data do not reflect the entire range of solvent extraction chemistry due to changes in the conditions of its implementation and, particularly, changes in the molar ratios of Ln(III)-TAMANO
3-TBP.
S.I. Stepanov et al. applied an approach that allows definition of the variety of extractable compounds in a wide range of a mixture’s molar ratios of lanthanide and solvents [
15,
16,
17]. In studies [
16,
17], the SE chemistry of La(III), Ce(III), Pr(III), and Nd(III) using TOMANO
3-TBP mixtures was investigated applying a wide range of changes in their molar ratios in toluene solutions using the isomolar series method. By studying the SE of these four REEs using TOMANO
3-TBP mixtures, the manifestation of a synergistic effect (S
Ln) was established, and the areas of synergistic SE and the compositions of extractable mixed complexes in maxima on the curves S
Ln =
f(composition of the mixture) were determined.
The SE of La(III) from a solution containing 0.88 mol/L La(NO
3)
3, 4.0 mol/L NH
4NO
3, and 0.01 mol/L HNO
3 using 1.0 mol/L isomolar mixtures of TOMANO
3-TBP in toluene was accompanied by the manifestation on the synergetic curve of two maxima corresponding to the formation of the complexes (R
4N)[La(NO
3)
4·(2–3)TBP] and (R
4N)
2[La(NO
3)
5·2TBP] for synergetic compositions 0.15 mol/L TOMANO
3-0.85 mol/L TBP and 0.9 mol/L TOMANO
3-0.1 mol/L TBP [
16].
The SE of Ce(III), using 1.0 mol/L TOMANO
3-TBP mixtures in toluene from solutions containing 1.0 mol/L Ce(NO
3)
3, 4.0 mol/L NH
4NO
3, and 0.01 mol/L HNO
3, was accompanied by the consecutive formation of complexes with the following compositions: R
4N[Ce(NO
3)
4·4TBP], (R
4N)
2[Ce(NO
3)
5·3TBP], (R
4N)
2[Ce(NO
3)
5·2TBP], (R
4N)
3[Ce(NO
3)
6·TBP], and (R
4N)
3[Ce(NO
3)
6]. The synergic SE domain ranged from the composition 0.2 mol/L TOMANO
3-0.8 mol/L TBP to 0.7 mol/L TOMANO
3-0.3 mol/L TBP [
16].
For Pr(III), during SE from a solution containing 0.487 mol/L Pr(NO
3)
3, 4.0 mol/L NH
4NO
3, 0.01 mol/L HNO
3, 1.0 mol/L using TOMANO
3-TBP mixtures in toluene, the synergic curve featured four maxima in TOMANO
3 concentration changes ranging from 0.2 mol/L to 0.65 mol/L. These maxima are consistent with sequential formation (with growing TOMANO
3 concentration) of the Pr(III) mixed complexes, R
4N[Pr(NO
3)
4·4TBP], R
4N[Pr(NO
3)
4·3TBP], R
4N[Pr(NO
3)
4·2TBP], (R
4N)
2[Pr(NO
3)
5·3TBP], (R
4N)
2[Pr(NO
3)
5·2TBP] and (R
4N)
3[Pr(NO
3)
6·2TBP], which confirms the mechanism of substitution of the phosphoryl ligand of TBP molecules by NO
3 ligands of TOMANO
3 [
17].
During SE of Nd(III), from a solution containing 0.429 mol/L Nd(NO
3)
3, 4.0 mol/L NH
4NO
3, 0.01 mol/L HNO
3 using 1.0 mol/L isomolar TOMANO
3-TBP mixtures in toluene, the synergic curve fully fitted in the area S
Nd(III) > 1 which indicates the occurrence of synergic SE for all mixture compositions studied. Four maxima, ranging from 0.2 mol/L TOMANO
3-0.8 mol/L TBP to 0.85 mol/L TOMANO
3-0.15 mol/L TBP corresponded to the sequential formation of the following compounds: R
4N[Nd(NO
3)
4·4TBP], (R
4N)
2[Nd(NO
3)
5·3TBP], (R
4N)
3[Nd(NO
3)
6·2TBP], and (R
4N)
4[Nd(NO
3)
7·TBP]. The latter complex can be considered a three-charge mixed complex (R
4N)
3[Nd(NO
3)
6·TBP]·R
4NNO
3, additionally solvated with excess solvents molecules, both TOMANO
3 and TBP. At high TOMANO
3 concentrations, the formation and extraction of a four-charge nitrate complex were accompanied by the displacement of TBP molecules from their coordination sphere. The manifestation of a synergistic effect for these compositions of mixtures can be due to the solvation of the three-charge complex (R
4N)
3[Nd(NO
3)
6] by free TOMANO
3 and TBP molecules with the formation of the solvate (R
4N)
3[Nd(NO
3)
6]·R
4NNO
3·pTBP, and by solvation by TBP molecules of a four-charge nitrate complex with the formation of the solvate (R
4N)
4[Nd(NO
3)
7]·pTBP [
17].
As implied by the material considered, the main motive for changing the composition of Nd(III) extractable mixed complexes with TOMANO3 and TBP is the displacement of phosphoryl ligands from the coordination sphere by nitrate ligands with an increase in the proportion of TOMANO3 in the mixture with the formation of more highly charged nitrate complexes in the organic phase. Conversely, with an increase in the concentration of TBP in the mixture, the main motive is to replace the nitrate ligands of TOMANO3 with phosphoryl ligands of TBP with a decrease in charge of the nitrate anionic complexes of Nd(III).
According to most of the research cited above on the extraction of REE nitrates by mixtures of quaternary ammonium nitrates and neutral organophosphorus compounds (NOPC) [
16,
17,
18,
19,
20,
21,
22,
23], the general formula of the resulting compounds can be written as follows: (R
4N)
n[Ln(NO
3)
3+n·mS], where S is the NOPC molecule,
n = 1–3,
m = 1–4. Various studies have shown that the nitrate groups of TOMANO
3 and phosphoryl groups of NOPC are included in the internal coordination sphere of the mixed complex. The main postulated mechanism of formation of such mixed complexes is the substitution of NOPC molecules in Ln(NO
3)
3·3S trisolvates with R
4NNO
3 nitrate ligands [
8,
15,
16,
18]. The main differences in the compositions of the extractable mixed REE compounds determined in the various studies are in the number of nitrate and phosphoryl groups that are simultaneously part of the complex. As shown above, these discrepancies are primarily due to different concentrations of lanthanide, nitrate QAC, and NOPC when determining the extracted complex.
Another important aspect of REE solvent extraction by mixtures of nitrates, QACs and NOPCs, including TOMANO
3-TBP, is the possibility of achieving high separation properties against the background of a significant synergistic effect, which leads to an increase in the separation factors of the neighboring REEs. For the REEs of the middle group, TAMANO
3-TBP mixtures proved to be optimal for separating Sm(III) and Gd(III) with β
Sm(III)/Gd(III) ~3–4 [
14,
19]. This has made it possible to use solutions of TAMANO
3-TBP mixtures in hydrocarbon diluents to obtain pure Sm
2O
3 and Gd
2O
3 oxides from concentrates containing samarium, gadolinium, and small quantities of europium on an industrial scale [
4,
24]. Good results on the SE separation of REEs of the middle group with Aliquat 336 and TBP mixtures using cascades of centrifugal extractors have been achieved in recent years by S.S. Shulin et al. [
25,
26]. However, the separating properties of the extractant mixtures described above cannot be limited to only the elements of the middle group. The occurrence of a synergistic effect and a wide variety of extracted REE compounds of the light group described above, based on studies [
15,
16,
17], allow us to consider these mixtures as having promise for the separation of La(III), Ce(III), Pr(III), and Nd(III).
The purpose of this research was to determine the possibility of using TOMANO3-TBP mixtures for the solvent extraction separation of REEs of the light group from nitrate low-acid solutions to obtain individual compounds of La(III), Ce(III), Pr(III), and Nd(III).
2. Results and Discussion
2.1. Solvent Extraction Separation of La(III), Ce(III), Pr(III), and Nd(III) Using TOMANO3-TBP Mixtures
Experimental data obtained previously on SE of La(III), Ce(III), Pr(III), and Nd(III) from individual weakly acidic nitrate solutions with a salting-out agent was used to evaluate separation factors of REEs depending on the composition of TOMANO
3-TBP mixtures [
16,
17]. For the La(III)/Ce(III) pair, La(III) was extracted more effectively into the organic phase, with values β
La(III)/Ce(III) ~1.2–1.4 practically throughout the solvent composition change area. For the Ce(III)/Pr(III) pair, Pr(III) was also extracted more effectively into the organic phase, with β
Pr(III)/Ce(III) ~1.5–2.3 throughout the solvent composition change area. For the La(III)/Pr(III) pair, extraction should proceed in favor of Pr(III) with β
La(III)/Pr(III) ~1.1–1.6 throughout the solvent composition change area. For the Pr(III)/Nd(III) pair, Nd(III) is more effectively extracted, the β
Nd(III)/Pr(III) value was 1.3–1.5. Such data estimates enable prediction of the possibility of effective SE separation of light REEs with TOMANO
3-TBP mixtures for all the pairs if the solvent composition is correctly selected. These mixtures can be used for REE extraction from weakly acidic nitrate media containing not more than 0.2 mol/L HNO
3, as opposed to TBP, and other NPOEs, extraction of REEs from solutions containing 1–4 mol/L HNO
3.
When calculated for single-element solutions, β
Ln1/Ln2 values significantly change on transition to a mixture of light REEs showing that they depend on the REE concentration ratio in the aqueous phase. To determine the composition of TOMANO
3-TBP mixtures with the most significant differences in distribution ratios, the dependency D
Ln during SE with 1.0 mol/L isomolar TOMANO
3-TBP mixtures in toluene from mixed nitrate solution containing 219 g Ce(III)/L, 106 g La(III)/L, 20 g Pr(III)/L, 55 g Nd(III)/L, and 0.1 mol/L HNO
3 was studied. This aqueous solution composition was chosen as a model solution that forms after the dissolution of a light REE concentrate separated from a loparite concentrate [
3].
Table 2 presents the dependencies of separation factors using 1.0 mol/L isomolar TOMANO
3-TBP mixtures in toluene.
For the La(III)/Ce(III) pair, the βLa(III)/Ce(III) value was >1 (but did not exceed 1.25) in the range of compositions 0.05–0.20 mol/L TOMANO3-0.95–0.80 mol/L TBP. In the range of 0.75–0.95 mol/L TOMANO3 and 0.25–0.05 mol/L TBP concentrations, inversion in the distribution of La(III) and Ce(III) was observed, and βCe(III)/La(III) values of 2.5–4 were achieved. This range may be used for separation of Ce(III)/La(III) in the case of distribution of Ce(III) into the organic phase.
Separation for Pr(III)/Nd(III) can be performed over a wide composition range— from 0.15 mol/L TOMANO3-0.85 mol/L TBP to 0.85 mol/L TOMANO3-0.15 mol/L TBP. The value of βPr(III)/Nd(III) in this range changed from 2.2 to 4.9, indicating high separation efficacy. For separation of the pair Ce(III)/Pr(III), the composition ranges of 0.15–0.30 mol/L TOMANO3-0.85–0.70 mol/L TBP can be selected. In this case, La(III) and Ce(III) will go to the organic phase, while Pr(III) and Nd(III) will stay in the raffinate.
To evaluate the possibility of separation of light REEs including Ce(III)/Pr(III), theoretical calculations were performed for a multistage counter-current SE cascade with scrubbing, according to the methodology suggested by G.V. Korpusov [
27]. In cases where the initial solution of REEs with the composition described above is direct to the middle section of the SE cascade (last step of loading), and used a solvent with composition 0.15 mol/L TOMANO
3-0.85 mol/L TBP in toluene, the number of steps of extraction (loading) of the SE cascade would be ten at an organic to aqueous phase volume ratio (O/A) equal to 18/1, the scrubbing section would have 7–8 steps at O/A equal to 10/1, with the total number of steps equal to 17–18. High O/A ratios are related primarily to low REE distribution ratios that do not exceed 0.1. Such O/A ratio values are not acceptable for industrial SE techniques.
An increase of DLn in the SE systems considered above can occur through an increase in initial solvent concentration or a change in aqueous phase composition or a rise in total REE concentration, or the introduction of a salting-out agent, for example, NH4NO3 or other suitable nitrates of an alkali or alkaline-earth metal.
In the case of a concentration increase of the mixture TOMANO3/TBP with molar ratio 0.15/0.85 from 1.0 mol/L to 3.3 mol/L in dodecane, the DLn value increased by an order of magnitude (DCe(III) = 0.534, DPr(III) = 0.324), while βCe(III)/Pr(III) fell to 1.65. Theoretical calculation of a multistage counter-current SE cascade with scrubbing for all newly obtained values of DCe(III), DPr(III), and βCe(III)/Pr(III) allowed the determination that the number of steps in the extraction section should be 32 at O/A equal to 2/1, in the scrubbing section, 24 steps at O/A equal to 4/1, while the total number of cascade steps should be equal to 56.
The addition of a salting-out agent (1.0–2.0 mol/L NH4NO3) promoted increase in DCe(III) to 1.1, DPr(III) to 0.34, and βCe(III)/Pr(III) to 3.3. In this case, the multistage counter-current SE cascade parameters will be as follows: total steps—24, including 12 steps for the loading section at O/A equal to 1.5/2 and 12 steps for the scrubbing section at O/A equal to 1.5/1.
Thus, the addition of the salting-out agent into weakly acidic solutions containing La(III), Ce(III), Pr(III), and Nd(III) enabled reduction in the total number of steps with a relative decrease in O/A ratio, both in loading and scrubbing sections of the multistage counter-current SE cascade.
2.2. Solvent Extraction Separation of La(III), Pr(III), and Nd(III) Using TOMANO3-TBP Mixtures
At high concentrations of Ce(III) in the raw feedstock, including in the collective REE concentrate obtained from loparite concentrate, cerium is recovered by a precipitation technique, for example, by oxidation to Ce(IV) and precipitation of hydroxide [
3]. Further, to separate the three-component mixture of La(III), Pr(III), and Nd(III), applying the solvent extraction technique using TBP, the first separation is carried out along the La(III)/Pr(III) line and then along the Pr(III)/Nd(III) line to obtain pure La(III), Pr(III), and Nd(III) compounds. According to data in
Table 2, for a solution containing all four light REEs, high values of β
La(III)/Pr(III) were observed for the range 0.15–0.20 mol/L TOMANO
3-0.5–0.80 mol/L TBP. In the range of solvent compositions 0.75–0.95 mol/L TOMANO
3-0.25–0.05 mol/L TBP, Pr(III) was better extracted, while β
Pr(III)/La(III) achieved a value equal to 2.2.
When passing to triple-component mixtures formed after recovery of cerium by precipitation, the composition of the solution changed, resulting in changes in the distribution ratios and separation factors.
Table 3 presents the values of D
Ln(III) and β
Pr(III)/La(III) obtained during SE of La(III), Pr(III), and Nd(III) from a model triple-component solution prepared from a collective REE concentrate. This collective REE concentrate was obtained by loparite concentrate reprocessing and cerium removal. The transition from toluene to dodecane was due to practical considerations since hydrocarbon diluents have been more widely applied in practical SE techniques than aromatic ones, including toluene. Experimental data obtained during single contact of aqueous and organic phases showed that D
Pr(III) > D
La(III), which is in contrast to data in
Table 2 for the same range of solvent compositions.
The behavior of La(III) and Pr(III) may be due to changes in the composition of a stock solution with high La(III) concentration, which exceeds by almost five times the initial concentration of Pr(III). At such high La(III) concentrations in the aqueous phase, the organic phase will be close to saturation by La(III), resulting in a significant decrease in DLa(III).
For the correct determination of β
La(III)/Pr(III) values, the organic phase was mixed with the stock solution three times, thus getting close to solvent saturation with all components of the mixed nitrate solution, as shown in
Table 4. TOMANO
3-TBP mixtures with molar ratio TOMANO
3/TBP equal to 2/8 or 1/4 in dodecane were used as solvents. In the case when solvent mixture saturation with all solution components was achieved, the D
La(III) value exceeded D
Pr(III) and D
Nd(III). This resulted in the inversion of separation factors for La(III)/Pr(III) in favor of La(III). Moreover, growth of β
La(III)/Pr(III) with saturation of the solvent by REEs and expansion of initial total concentration solvents occurred. The maximum β
La(III)/Pr(III) value for 2.0 mol/L solution of TOMANO
3-TBP mixture will reach 1.5, which will lead to growth in the number of steps of the SE cascade.
Effective SE separation of REEs for La(III)/Pr(III) may be achieved up to the value of βLa(III)/Pr(III) ≥ 1.6. The saturation of 3.0 mol/L solution of TOMANO3-TBP mixture in dodecane can ensure high values for separation factors equal to 1.62–2.43.
Empirical separation factors for La(III)/Pr(III) values calculated for saturation conditions for TOMANO
3-TBP mixtures in dodecane were used to design the multistage counter-current SE cascade with scrubbing section.
Table 5 presents the input data for calculating the multistage counter-current SE cascade using the original PC software “KASKAD”.
Figure 1 provides the results of calculations using the «KASKAD» original PC software in the form of dependencies of the total number of extraction (N) and scrubbing (M) steps (N + M) and parameter ((W + V)/V
0) from relative selection (θ).
At the intersection of curves in
Figure 1, the value θ was 0.795, N + M was 70.25, and (V + W)/V
0 was 3.64. Based on these calculation values, the following parameters of an operating SE cascade for extraction section were selected: N equal to 40 at O/A equal to 1/1; for the scrubbing section: M equal to 32 at O/A equal to 2/1.
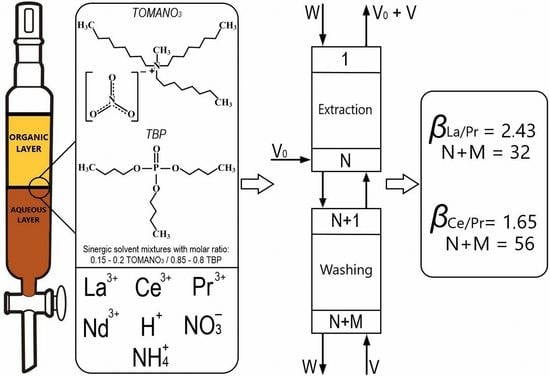
Figure 2 shows the operational scheme of the counter-current solvent extraction cascade, which was used to separate the REEs along the line La(III)/Pr(III).
In the case of applying the mixture containing 0.6 mol/L TOMANO3-2.4 mol/L TBP in dodecane, for which βLa(III)/Pr(III) was 2.43, similar calculations were carried out. The optimal values of relative selection at the intersection of curves (N + M) = f(θ) and (V + W)/V0 = f(θ) was 0.815, N + M was 30.5, and (V + W)/V0 was 1.4. Based on the calculated values, the following parameters of an operating SE cascade were selected: for extraction (loading) section, N equal to 18 at O/A equal to 2/1, and for the scrubbing section, M equal to 14 at O/A equal to 2.8/1.
Thus, an increase in separation factors for the pair La(III)/Pr(III) for SE with mixture 0.6 mol/L TOMANO3-2.4 mol/L TBP in dodecane enabled reduction in the number of steps in the multistage counter-current SE cascade from 72 to 32 with an increase of O/A for the extraction and scrubbing sections by 2 and 1.4 times, respectively.
It should be noted that, in the initial stage of research, the experimental results obtained earlier [
16,
17] were used to develop a counter-current multistage solvent extraction process for the separation of La(III), Ce(III), Nd(III), and Pr(III). These data made it possible to determine the synergic compositions of solvent mixtures, to establish the main characteristics of the separation process of La(III), Ce(III), Nd(III), Pr(III), and the modification of the operational scheme of the solvent extraction cascade. The results of the study presented in this article made it possible to fully substantiate the previously obtained data and optimize the separation process using a calculated and mounted multistage solvent extraction laboratory unit. The results obtained are important for developing and optimizing new solvent extraction selective systems for REE separation in weakly acidic nitrate media.
3. Materials and Methods
For this study, the oxides, La2O3, Pr6O11, Nd2O3, and compounds Ce(NO3)3·6H2O, HNO3, NH4OH, Trilon B (2-aqueous disodium ethylene diamine-N,N,N′,N′-tetraacetic acid), xylenol orange, sodium salt of diphenylamine-4-sulphonic acid, and chemically pure grade AgNO3 were used.
As solvents, methyltri-n-octylammonium (MTOA) nitrate and pure grade tri-n-butyl phosphate were used (see
Figure 3). MTOA nitrate was synthesized as per original techniques from MTOA methylsulphate (product of tri-n-octylamine alkylation with dimethylsulphate) with 99.9% content of CH
3(C
8H
17)
3NNO
3 (in terms of dry product). Toluene and dodecane of chemically pure grade were used as diluents. By dissolving a precisely weighed relevant oxide in the estimated quantity of concentrated HNO
3 solution stock individual nitrate solutions of La(III), Pr(III), and Nd(III) were prepared. After completely dissolving the REE oxides, ammonium nitrate was added to the solution. To the resulting solution, a calculated and exactly weighed quantity of solid NH
4NO
3 was added and dissolved. The pH of the solution was adjusted to 2 (by addition of 1.0 mol/L NH
4OH solution), corresponding to an HNO
3 concentration equal to 0.01 mol/L. For the preparation of Ce(III)-containing nitrate solution, a precisely weighed quantity of crystalline Ce(NO
3)
3·6H
2O was dissolved in an aqueous solution containing 4.0 mol/L NH
4NO
3 with pH ~2 (correction using HNO
3 solution).
By dissolving precisely weighed quantities of REE oxides in the estimated amount of concentrated HNO3 solution stock mixed nitrate solutions of La(III), Pr(III), and Nd(III) were prepared. After complete dissolution of the REE oxides, an exactly weighed amount of crystalline Ce(NO3)3·6H2O was added to the solution. After Ce(NO3)3·6H2O dissolution, the content of HNO3 in nitrate solution was adjusted to 0.1–0.2 mol/L by adding an estimated quantity of 1.0 mol/L HNO3 or by neutralizing of excessive acid with 1.0 mol/L NH4OH solution.
Organic solutions of the solvent mixtures were prepared by dissolving precisely weighed quantities of 1.96 mol/L TOMANO3 and 3.65 mol/L TBP in toluene or dodecane in a 50 mL or 100 mL volumetric flask. Estimated quantities of 3.65 mol/L (100%) TBP and 1.96 mol/L (100%) MTOA nitrate were put into the flask, following which the mixture was diluted with the diluent to the required volume. The content of TOMANO3 in mixed solutions was determined by potentiometric titration of the iodide form of the quaternary salt with 0.1 mol/L aqueous solutions of AgNO3 in the presence of an iodide-selective electrode (Radelkis, Hungary), in 50% v/v n-propanol + 50% v/v aqueous medium.
Solvent extraction from individual REE solutions was performed in 10–25 mL glass-stoppered tubes at room temperature 20 ± 2 °C, while intensively stirring at O/A equal to 1/1 (5–10 mL per phase). The phase contact time was 15 min and the phase separation time was 10–20 min. After which a sample of aqueous phase aliquot was taken and tested for metal content using the Trilon B titration method in the presence of xylenol orange [
28].
Solvent extraction from mixed REE solutions was performed in 250 mL separating glass funnels equipped with mechanic stirrers at 20 ± 2 °C at required O/A. The phase contact time was 15 min and the phase separation time was 15–20 min. On phase disengagement, the aqueous phase was separated from the organic phase and tested for content of the total of elements La(III), Ce(III), Pr(III), and Nd(III) with the Trilon B titration method in the presence of xylenol orange [
28]. The content of Pr(III) and Nd(III) in mixed solutions was determined spectrophotometrically based on absorption of individual bands in the electronic spectrum, at 444 nm for Pr(III) and at 794 nm for Nd(III). The concentration of Ce(III) in mixed solutions was determined by redox titration using ferrous ammonium sulfate in the presence of the sodium salt of diphenylamine-4-sulphonic acid as an indicator [
29]. The concentration of La(III) in mixed solutions was calculated by the difference between the total concentration of all the four metals and the concentration of Ce(III), Pr(III), and Nd(III).
The content of La(III), Ce(III), Pr(III), and Nd(III) in phases at equilibrium was calculated from differences in concentrations in the stock and aqueous solutions at equilibrium. In the event of multiple contacts of mixed solutions with the organic phase, the concentration of metals in the organic phase was determined in aqueous phase after double stripping with 4.0 mol/L aqueous solution of HNO3 at O/A equal to 1/1, using the methods described above. For verification and confirmation of the obtained experimental data, a comparison was carried out between the calculated and experimentally determined concentrations of each REE.
Distribution ratios of Ln (D
Ln) were calculated by the formula:
where C
INIT, C
AQ, and C
ORG are initial concentrations of Ln in initial aqueous nitrate solution, aqueous and organic phases after extraction, respectively.
Synergistic effect (factor) S
Ln was calculated by the formula:
where D
exp is the experimentally determined distribution ratio of Ln for the solution of solvent mixture and D
1 and D
2 are the distribution ratios of Ln for an individual solution of each solvent at their equal concentrations in the mixed solution. At S > 1, a synergistic effect is observed, while at S < 1, an antagonistic effect is observed [
15,
17].
Calculations of multistage counter-current SE cascades containing the loading and scrubbing section during delivery of stock nitrate REE-containing solution into the middle cascade section (the last stage of loading section) were performed using KASKAD original PC software. The KASKAD program was developed according to the theoretical methodology by G.V. Korpusov [
27]. Calculation of cascade steps and O/A ratio in the loading and scrubbing section of the SE cascade was performed by the equations binding these parameters with the recovery parameters of the extractable components, firstly, with the relative selection (θ), enrichment level (Q), and separation factor (β
Ln1/Ln2). Enrichment level (Q) is equal to change in the relative concentration of a component. Relative selection (θ) value is calculated according to the following formula:
where θ′ (selection) is defined as the proportion of the pure component extracted into the raffinate or extract in relation to the total amount of this component feeding to the extraction or scrubbing, respectively. The maximum possible selection value (θ′
max) is assumed to be equal to (β
Ln1/Ln2 − 1)/β
Ln1/Ln2 (when obtaining a purified individual REE compound) [
27].
Then the dependencies of the total number of loading (N) and scrubbing (M) steps (N + M) and the ratio (W + V)/V0 are plotted, where W, V, and V0 are flows of the organic phase, scrubbing solution, and REEs-containing nitrate solution, respectively. The optimal value of relative selection is determined at the intersection point of the curves (N + M) = f(θ) and (V + W)/V0 = f(θ). The optimal value of relative selection is used to calculate the optimal number of steps and O/A ratio in each of the SE cascade sections.