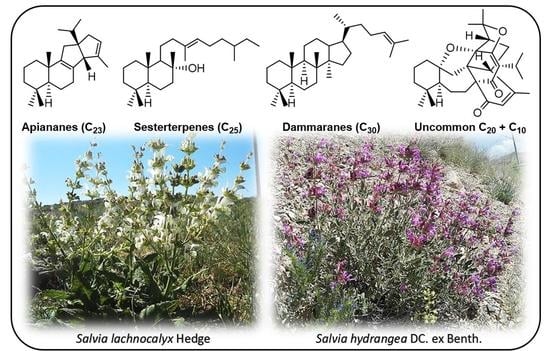
Uncommon Terpenoids from Salvia Species: Chemistry, Biosynthesis and Biological Activities
Abstract
:1. Introduction
2. C23-Terpenoids
3. Sesterterpenoids
3.1. Bicyclic Sesterterpenoids
3.1.1. Bicyclic Sesterterpene Lactones Containing C-23 Methyl Group
3.1.2. Bicyclic Sesterterpene Lactones Containing C-23 Hydroxymethyl Group
3.1.3. Bicyclic Sesterterpene Lactones Containing C-23 Formyl Group
3.1.4. Bicyclic Sesterterpene Lactones Containing C-23 Carboxylic Acid
3.1.5. Bicyclic Sesterterpene Lactones Containing C-23 Methyl Ester of Carboxylic Acid
3.1.6. Bicyclic Sesterterpenes Containing C-6, 23- and C-16, 19-Diolide
3.1.7. Bicyclic Sesterterpenes Containing C-19,16-Olide and C-6,23-Tetrahydrofuran
3.2. Tricyclic Sesterterpenoids
3.3. Norsesterterpenes
4. Dammarane Triterpenoids
5. Triterpenoids with Novel Skeleton
6. Biological and Pharmacological Activities of Uncommon Terpenoids from Salvia Species
6.1. Antioxidant Activity
6.2. Antiviral Activity
6.3. Cytotoxic Activity
6.4. Antiparasitic Activity
6.5. Antibacterial Activity
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drew, B.; González-Gallegos, J.G.; Xiang, C.; Kriebel, R.; Drummond, C.; Walker, J.; Sytsma, K. Salvia united: The greatest good for the greatest number. Taxon 2017, 66, 133–145. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.B.; Ni, Z.Y.; Shi, Q.W.; Dong, M.; Kiyota, H.; Gu, Y.C.; Cong, B. Constituents from Salvia species and their biological activities. Chem. Rev. 2012, 112, 5967–6026. [Google Scholar] [CrossRef] [PubMed]
- Will, M.; Claßen-Bockhoff, R. Time to split Salvia s.l. (Lamiaceae)—New insights from Old World Salvia phylogeny. Mol. Phylogenet. Evol. 2017, 109, 33–58. [Google Scholar] [CrossRef] [PubMed]
- Jamzad, Z. Lamiaceae. In Flora of Iran, 1st ed.; Assadi, M., Maassoumi, A.A., Mozaffarian, V., Eds.; Research Institute of Forests and Rangelands Publications Research Institute of Forests and Rangelands Publications: Tehran, Iran, 2012; Volume 76, pp. 948–950. [Google Scholar]
- Li, M.; Li, Q.; Zhang, C.; Zhang, N.; Cui, Z.; Huang, L.; Xiao, P. An ethnopharmacological investigation of medicinal Salvia plants (Lamiaceae) in China. Acta Pharm. Sin. B 2013, 3, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Kamatou, G.; Makunga, N.; Ramogola, W.; Viljoen, A. South African Salvia species: A review of biological activities and phytochemistry. J. Ethnopharmacol. 2008, 119, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L. Salvia (Sage): A review of its potential cognitive-enhancing and protective effects. Drugs R&D 2016, 17, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, M.; Ozcelik, B.; Altın, G.; Daşkaya-Dikmen, C.; Martorell, M.; Ramírez-Alarcón, K.; Alarcón-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.; Leal, A.L.A.B.; et al. Salvia spp. plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol. 2018, 80, 242–263. [Google Scholar] [CrossRef]
- Valdivia-López, M.; Tecante, A. Chia (Salvia hispanica): A Review of Native Mexican Seed and its Nutritional and Functional Properties. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2015; Volume 75, pp. 53–75. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Qian, S.S.; Zhang, Y.J.; Wang, R.Q. Salvia miltiorrhiza: A source for anti-Alzheimer’s disease drugs. Pharm. Biol. 2015, 54, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Akhondzadeh, S.; Noroozian, M.; Mohammadi, M.; Ohadinia, S.; Jamshidi, A.H.; Khani, M. Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: A double blind, randomized and placebo-controlled trial. J. Clin. Pharm. Ther. 2003, 28, 53–59. [Google Scholar] [CrossRef]
- Moss, L.; Rouse, M.; Wesnes, K.; Moss, M. Differential effects of the aromas of Salvia species on memory and mood. Hum. Psychopharmacol. Clin. Exp. 2010, 25, 388–396. [Google Scholar] [CrossRef]
- Seol, G.H.; Shim, H.S.; Kim, P.-J.; Moon, H.K.; Lee, K.H.; Shim, I.; Suh, S.H.; Min, S.S. Antidepressant-like effect of Salvia sclarea is explained by modulation of dopamine activities in rats. J. Ethnopharmacol. 2010, 130, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Mora, S.; Millán, R.; Lungenstrass, H.; Díaz-Véliz, G.; Morán, J.; Herrera-Ruiz, M.; Tortoriello, J. The hydroalcoholic extract of Salvia elegans induces anxiolytic- and antidepressant-like effects in rats. J. Ethnopharmacol. 2006, 106, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Vuksan, V.; Whitham, D.; Sievenpiper, J.L.; Jenkins, A.L.; Rogovik, A.L.; Bazinet, R.P.; Vidgen, E.; Hanna, A. Supplementation of conventional therapy with the novel grain Salba (Salvia hispanica L.) improves major and emerging cardiovascular risk factors in type 2 diabetes. Diabetes Care 2007, 30, 2804–2810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solares-Pascasio, J.; Ceballos, G.; Calzada, F.; Barbosa, E.; Velazquez, C. Antihyperglycemic and lipid profile effects of Salvia amarissima Ortega on streptozocin-induced type 2 diabetic mice. Molecules 2021, 26, 947. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.M.; Sasmal, D.; Mazumder, P.M. The antihyperglycemic effect of aerial parts of Salvia splendens (scarlet sage) in streptozotocin-induced diabetic-rats. Pharmacogn. Res. 2010, 2, 190–194. [Google Scholar] [CrossRef] [Green Version]
- Zarei, A.; Vaezi, G.; Malekirad, A.A.; Abdollahi, M. Hypoglycemic and hypolipidemic activities of Salvia hydrangea in streptozotocin-induced diabetes in rats. Iran. J. Basic Med. Sci. 2015, 18, 417–422. [Google Scholar] [CrossRef]
- Alfieri, A.; Maione, F.; Bisio, A.; Romussi, G.; Mascolo, N.; Cicala, C. Effect of a diterpenoid from Salvia cinnabarina on arterial blood pressure in rats. Phytotherapy Res. 2007, 21, 690–692. [Google Scholar] [CrossRef]
- Fernando, M.-P.; Alberto, H.-L.; Guadalupe, V.-D.M.; Agustina, C.-M.; Fernando, N.-G.; Eva, A.-H.; Hermelinda, S.-C.; Eva, G.-T.M. Neo-clerodane diterpenic influence in the antinociceptive and anti-inflammatory properties of Salvia circinnata Cav. J. Ethnopharmacol. 2020, 268, 113550. [Google Scholar] [CrossRef]
- Brindisi, M.; Bouzidi, C.; Frattaruolo, L.; Loizzo, M.R.; Tundis, R.; Dugay, A.; Deguin, B.; Cappello, A.R.; Cappello, M.S. Chemical profile, antioxidant, anti-inflammatory, and anti-cancer effects of Italian Salvia rosmarinus Spenn. methanol leaves extracts. Antioxidants 2020, 9, 826. [Google Scholar] [CrossRef]
- Jang, H.-J.; Lee, S.; Lee, S.J.; Lim, H.-J.; Jung, K.; Kim, Y.H.; Lee, S.W.; Rho, M.-C. Anti-inflammatory activity of eudesmane-type sesquiterpenoids from Salvia plebeia. J. Nat. Prod. 2017, 80, 2666–2676. [Google Scholar] [CrossRef]
- Righi, N.; Boumerfeg, S.; Deghima, A.; Fernandes, P.A.; Coelho, E.; Baali, F.; Cardoso, S.M.; Coimbra, M.A.; Baghiani, A. Phenolic profile, safety assessment, and anti-inflammatory activity of Salvia verbenaca L. J. Ethnopharmacol. 2021, 272, 113940. [Google Scholar] [CrossRef]
- Hafez Ghoran, S.; Firuzi, O.; Jassbi, A.R. Phytoconstituents from the Aerial Parts of Salvia dracocephaloides Boiss. and their Biological Activities. J. Environ. Treat. Tech. 2020, 8, 1274–1278. [Google Scholar] [CrossRef]
- Rowshan, V.; Najafian, S. Polyphenolic contents and antioxidant activities of aerial parts of Salvia multicaulis from the Iran flora. Nat. Prod. Res. 2019, 34, 2351–2353. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I.; Tel, A.Z.; Goren, A.C.; Taslimi, P.; Alwasel, S.H. Sage (Salvia pilifera): Determination of its polyphenol contents, anticholinergic, antidiabetic and antioxidant activities. J. Food Meas. Charact. 2019, 13, 2062–2074. [Google Scholar] [CrossRef]
- Pereira, O.R.; Catarino, M.D.; Afonso, A.F.; Silva, A.M.S.; Cardoso, S.M. Salvia elegans, Salvia greggii and Salvia officinalis decoctions: Antioxidant activities and inhibition of carbohydrate and lipid metabolic enzymes. Molecules 2018, 23, 3169. [Google Scholar] [CrossRef] [Green Version]
- Eltawaty, S.I.; Yagoub, S.O.; Shouman, S.A.; Ahmed, A.; Omer, F.A. Anticancer effects of methanol extract of Libyan Salvia fruticosa Mill on Mcf7, T47D and (Mda-Mb-468) breast cells lines. Eur. J. Pharm. Med. Res. 2020, 7, 165–169. [Google Scholar]
- Balaei-Kahnamoei, M.; Eftekhari, M.; Ardekani, M.R.S.; Akbarzadeh, T.; Saeedi, M.; Jamalifar, H.; Safavi, M.; Sam, S.; Zhalehjoo, N.; Khanavi, M. Phytochemical constituents and biological activities of Salvia macrosiphon Boiss. BMC Chem. 2021, 15, 4. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Emami, S.A.; Tayarani-Najaran, Z.; Iranshahi, M.; Shakeri, A.; Hohmann, J.; Asili, J. Cytotoxic diterpene quinones from Salvia tebesana Bunge. Fitoterapia 2018, 128, 97–101. [Google Scholar] [CrossRef]
- Shakeri, A.; Farahmand, S.S.; Tayarani-Najaran, Z.; Emami, S.A.; Kúsz, N.; Hohmann, J.; Boozari, M.; Tavallaie, F.Z.; Asili, J. 4,5-Seco-5,10-friedo-abietane-type diterpenoids with anticancer activity from Salvia atropatana Bunge. Naunyn-Schmiedebergs Archiv für Experimentelle Pathologie und Pharmakologie 2020, 394, 241–248. [Google Scholar] [CrossRef]
- Bustos-Brito, C.; Joseph-Nathan, P.; Burgueño-Tapia, E.; Martínez-Otero, D.; Nieto-Camacho, A.; Calzada, F.; Yépez-Mulia, L.; Esquivel, B.; Quijano, L. Structure and absolute configuration of abietane diterpenoids from Salvia clinopodioides: Antioxidant, Antiprotozoal, and Antipropulsive Activities. J. Nat. Prod. 2019, 82, 1207–1216. [Google Scholar] [CrossRef]
- Cezarotto, C.S.; Dorneles, A.; Baldissera, F.G.; da Silva, M.B.; Markoski, M.M.; Junior, L.R.; Peres, A.; Fazolo, T.; Bordignon, S.A.; Apel, M.A.; et al. Leishmanicidal and antichemotactic activities of icetexanes from Salvia uliginosa Benth. Phytomedicine 2018, 58, 152748. [Google Scholar] [CrossRef] [PubMed]
- Kuźma, Ł.; Kaiser, M.; Wysokińska, H. The production and antiprotozoal activity of abietane diterpenes in Salvia austriaca hairy roots grown in shake flasks and bioreactor. Prep. Biochem. Biotechnol. 2016, 47, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Tabanca, N.; Demirci, B.; Baser, K.H.C.; Aytac, Z.; Ekici, M.; Khan, S.I.; Jacob, M.R.; Wedge, D.E. Chemical composition and antifungal activity of Salvia macrochlamys and Salvia recognita essential oils. J. Agric. Food Chem. 2006, 54, 6593–6597. [Google Scholar] [CrossRef] [PubMed]
- Soković, M.D.; Brkić, D.D.; Džamić, A.M.; Ristić, M.S.; Marin, P.D. Chemical composition and antifungal activity of Salvia desoleana Atzei & Picci essential oil and its major components. Flavour Fragr. J. 2009, 24, 83–87. [Google Scholar] [CrossRef]
- Liang, Y.-Y.; Li, K.-W.; Niu, F.-J.; Li, Y.; Wei, H.-C.; Dai, Y.-L.; Wang, Y.-Y.; Zhou, C.-Z.; Wan, X.-H. Salvia plebeia R. Br. polysaccharides (SPP) against RSV (respiratory syncytial virus) infection: Antiviral effect and mechanisms of action. Biomed. Pharmacother. 2021, 141, 111843. [Google Scholar] [CrossRef] [PubMed]
- Ustun, O.; Ozcelik, B. In vitro antiviral activity and cytotoxicity of the extracts of Salvia wiedemannii Boiss. Planta Med. 2011, 77, PL23. [Google Scholar] [CrossRef]
- Azad, M.M.; Al Mahmud, A.; Islam, S.; Gouhar, A.I. Common sage (Salvia officinalis) antiviral role: Potentiality of a Unani hand sanitizer in COVID-19 (corona virus) second wave control. Asian J. Med Biol. Res. 2021, 6, 611–617. [Google Scholar] [CrossRef]
- Najar, B.; Mecacci, G.; Nardi, V.; Cervelli, C.; Nardoni, S.; Mancianti, F.; Ebani, V.; Giannecchini, S.; Pistelli, L. Volatiles and antifungal-antibacterial-antiviral activity of South African Salvia spp. essential oils cultivated in uniform conditions. Molecules 2021, 26, 2826. [Google Scholar] [CrossRef]
- Güzel, S.; Özay, Y.; Kumaş, M.; Uzun, C.; Özkorkmaz, E.G.; Yıldırım, Z.; Ülger, M.; Güler, G.; Çelik, A.; Çamlıca, Y.; et al. Wound healing properties, antimicrobial and antioxidant activities of Salvia kronenburgii Rech. f. and Salvia euphratica Montbret, Aucher & Rech. f. var. euphratica on excision and incision wound models in diabetic rats. Biomed. Pharmacother. 2019, 111, 1260–1276. [Google Scholar] [CrossRef]
- Bisio, A.; De Mieri, M.; Milella, L.; Schito, A.M.; Parricchi, A.; Russo, D.; Alfei, S.; Lapillo, M.; Tuccinardi, T.; Hamburger, M.; et al. Antibacterial and hypoglycemic diterpenoids from Salvia chamaedryoides. J. Nat. Prod. 2017, 80, 503–514. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Zare, S.; Firuzi, O.; Xiao, J. Bioactive phytochemicals from shoots and roots of Salvia species. Phytochem. Rev. 2015, 15, 829–867. [Google Scholar] [CrossRef]
- Xu, J.; Wei, K.; Zhang, G.; Lei, L.; Yang, D.; Wang, W.; Han, Q.; Xia, Y.; Bi, Y.; Yang, M.; et al. Ethnopharmacology, phytochemistry, and pharmacology of Chinese Salvia species: A review. J. Ethnopharmacol. 2018, 225, 18–30. [Google Scholar] [CrossRef]
- Luis, J.G.; Lahlou, E.H.; Andrés, L.S.; Sood, G.H.; Ripoll, M.M. Apiananes: C23 terpenoids with a new type of skeleton from Salvia apiana. Tetrahedron Lett. 1996, 37, 4213–4216. [Google Scholar] [CrossRef]
- Luis, J.G.; Lahlou, E.H.; Andrés, L.S. Hassananes: C23 terpenoids with a new type of skeleton from Salvia apiana Jeps. Tetrahedron 1996, 52, 12309–12312. [Google Scholar] [CrossRef]
- Miura, K.; Kikuzaki, H.; Nakatani, N. Apianane terpenoids from Salvia officinalis. Phytochemistry 2001, 58, 1171–1175. [Google Scholar] [CrossRef]
- Xu, G.; Hou, A.-J.; Wang, R.-R.; Liang, G.-Y.; Zheng, Y.-T.; Liu, Z.-Y.; Li, X.-L.; Zhao, Y.; Huang, S.-X.; Peng, L.-Y.; et al. Przewalskin A: A new C23 terpenoid with a 6/6/7 carbon ring skeleton from Salvia przewalskii Maxim. Org. Lett. 2006, 8, 4453–4456. [Google Scholar] [CrossRef]
- Wu, W.L.; Chang, W.L.; Chen, C.F. Cytotoxic activities of tanshinones against human carcinoma cell lines. Am. J. Chin. Med. 1991, 19, 207–216. [Google Scholar] [CrossRef]
- Chen, Q.; Li, J.; Ma, Y.; Yuan, W.; Zhang, P.; Wang, G. Occurrence and biosynthesis of plant sesterterpenes (C25), a new addition to terpene diversity. Plant Commun. 2021, 2, 100184. [Google Scholar] [CrossRef]
- Guo, K.; Liu, Y.; Li, S.H. The untapped potential of plant sesterterpenoids: Chemistry, biological activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 2293–2314. [Google Scholar] [CrossRef]
- Bisio, A.; Schito, A.M.; Pedrelli, F.; Danton, O.; Reinhardt, J.K.; Poli, G.; Tuccinardi, T.; Bürgi, T.; De Riccardis, F.; Giacomini, M.; et al. Antibacterial and ATP synthesis modulating compounds from Salvia tingitana. J. Nat. Prod. 2020, 83, 1027–1042. [Google Scholar] [CrossRef]
- Cioffi, G.; Bader, A.; Malafronte, A.; Piaz, F.D.; De Tommasi, N. Secondary metabolites from the aerial parts of Salvia palaestina Bentham. Phytochemistry 2008, 69, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Piaz, F.D.; Vassallo, A.; Lepore, L.; Tosco, A.; Bader, A.; De Tommasi, N. Sesterterpenes as tubulin tyrosine ligase inhibitors. First insight of structure–activity relationships and discovery of new lead. J. Med. Chem. 2009, 52, 3814–3828. [Google Scholar] [CrossRef] [PubMed]
- Rustaiyan, A.; Sadjadi, A. Salvisyriacolide, a sesterterpene from Salvia syriaca. Phytochemistry 1987, 26, 3078–3079. [Google Scholar] [CrossRef]
- Piaz, F.D.; Imparato, S.; Lepore, L.; Bader, A.; De Tommasi, N. A fast and efficient LC–MS/MS method for detection, identification and quantitative analysis of bioactive sesterterpenes in Salvia dominica crude extracts. J. Pharm. Biomed. Anal. 2010, 51, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, F.M.; Amiri, R.; Alam, M.; Hossain, M.B.; van der Helm, D. Structure and absolute stereochemistry of salvimirzacolide, a new sesterterpene from Salvia mirzayanii. J. Nat. Prod. 1998, 61, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Rustaiyan, A.; Niknejad, A.; Nazarians, L.; Jakupovic, J.; Bohlmann, F. Sesterterpenes from Salvia hypoleuca. Phytochemistry 1982, 21, 1812–1813. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Zaynizadeh, B.; Rüedi, P. Salvileucolide methylester, a sesterterpene from Salvia Sahendica. Phytochemistry 1995, 39, 715–716. [Google Scholar] [CrossRef]
- Linden, A.; Juch, M.; Moghaddam, F.M.; Zaynizadeh, B.; Rüedi, P. The absolute configuration of salvileucolide methyl ester, a sesterterpene from Iranian Salvia species. Phytochemistry 1996, 41, 589–590. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Koussari, S. Further sesterterpenes from Salvia hypoleuca. Phytochemistry 1988, 27, 1767–1769. [Google Scholar] [CrossRef]
- Farimani, M.M.; Mazarei, Z. Sesterterpenoids and other constituents from Salvia lachnocalyx Hedge. Fitoterapia 2014, 98, 234–240. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Farimani, M.M.; Seirafi, M.; Taheri, S.; Khavasi, H.R.; Sendker, J.; Proksch, P.; Wray, V.; Edrada, R. Sesterterpenoids and other constituents of Salvia sahendica. J. Nat. Prod. 2010, 73, 1601–1605. [Google Scholar] [CrossRef]
- Hasan, M.R.; Al-Jaber, H.I.; Al-Qudah, M.; Abu Zarga, M.H. New sesterterpenoids and other constituents from Salvia dominica growing wild in Jordan. Phytochem. Lett. 2016, 16, 12–17. [Google Scholar] [CrossRef]
- Gonzalez, M.; Segundo, J.S.; Grande, M.; Medarde, M.; Bellido, I. Sesterterpene lactones from Salvia aethiopis. Salviaethiopisolide and 13-epi-salviaethiopisolide. Tetrahedron 1989, 45, 3575–3582. [Google Scholar] [CrossRef]
- Topcu, G.; Ulubelen, A.; Tam, T.C.-M.; Tao-Che, C. Sesterterpenes and other constituents of Salvia yosgadensis. Phytochemistry 1996, 42, 1089–1092. [Google Scholar] [CrossRef]
- Malafronte, A.; Piaz, F.D.; Cioffi, G.; Braca, A.; Leone, A.; De Tommasi, N. Secondary metabolites from the aerial parts of Salvia aethiopis L. Nat. Prod. Commun. 2008, 3. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, S.N.; Farimani, M.M.; Mirzania, F.; Soltanipoor, M.A.; De Mieri, M.; Hamburger, M. Manoyloxide sesterterpenoids from Salvia mirzayanii. J. Nat. Prod. 2014, 77, 848–854. [Google Scholar] [CrossRef]
- Topcu, G.; Ulubelen, A.; Tam, T.C.M.; Che, C.T. Norditerpenes and norsesterterpenes from Salvia yosgadensis. J. Nat. Prod. 1996, 59, 113–116. [Google Scholar] [CrossRef]
- Ulubelen, A.; Topcu, G.; Sönmez, U.; Eriş, C.; Özgen, U. Norsesterterpenes and diterpenes from the aerial parts of Salvia limbata. Phytochemistry 1996, 43, 431–434. [Google Scholar] [CrossRef]
- Ruan, J.; Zheng, C.; Qu, L.; Liu, Y.; Han, L.; Yu, H.; Zhang, Y.; Wang, T. Plant resources, 13C-NMR spectral characteristic and pharmacological activities of dammarane-type triterpenoids. Molecules 2016, 21, 1047. [Google Scholar] [CrossRef]
- Carradori, S.; Petzer, J.P. Novel monoamine oxidase inhibitors: A patent review (2012–2014). Expert Opin. Ther. Pat. 2015, 25, 91–110. [Google Scholar] [CrossRef]
- Pedreros, S.; Rodríguez, B.; De La Torre, M.C.; Bruno, M.; Savona, G.; Perales, A.; Torres, M.R. Dammarane triterpenes of Salvia hierosolymitana. Phytochemistry 1990, 29, 919–922. [Google Scholar] [CrossRef]
- Kolak, U.; Kabouche, A.; Öztürk, M.; Kabouche, Z.; Topçu, G.; Ulubelen, A. Antioxidant diterpenoids from the roots of Salvia barrelieri. Phytochem. Anal. 2009, 20, 320–327. [Google Scholar] [CrossRef]
- Yosioka, I.; Yamauchi, H.; Kitagawa, I. Lichen triterpenoids. V. On the neutral triterpenoids of Pyxine endochrysina NYL. Chem. Pharm. Bull. 1972, 20, 502–513. [Google Scholar] [CrossRef] [Green Version]
- Valverde, S.; Escudero, J.; López, J.C.; Rabanal, R.M. Two terpenoids from Salvia bicolor. Phytochemistry 1985, 24, 111–113. [Google Scholar] [CrossRef]
- Ahmad, Z.; Fatima, I.; Mehmood, S.; Ifzal, R.; Malik, A.; Afza, N. New epoxydammarane triterpenes from Salvia santolinifolia. Helvetica Chim. Acta 2008, 91, 73–78. [Google Scholar] [CrossRef]
- Esquivel, B.; Guerrero, F.; Toscano, R. Tri-nordammarane triterpenoids and neoclerodane diterpenoids from Salvia aspera (Labiatae). Nat. Prod. Lett. 2002, 16, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Harraz, F.M.; Ulubelen, A.; Öksüz, S.; Tan, N. Dammarane triterpenes from Cleome amblyocarpa. Phytochemistry 1995, 39, 175–178. [Google Scholar] [CrossRef]
- Ghoran, S.H.; Firuzi, O.; Asadollahi, M.; Stuppner, H.; Alilou, M.; Jassbi, A.R. Dammarane-type triterpenoid saponins from Salvia russellii Benth. Phytochemistry 2021, 184, 112653. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Zahid, M.; Ali, M.S.; Ali, Z.; Jassbi, A.R.; Abbas, M.; Clardy, J.; Lobkovsky, E.; Tareen, R.B.; Iqbal, M.Z. Salvadiones-A and -B: Two terpenoids having novel carbon skeleta from Salvia bucharica. J. Org. Chem. 1999, 64, 8465–8467. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Zahid, M.; Ali, M.S.; Choudhary, M.I.; Akhtar, F.; Ali, Z.; Iqbal, M.Z. Salvadiol: A novel triterpenoid from Salvia bucharica. Tetrahedron Lett. 1999, 40, 7561–7564. [Google Scholar] [CrossRef]
- Farimani, M.M.; Bahadori, M.B.; Taheri, S.; Ebrahimi, S.N.; Zimmermann, S.; Brun, R.; Amin, G.; Hamburger, M. Triterpenoids with rare carbon skeletons from Salvia hydrangea: Antiprotozoal activity and absolute configurations. J. Nat. Prod. 2011, 74, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Farimani, M.M.; Taheri, S.; Ebrahimi, S.N.; Bahadori, M.B.; Khavasi, H.R.; Zimmermann, S.; Brun, R.; Hamburger, M. Hydrangenone, a new isoprenoid with an unprecedented skeleton from Salvia hydrangea. Org. Lett. 2011, 14, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Tabefam, M.; Farimani, M.M.; Danton, O.; Ramseyer, J.; Ebrahimi, S.N.; Neuburger, M.; Kaiser, M.; Salehi, P.; Potterat, O.; Hamburger, M. Antiprotozoal isoprenoids from Salvia hydrangea. J. Nat. Prod. 2018, 81, 2682–2691. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Kikuzaki, H.; Nakatani, N. Antioxidant activity of chemical components from sage (Salvia officinalis L.) and thyme (Thymus vulgaris L.) measured by the oil stability index method. J. Agric. Food Chem. 2002, 50, 1845–1851. [Google Scholar] [CrossRef]
- Pais, G.C.G.; Zhang, X.; Marchand, C.; Neamati, N.; Cowansage, K.; Svarovskaia, E.S.; Pathak, V.K.; Tang, Y.; Nicklaus, M.; Pommier, Y.; et al. Structure activity of 3-aryl-1,3-diketo-containing compounds as HIV-1 integrase inhibitors. J. Med. Chem. 2002, 45, 3184–3194. [Google Scholar] [CrossRef] [PubMed]

























| Compounds | Plant Species | Used Part | Biological Activity | Ref. |
|---|---|---|---|---|
| C23 terpenoids | ||||
| 14-hydroxy-7-methoxy-11,16-diketo-apian-8-en-(22,6)-olide (1) | S. apiana Jeps | Aerial Parts | - | [45] |
| 7-methoxy-11,16-diketo-apian-8,14-dien-(22,6)-olide (2) | ||||
| 13,14-dioxo-11-hydroxy-7-methoxy-hassane-8,11,15-trien-(22,6)-olide (3) | S. apiana Jeps | Aerial Parts | - | [46] |
| rel-(5S,6S,7S,10R,12S,13R)-7-hydroxyapiana-8,14-diene-11,16-dion-(22,6)-olide (4) | S. officinalis L. | Leaves | Weak antioxidant activity | [47,86] |
| rel-(5S,6S,7R,10R,12S,13R)-7-hydroxyapiana-8,14-diene-11,16-dion-(22,6)-olide (5) | - | |||
| rel-(5S,6S,7S,10R,12R,13S)-7-hydroxyapiana-8,14-diene-11,16-dion-(22,6)-olide (6) | ||||
| Przewalskin A (7) and its oxidation derivative (8) | S. przewalskii Maxim | Aerial parts | Anti-HIV-1 activity | [48] |
| Sesterterpenoids | ||||
| (13E)-labd-13(14),17(18)-dien-8α,16,19-triol (9) | S. tingitana Etl., | Aerial parts | Antibacterial and ATP modulation activity | [52] |
| 3α,8α,13,14-erythro-tetrahydroxy-labd-15,17-dien-16,19-olide (10) | S. palaestina Bentham | Aerial parts | - | [53] |
| 6α,8α,15(S)-trihydroxy-labd-13(14),17-dien-16(S),19-olide (11) | S. dominica L. | Aerial parts | Inhibition of Tubulin Tyrosine ligase | [54] |
| (13E)-8α,23-dihydroxy-labd-13(14),17(18)-dien-16,19-olide (12) | S. tingitana Etl. | Aerial parts | Antibacterial and ATP modulation activity | [52] |
| Salvisyriacolide (13) | S. syriaca L. | Aerial parts | - | [55] |
| 6α,8α,15(S),23-tetrahydroxy-labd-13(14),17-dien-16(S),19-olide (14) | S. dominica L. | Aerial parts | Inhibition of Tubulin Tyrosine ligase | [54] |
| 6α,8α,23-trihydroxy-labd-13(14),17-dien-16(R),19-olide (15) | ||||
| 6α,15(S),23-trihydroxy-labd-8(22),13(14),17-trien-16(S),19-olide (16) | ||||
| 6α,8α,23-trihydroxy-labd-13(14),15,17-trien-16,19-olide (17) | ||||
| 6α,8α,23,14,15-threo-pentahydroxy-labd-13(21),17-dien-16,19-olide (18) | ||||
| 6α,8α,23,14,15-erythro-pentahydroxy-labd-13(21),17-dien-16,19-olide (19) | ||||
| 6α,8α,13,23,14,15-threo-hexahydroxy-labd-17-en-16,19-olide (20) | S. dominica L. | Aerial parts | Inhibition of Tubulin Tyrosine ligase | [56] |
| 6α,8α,15(S)-trihydroxy-23-oxo-labd-13(14),17-dien-16(S),19-olide (21) | S. dominica L. | Aerial parts | Inhibition of Tubulin Tyrosine ligase | [54] |
| 6α,8α-dihydroxy-23-oxo-labd-13(14),17-dien-16(R),19-olide (22) | ||||
| 6α,15(S)-dihydroxy-23-oxo-labd-8(22),13(14),17-trien-16(S),19-olide (23) | ||||
| 6α,8α-dihydroxy-23-oxo-labd-13(14),15,17-trien-16,19-olide (24) | ||||
| 6α,8α,14,15-threo-tetrahydroxy-23-oxo-labd-13(21),17-dien-16,19-olide (25) | ||||
| 6α,8α,13,14,15-threo-23-oxo-pentahydroxy-labd-17-en-16,19-olide (26) | S. dominica L. | Aerial parts | Inhibition of Tubulin Tyrosine ligase | |
| Salvimirzacolide (27) | S. mirzayanii Rech. and Esfandieri. | Aerial parts | - | [57] |
| 8α,13,14-threo-trihydroxy-labd-15,17-dien-16,19-olide-23-oic acid (28) | S. palaestina Bentham | Aerial parts | - | [53] |
| 8α,13,14-erythro-trihydroxy-labd-15,17-dien-16,19-olide-23-oic acid (29) | ||||
| 6α,8α,15(S)-trihydroxy-23-carboxy-labd-13(14),17-dien-16(S),19-olide (30) | S. dominica L. | Aerial parts | Inhibition of Tubulin Tyrosine ligase | [54] |
| 6α,8α-dihydroxy-23-carboxy-labd-13(14),17-dien-16,19-olide (31) | ||||
| 6α,8α-dihydroxy-23-carboxy-labd-13(14),15,17-trien-16,19-olide (32) | ||||
| Salvileucolide methyl ester (33) | S. syriaca L. | Aerial parts | - | [55] |
| S. hypoleuca Benth. | Aerial parts | - | [58] | |
| S. sahendica Boiss & Buhse | Aerial parts | - | [59] | |
| S. lachnocalyx Hedge | Aerial parts | Cytotoxic activity | [62] | |
| 14-hydroperoxy-13(21)-dehydro-SME (34) | S. hypoleuca Benth. | Aerial parts | - | [58] |
| 13-hydroperoxy-14-ene-SME (35) | ||||
| 13-epi-hydroperoxy-14-ene-SME (36) | ||||
| 14,17-cycloperoxy-13(21)-dehydro-SME (36) | ||||
| 6α,8α,15(S)-trihydroxy-23-carboxymethyl-labd-13(14),17-dien-16(S),19-olide (38) | S. dominica L. | Aerial parts | Inhibition of Tubulin Tyrosine ligase | [54] |
| (4R,5R,8R,9R,10S,16R,13E)-8-hydroxy-23-carboxymethyl-labd-13(14),17(18)-dien-16,19-olide (39) | S. tingitana Etl. | Aerial parts | Antibacterial and ATP modulation activity | [52] |
| (4R,5R,6S,8R,9R,10S,15S,16S,13E)-8,15-dihydroxy-23-carboxymethyl-labd-13(14),17(18)-dien-16,19-olide (40) | ||||
| Salvileucolide-6,23-lactone (41) | S. hypoleuca Benth. | Aerial parts | - | [58] |
| S. lachnocalyx Hedge | Aerial parts | Cytotoxic activity | [62] | |
| S. tingitana Etl. | Aerial parts | Antibacterial and ATP modulation activity | [52] | |
| 15,16-dehydrosalvileucolide-6,23-lactone-trans-epoxide (42) | S. hypoleuca Benth. | Aerial parts | - | [58] |
| 15,16-dehydrosalvileucolide-6,23-lactone-cis-epoxide (43) | ||||
| 15,16-dehydrosalvileucolide-6,23-lactone-13,14-bis-epi-trans-epoxide (44) | ||||
| 14-hydroperoxy-13(21)-dehydro-13,14-dihydro-salvileucolide-6,23-lactone (45) | ||||
| Salvileucolide (46) | S. tingitana Etl. | Aerial parts | Antibacterial and ATP modulation activity | [52] |
| Lachnocalyxolide B (47) | S. lachnocalyx Hedge | Aerial parts | Cytotoxic activity | [62] |
| 8α,15(S)-dihydroxy-labd-13(14),17-dien-23,6α-16(S),19-diolide (48) | S. dominica L. | Aerial parts | Inhibition of Tubulin Tyrosine ligase | [54] |
| 8α-hydroxy-labd-13(14),15,17-trien-6α,23-16,19-diolide (49) | ||||
| 8α-hydroxy-13-hydroperoxylabd-14,17-dien-19,16;23,26α-diolide (50) | S. sahendica Boiss & Buhse | Aerial parts | - | [63] |
| 23,6α-epoxy-labd-8,13(14),17-trien-16(R),19-olide (51) | S. dominica L. | Aerial parts | Inhibition of Tubulin Tyrosine ligase | [54] |
| 15(S)-dihydroxy-23,6α-epoxy-labd-13(14),17-dien-16(S),19-olide (52) | ||||
| 8α,15(S),23α-trihydroxy-23,6α-epoxy-labd-13(14),17-dien-16(S),19-olide (53) | ||||
| 8α,15(S)-dihydroxy-23α-O-ethyl-23,6α-epoxy-labd-13(14),17-dien-16(S),19-olide (54) | ||||
| 8α-hydroxy-23α-O-ethyl-23,6α-epoxy-labd-13(14),17-dien-16(R),19-olide (55) | ||||
| 8α,23-dihydroxy-23,6α-epoxy-labd-13(14),15,17-trien-16,19-diolide (56) | ||||
| (4R,5R,6S,8R,9R,10S,16R,23S,13E)-8,23-dihydroxy-23,6-epoxy-labd-13(14),17(18)-dien-16,19-olide (57) | S. tingitana Etl. | Aerial parts | Antibacterial and ATP modulation activity | [52] |
| (13E)-8α-hydroxy-23α-O-methyl-23,6α-epoxy-labd-13(14),17(18)-dien-16,19-olide (58) | ||||
| (4R,5R,6S,8R,9R,10S,15S,16S,23S,13E)-8,15-dihydroxy-23-O-methyl-23,6-epoxy-labd-13(14),17(18)-dien-16,19-olide (59) | ||||
| Salvidominicolide B (60) | S. dominica L. | Whole parts | - | [64] |
| 13-epi-salviaethiopisolide (61 and 61′) | S. aethiopis | Aerial parts | - | [65] |
| Salviaethiopisolide (62 and 62′) | ||||
| Yosgadensolide A (6α,14-dihydroxymanoyloxide-15,17-dien-16,19-olide; 63) | S. yosgadensis Freyn et Bornm. | Aerial parts | - | [66] |
| yosgadensolide B (6α,16-dihydroxymanoyloxide-14,17-dien-16,19-olide; 64) | ||||
| 3β-hydroxymanoyloxide-14(E),17-dien-16-oxo-19-oic acid (65) | S. aethiopis L. | Aerial parts | - | [67] |
| Hydroxymanoyloxide-14,17-dien-16-oxo-19-oic acid (66) | ||||
| Hydroxymanoyloxide-14,17-dien-16-oxo-19,23-dioic acid (67) | ||||
| (4R,5R,8R,9R,10S,13S,14S)-14-hydroxymanoyloxide-15,17-dien-15(Z)-16,19-olide (68) | S. mirzayanii Rech. and Esfandieri. | Aerial parts | - | [68] |
| (4R,5R,8R,9R,10S,13R,14S)-14-hydroxymanoyloxide-15,17-dien-15(Z)-16,19-olide (69) | ||||
| (4R,5R,8R,9R,10S,13S,14R)-14-hydroxymanoyloxide-15,17-dien-15(Z)-16,19-olide (70) | ||||
| (4R,5R,8R,9R,10S,13R,16R)-14-hydroxymanoyloxide-17-en-16,19-olide (71) | ||||
| (4R,5R,8R,9R,10S,13R)-manoyloxide-15,17-dien-15(Z)-16,19-olide (72) | ||||
| (14E)-methylmanoyloside-14,16,18-trien-16,19-oxide-23-carboxilate (73) | S. tingitana Etl. | Aerial parts | Antibacterial and ATP modulation activity | [52] |
| Lachnocalyxolide A (74) | S. lachnocalyx Hedge | Aerial parts | Cytotoxic activity | [62] |
| Lachnocalyxolide C (75) | ||||
| Salvidominicolide A (76) | S. dominica L. | Whole parts | - | [64] |
| (13E)-4α,6α,8α-trihydroxy-labd-13(14),17(18)-dien-16,19-olide (77) | S. tingitana Etl. | Aerial parts | Antibacterial and ATP modulation activity | [52] |
| Yosgadensonol (78) | S. yosgadensis Freyn et Bornm. | Aerial parts | - | [69] |
| 13-epi-yosgadensonol (79) | ||||
| 6-dehydroxy-yosgadensonol (80) | S. limbata C. A. Meyer | Aerial parts | - | [70] |
| 6-dehydroxy-13-epi-yosgadensonol (81) | ||||
| (17,18,19,20-tetranor-13-epi-manoyloxide-14-en-16-oic acid-23,6α-olide; 82) | S. sahendica Boiss & Buhse | Aerial parts | - | [63] |
| Dammarane triterpenoids | ||||
| Salvilymitol (83) | S. hierosolymitana Boiss. | Aerial parts | - | [73] |
| Salvilymitone (84) | ||||
| Pixynol (85) | S. barrelieri Ettling | Roots | Weak antioxidant activity | [74,75] |
| (20S,24R)-epoxydammar-12β,25-diol-3-one (86) | S. bicolor | Whole parts | - | [76] |
| Santolin A (87) | S. santolinifolia Boiss. | Whole parts | - | [77] |
| Santolin B (88) | ||||
| Santolin C (89) | ||||
| Amblyol (90) | S. aspera M. et G. | Aerial parts | - | [79] |
| Amblyone (91) | ||||
| Russelliinoside A (92) | S. Russellii Beneth. | Aerial parts | Cytotoxic activity | [80] |
| Russelliinoside B (93) | ||||
| Russelliinoside C (94) | ||||
| Uncommon triterpenoids | ||||
| Salvadione A (95) | S. bucharica M. Pop | Aerial parts | - | [81] |
| Salvadione B (96) | ||||
| Salvadiol (97) | S. bucharica M. Pop | Aerial parts | - | [82] |
| Salvadione C (98) | S. hydrangea DC. ex. Benth. | Aerial parts | Antiparasitic activity | [83] |
| Perovskone B (99) | ||||
| Hydrangenone (100) | S. hydrangea DC. ex. Benth. | Aerial parts | Antiparasitic activity | [84] |
| Salvadione A (95) | S. hydrangea DC. ex. Benth. | Aerial parts | Antiparasitic activity | [85] |
| Hydrangenone B (101) | ||||
| Pervoskones C (102) | ||||
| Pervoskones D (103) | ||||
| Pervoskones E (104) | ||||
| Pervoskones F (105) | ||||
| Salvadione D (106) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafez Ghoran, S.; Taktaz, F.; Mozafari, A.A.; Tunçtürk, M.; Sekeroglu, N.; Kijjoa, A. Uncommon Terpenoids from Salvia Species: Chemistry, Biosynthesis and Biological Activities. Molecules 2022, 27, 1128. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27031128
Hafez Ghoran S, Taktaz F, Mozafari AA, Tunçtürk M, Sekeroglu N, Kijjoa A. Uncommon Terpenoids from Salvia Species: Chemistry, Biosynthesis and Biological Activities. Molecules. 2022; 27(3):1128. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27031128
Chicago/Turabian StyleHafez Ghoran, Salar, Fatemeh Taktaz, Ali Akbar Mozafari, Murat Tunçtürk, Nazim Sekeroglu, and Anake Kijjoa. 2022. "Uncommon Terpenoids from Salvia Species: Chemistry, Biosynthesis and Biological Activities" Molecules 27, no. 3: 1128. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27031128