Magnetic and Luminescence Properties of 8-Coordinate Holmium(III) Complexes Containing 4,4,4-Trifluoro-1-Phenyl- and 1-(Naphthalen-2-yl)-1,3-Butanedionates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Physical Measurements
2.2. Synthesis of the Complexes
2.2.1. [Ho(btfa)3(H2O)2] (1a)
2.2.2. [Ho(ntfa)3(MeOH)2] (1b)
2.2.3. [Ho(btfa)3(L)] (2: L = phen; 3: L = bipy; 4: L = di-tBubipy)
2.2.4. [Ho(ntfa)3(5,5′-Me2bipy)] (5)
2.2.5. [Ho(ntfa)3(bipy)] (6)
2.3. X-Ray Crystal Structure Analysis
3. Results and Discussion
3.1. Synthesis and IR Spectra of the Complexes
3.2. Description of the Crystal Structures 2–6
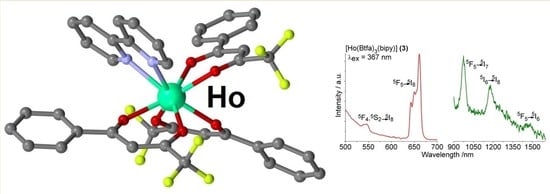
3.3. Photoluminescence of the Complexes
3.4. Magnetic Properties of the Complexes
3.4.1. Ac Magnetic Susceptibility Studies
3.4.2. Dc Magnetic Susceptibility Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bünzli, J.-C.G.; McGill, I.I. Rare Earth Elements. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2018; pp. 1–53. [Google Scholar]
- Bünzli, J.-C.G. Lanthanide Photonics: Shaping the nano world. Trends Chem. 2019, 1, 751–762. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. Lanthanides, Kirk-Othmer Encyclopedia of Chemical Technology; Wiley Online Library: New York, NY, USA, 2013; pp. 1–43. [Google Scholar]
- Cotton, S. Lanthanide and Actinide Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2006. [Google Scholar]
- Harrowfield, J.M.; Silber, H.B.; Paquette, S.J. Metal Ions in Biological Systems; Sigel, A., Sigel, H., Eds.; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Bünzli, J.-C.G. Lanthanide luminescence for biomedical analyses and imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef] [PubMed]
- Brayshaw, L.L.; Smith, R.-C.G.; Badaoui, M.; James, A.; Irving, J.A.; Price, R.S. Lanthanides compete with calcium for binding to cadherins and inhibit cadherin-mediated cell adhesion. Metallomics 2019, 11, 914–924. [Google Scholar] [CrossRef] [Green Version]
- Allen, K.N.; Imperiali, B. Lanthanide-tagged proteins—An illuminating partnership. Curr. Opin. Chem. Biol. 2010, 15, 247–254. [Google Scholar] [CrossRef]
- Pałasz, A.; Segovia, Y.; Skowronek, R.; Worthington, J.J. Molecular neurochemistry of the lanthanides. Synapse 2019, 73, e22119. [Google Scholar] [CrossRef] [PubMed]
- Jastrza, R.; Nowak, M.; Skrobańska, M.; Tolińska, A.; Zabiszak, M.; Gabryel, M.; Marciniak, Ł.; Kaczmarek, M.T. DNA as a target for lanthanide (III) complexes influence. Coord. Chem. Rev. 2019, 382, 145–159. [Google Scholar] [CrossRef]
- Campello, M.P.C.; Palma, E.; Correia, I.; Paulo, P.M.R.; Matos, A.; Rino, J.; Coimbra, J.; Pessoa, J.C.; Gambino, D.; Paulo, A.; et al. Lanthanide complexes with phenanthroline-based ligands: Insights into cell death mechanisms obtained by microscopy techniques. Dalton Trans. 2019, 48, 4611–4624. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.P.; Wang, Z.F.; Tan, M.X.; Huang, X.L.; Zou, H.H.; Zou, B.Q.; Shi, B.B.; Zhang, S.H. Complexes of lanthanides(III) with mixed 2,2′-bipyridyl and 5,7-dibromo-8-quinolinoline chelating ligands as a new class of promising anti-cancer agents. Metallomics 2019, 11, 1005–1015. [Google Scholar] [CrossRef]
- Dos Santos, C.M.G.; Harte, A.J.; Quinn, S.J.; Gunnlaugson, T. Recent developments in the field of supramolecular lanthanide luminescent sensors and self-assemblies. Coord. Chem. Rev. 2008, 252, 2512–2527. [Google Scholar] [CrossRef]
- Staszak, K.; Wieszczycka, K.; Marturano, V.; Tylkowski, B. Lanthanides complexes—Chiral sensing of biomolecules. Coord. Chem. Rev. 2019, 397, 76–90. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bünzli, J.-C.G. Rare earths: Jewels for functional materials of the future. New J. Chem. 2011, 35, 1165–1176. [Google Scholar] [CrossRef] [Green Version]
- Carlos, L.D.; Ferreira, R.A.S.; De Zea Bermudez, V.; Julian-Lopez, B.; Escribano, P. Progress on lanthanide-based organic–inorganic hybrid phosphors. Chem. Soc. Rev. 2011, 40, 536–549. [Google Scholar] [CrossRef] [Green Version]
- Ward, M.D. Mechanisms of sensitization of lanthanide (III)-based luminescence in transition metal/lanthanide and anthracene/lanthanide dyads. Coord. Chem. Rev. 2010, 254, 2634–2642. [Google Scholar] [CrossRef]
- Chen, F.-F.; Chen, Z.-Q.; Bian, Z.-Q.; Huang, C.-H. Sensitized luminescence from lanthanides in d–f bimetallic complexes. Coord. Chem. Rev. 2010, 254, 991–1010. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal–organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Gao, W.; Zhang, X.-M.; Zhou, A.-M.; Liu, J.-P. 3D LnIII-MOFs: Displaying slow magnetic relaxation and highly sensitive luminescence sensing of alkylamines. CrystEngComm 2019, 21, 694–702. [Google Scholar] [CrossRef]
- Greenspon, A.S.; Marceaux, B.L.; Hu, E.L. Robust lanthanide emitters in polyelectrolyte thin films for photonic applications. Nanotechnology 2018, 29, 075302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrester, P.R.; Patthey, F.; Fernandes, E.; Sblendorio, D.P.; Brune, H.; Natterer, F.D. Quantum state manipulation of single atom magnets using the hyperfine interaction. Phys. Rev. B 2019, 100, 180405. [Google Scholar] [CrossRef] [Green Version]
- Coldeway, D. Storing Data in a Single Atom Proved Possible by IBM Researchers. TechCrunch. 9 March 2017. Available online: https://techcrunch.com/2017/03/08/storing-data-in-a-single-atom-proved-possible-by-ibm-researchers/ (accessed on 10 March 2017).
- Hoard, R.W.; Mance, S.C.; Leber, R.L.; Dalder, E.N.; Chaplin, M.R.; Blair, K.; Nelson, D.H.; Van Dyke, D.A. Field enhancement of a 12.5-T magnet using holmium poles. IEEE Trans. Magn. 1985, 21, 448–450. [Google Scholar] [CrossRef] [Green Version]
- Wollin, T.A.; Denstedt, J.D. The holmium laser in urology. J. Clin. Laser Med. Surg. 1998, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.; Lucas, P.; Le Mercier, T.; Rollat, A.; Davenport, W. Rare earth doped lasers and optical amplifiers. In Rare Earths; Elsevier: Amsterdam, The Netherlands, 2015; pp. 319–332. [Google Scholar] [CrossRef]
- Placer, J.; Gelabert-Mas, A.; Vallmanya, F.; Manresa, J.M.; Menéndez, V.; Cortadellas, R.; Arango, O. Holmium laser enucleation of prostate: Outcome and complications of self-taught learning curve. Urology 2009, 73, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Da Rosa, P.P.F.; Kitagawa, Y.; Hasegawa, Y. Luminescent lanthanide complex with seven-coordination geometry. Coord. Chem. Rev. 2020, 406, 213153. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Kitagawa, Y.; Nakanishi, T. Effective photosensitized, electrosensitized, and mechanosensitized luminescence of lanthanide complexes. NPG Asia Mater. 2018, 10, 52–70. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.-F.; Liu, Z.; Ren, P.; Liu, X.-H.; Wang, N.; Cui, H.-J.Z.; Gao., L. A new family of dinuclear lanthanide complexes constructed from 8-hydroxyquinoline Schiff base and β-diketone: Magnetic properties and near-infrared luminescence. Dalton Trans. 2019, 48, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Carlos, L.D.; Ferreira, R.A.S.; De Zea Bermudez, V.; Ribeiro, S.J.L. Lanthanide-containing light-emitting organic–inorganic hybrids: A bet on the future. Adv. Mater. 2009, 21, 509–534. [Google Scholar] [CrossRef]
- Lis, S.; Elbanowski, M.; Makowska, B.; Hnatejko, Z. Energy transfer in solution of lanthanide complexes. J. Photochem. Photobiol. A Chem. 2002, 150, 233–247. [Google Scholar] [CrossRef]
- De Sa, G.F.; Malto, O.L.; De Mello Donega, C.; Simas, A.M.; Longo, R.L.; Santa-Cruz, P.A.; Da Silva, E.R., Jr. Spectroscopic properties and design of highly luminescent lanthanide coordination complexes. Coord. Chem. Rev. 2000, 196, 165–195. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef]
- Su, C.Y.; Kang, B.S.; Liu, H.Q.; Wang, Q.G.; Chen, Z.N.; Lu, Z.L.; Tong, Y.X.; Mak, T.C.W. Luminescent lanthanide complexes with encapsulating polybenzimidazole tripodal ligands. Inorg. Chem. 1999, 38, 1374–1375. [Google Scholar] [CrossRef]
- Alpha, B.; Lehn, J.M.; Mathis, G. Energy transfer luminescence of europium(III) and terbium(III) cryptates of macrobicyclic polypyridine ligands. Angew. Chem. Int. Ed. 1987, 26, 266–267. [Google Scholar] [CrossRef]
- Ziessel, R.; Maestri, M.; Prodi, L.; Balzani, V.; Dorsselaer, A. Dinuclear europium (3+), terbium (3+) and gadolinium (3+) complexes of a branched hexaazacyclooctadecane ligand containing six 2,2′-bipyridine pendant units. Inorg. Chem. 1993, 32, 1237–1241. [Google Scholar] [CrossRef]
- Armelao, L.; Quici, S.; Barigelletti, F.; Accorsi, G.; Bottaio, G.; Cavazzini, M.; Tondello, E. Design of luminescent lanthanide complexes: From molecules to highly efficient photo-emitting materials. Coord. Chem. Rev. 2010, 254, 487–505. [Google Scholar] [CrossRef]
- Binnemans, K. Rare earth β-diketonates. In Gschneider, Design of Luminescent Lanthanide Complexes: From Molecules to Highly Efficient Photo-Emitting Materials; Handbook on the Physics and Chemistry of Rare Earths; Bünzli, J.-C.G., Pecharsky, V.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 35, pp. 107–272. [Google Scholar]
- Hasegawa, Y.; Nakagawa, T.; Kawai, T. Recent progress of luminescent metal complexes with photochromic units. Coord. Chem. Rev. 2010, 254, 2643–2651. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, H.; Fu, L.; Deng, R.; Zhou, L.; Li, H.; Liu, F.; Fu, H. Synthesis, structure and luminescent properties of a new praseodymium(III) complex with β-diketone. Inorg. Chem. Commun. 2003, 6, 852–854. [Google Scholar] [CrossRef]
- Vicente, R.; Tubau, À.; Speed, S.; Mautner, F.A.; Bierbaumer, F.; Fischer, R.C.; Massoud, S.S. Slow magnetic relaxation and luminescence properties in neodymium(III)-4,4,4-trifluoro-1-(2-naphthyl)butane-1,3-dionato complexes incorporating bipyridyl ligands. New J. Chem. 2021, 45, 14713–14723. [Google Scholar] [CrossRef]
- Hyre, A.S.; Doerrer, L.H. A structural and spectroscopic overview of molecular lanthanide complexes with fluorinated O-donor ligands. Coord. Chem. Rev. 2020, 404, 213098. [Google Scholar] [CrossRef]
- Gao, H.-L.; Wang, N.-N.; Wang, W.-M.; Shen, H.-Y.; Zhou, X.-P.; Chang, Y.-X.; Zhang, R.X.; Cui, J.-Z. Fine-tuning the magnetocaloric effect and SMMs behaviors of coplanar RE4 complexes by β-diketonate coligands. Inorg. Chem. Front. 2017, 4, 860–870. [Google Scholar] [CrossRef]
- Chang, Y.-X.; Gao, N.; Wang, M.-Y.; Wang, W.-T.; Fan, Z.-W.; Ren, D.-D.; Wu, Z.-L.; Wang, W.-M. Two phenoxo-O bridged dinuclear Dy(III) complexes exhibiting distinct slow magnetic relaxation induced by different β-diketonate ligands. Inorg. Chim. Acta 2020, 505, 119499. [Google Scholar] [CrossRef]
- Mautner, F.A.; Bierbaumer, F.; Fischer, R.C.; Torvisco, A.; Vicente, R.; Font-Bardía, M.; Tubau, À.; Speed, S.; Massoud, S.S. Diverse coordination numbers and geometries in pyridyl adducts of lanthanide(III) complexes based on β-diketonate. Inorganics 2021, 9, 74. [Google Scholar] [CrossRef]
- Mautner, F.A.; Bierbaumer, F.; Fischer, R.C.; Vicente, R.; Tubau, À.; Ferran, A.; Massoud, S.S. Structural characterization, magnetic and luminescent properties of praseodymium(III)-4,4,4-trifluoro-1-(2-naphthyl)butane-1,3-dionato(1-) complexes. Crystals 2021, 11, 179. [Google Scholar] [CrossRef]
- Mautner, F.A.; Bierbaumer, F.; Gyurkac, M.; Fischer, R.C.; Torvisco, A.; Massoud, S.S.; Vicente, R. Synthesis and characterization of lanthanum(III) complexes containing 4,4,4-trifluoro-1-(2-naphthalen-yl)-butane-1,3-dionate. Polyhedron 2020, 179, 114384. [Google Scholar] [CrossRef]
- Zhang, S.; Ke, H.; Shi, Q.; Zhang, J.; Yang, Q.; Wei, Q.; Xie, G.; Wang, W.; Yang, D.; Chen, S. Dysprosium(iii) complexes with a square-antiprism configuration featuring mononuclear single-molecule magnetic behaviours based on different β-diketonate ligands and auxiliary ligands. Dalton Trans. 2016, 45, 5310–5320. [Google Scholar] [CrossRef]
- Li, D.-P.; Zhang, X.-P.; Wang, T.-W.; Ma, B.-B.; Li, C.-H.; Li, Y.-Z.; You, X.-Z.; You, X.-Z. Distinct magnetic dynamic behavior for two polymorphs of the same Dy(III) complex. Chem. Commun. 2011, 47, 6867–6869. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Deng, R.; Sun, L.; Li, Z.; Zhang, H. Photophysical properties of a series of high luminescent europium complexes with fluorinated ligands. J. Lumin. 2011, 131, 328–335. [Google Scholar] [CrossRef]
- Fernandes, J.A.; Ferreira, R.A.S.; Pillinger, M.; Carlos, L.D.; Jepsen, J.; Hazell, A.; Ribeiro-Claro, P.; Goncalves, I.S. Investigation of europium(III) and gadolinium(III) complexes with naphthoyltrifluoroacetone and bidentate heterocyclic amines. J. Lumin. 2005, 113, 50–63. [Google Scholar] [CrossRef]
- Thompson, L.C.; Atchison, F.W.; Young, V.G. Isomerism in the adduct of tris(4, 4,4,-trifluoro-1-(2-naphthyl)-1,3-butanedionato) europium(III) with dipyridyl. J. Alloys Compd. 1998, 275, 765–768. [Google Scholar] [CrossRef]
- Trieu, T.-N.; Dinh, T.-H.; Nguyen, H.-H.; Abram, U.; Nguyen, M.-H. Novel lanthanoide(III) ternary complexes with naphtoyltrifluoroacetone: A synthetic and spectroscopic study. Z. Anorg. Allg. Chem. 2015, 641, 1934–1940. [Google Scholar] [CrossRef]
- Taydakov, I.V.; Akkuzina, A.; Avetisov, R.I.; Khomyakov, A.V.; Saifutarov, R.R.; Avetissov, I.C. Effective electroluminescent materials for OLED applications based on lanthanide 1,3-diketonates bearing pyrazole moiety. J. Lumin. 2016, 177, 31–39. [Google Scholar] [CrossRef]
- Maggini, I.; Traboulsi, H.; Yoosaf, K.; Mohanraj, J.; Wouters, J.; Pietraszkiewicz, O.; Pietraszkiewicz, M.; Armaroli, N.; Bonifazi, D. Electrostatically-driven assembly of MWCNTs with a europium complex. Chem. Commun. 2011, 47, 1625–1627. [Google Scholar] [CrossRef] [PubMed]
- Lunstroot, L.; Nockemann, P.; Van Hecke, K.; Van Meervelt, L.; Gorller-Walrand, C.; Binnemans, K.; Driesen, K. Visible and near-infrared emission by samarium (III)-containing ionic liquid mixtures. Inorg. Chem. 2009, 48, 3018–3026. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.M.; Ferreira, R.A.S.; Paz, F.A.A.; Carlos, L.D.; Pillinger, M.; Ribeiro-Claro, P.; Goncalves, I.S. Structural and photoluminescence studies of a europium(III) tetrakis(β-diketonate) complex with tetrabutylammonium, imidazolium, pyridinium and silica-supported imidazolium counterions. Inorg. Chem. 2009, 48, 4882–4895. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.-R.; Sun, W.-B.; Li, H.-F.; Chen, P.; Tian, Y.-M.; Zhang, W.-Y.; Zhang, Y.-Q.; Yan, P.-F. Complementation and joint contribution of appropriate intramolecular coupling and local ion symmetry to improve magnetic relaxation in a series of dinuclear Dy2 single-molecule magnets. Inorg. Chem. Front. 2017, 4, 499–508. [Google Scholar] [CrossRef]
- Martin-Ramos, P.; Coya, C.; Alvarez, A.L.; Ramos-Silva, M.; Zaldo, C.; Paixao, J.A.; Chamorro-Posada, P.; Martin-Gil, J. Charge transport and sensitized 1.5 μm electroluminescence properties of full solution-processed NIR-OLED based on novel Er(III) fluorinated β-diketonate ternary complex. J. Phys. Chem. C 2013, 117, 10020–10030. [Google Scholar] [CrossRef]
- Dasari, S.; Singh, S.; Sivakumar, S.; Patra, A.K. Dual-sensitized luminescent europium(III) and terbium(III) complexes as bioimaging and light-responsive therapeutic agents. Chem. Eur. J. 2016, 22, 7387–17396. [Google Scholar] [CrossRef]
- Bruno, S.M.; Ananias, D.; Paz, F.A.A.; Pillinger, M.; Valente, A.A.; Carlos, L.D.; Goncalves, I.S. Crystal structure and temperature-dependent luminescence of a heterotetranuclear sodium–europium(III) β-diketonate complex. Dalton Trans. 2015, 44, 488–492. [Google Scholar] [CrossRef]
- Fernandes, J.A.; Braga, S.S.; Pillinger, M.; Ferreira, R.A.S.; Carlos, L.D.; Hazell, A.; Ribeiro-Claro, P.; Goncalves, I.S. β-Cyclodextrin inclusion of europium(III) tris(β-diketonate)-bipyridine. Polyhedron 2006, 25, 1471–1476. [Google Scholar] [CrossRef]
- Shen, F.; Hu, J.; Xie, M.; Wang, S.; Huang, X. Synthesis and structural investigation of lanthanide organometallics involving cyclopentadienyl and 2-napthoyltrifluoroacetonato chelate ligands Synthesis and structural investigation of lanthanide organometallics involving cyclopentadienyl and 2-napthoyl-trifluoroacetonato chelate ligands. J. Organomet. Chem. 1995, 485, C6–C9. [Google Scholar]
- Shen, F.; Hu, J.; Xie, M.; Wang, S.; Huang, X. Synthesis and structural study of cyclopentadienyl lanthanide derivatives containing the 2-naphthoyltrifluoro-acetonato ligand. Polyhedron 1996, 15, 1151–1155. [Google Scholar] [CrossRef]
- Bruker. APEX, SAINT v. 8.37A; Bruker AXS, Inc.: Madison, WI, USA, 2015. [Google Scholar]
- Sheldrick, G.M. SADABS v. 2; University of Goettingen: Goettingen, Germany, 2001. [Google Scholar]
- Sheldrick, G.M. A Short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, T.; Van de Streek, J.J. Mercury: Visualization and analysis of crystal structures. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L. PLATON, a Multipurpose Crystallographic Tool; Utrecht University: Utrecht, The Netherlands, 1999. [Google Scholar]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape maps and polyhedral interconversion paths in transition metal chemistry. Chem. Soc. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Cirera, J.; Alvarez, S. Stereospinomers of pentacoordinate iron porphyrin complexes: The case of the [Fe(porphyrinato)(CN)]− anions. Dalton Trans. 2013, 42, 7002–7008. [Google Scholar] [CrossRef]
- Boyer, J.C.; Vetrone, F.; Capobianco, J.A.; Speghini, A.; Zambelli, M.; Bettinelli, M. Investigation of the upconversion processes in nanocrystalline Gd3Ga5O12:Ho3+. J. Lumin. 2004, 106, 263–268. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, J.; Jiang, J.; Pan, R.; Zhang, B. Microstructure and photoluminescence properties of Ho-doped (Ba,Sr)TiO3 thin films. Thin Solid Films 2007, 515, 7721–7725. [Google Scholar] [CrossRef]
- Lim, C.S.; Aleksandrovsky, A.; Molokeev, M.; Oreshonkov, A.; Atuchin, V. The modulated structure and frequency upconversion properties of CaLa2 (MoO4)4: Ho3+/Yb3+ phosphors prepared by microwave synthesis. Phys. Chem. Chem. Phys. 2015, 17, 19278–19287. [Google Scholar] [CrossRef]
- Susumu, S.; Masanobu, W. Relations between intramolecular energy transfer efficiencies and triplet state energies in rare earth β-diketone chelates. Bull. Chem. Soc. Jpn. 1970, 43, 1955–1962. [Google Scholar]
- Latva, M.; Takalo, H.; Mukkala, V.-M.; Matachescu, C.; Rodríguez-Ubis, J.C.; Kankare, J. Luminescent Lanthanoid Calixarene Complexes and Materials. J. Lumin. 1997, 75, 149–169. [Google Scholar] [CrossRef]
- Quici, S.; Cavazzini, M.; Marzanni, G.; Accorsi, G.; Armaroli, N.; Ventura, B.; Barigelletti, F. Visible and near-infrared intense luminescence from water-soluble lanthanide [Tb(III), Eu(III), Sm(III), Dy(III), Pr(III), Ho(III), Yb(III), Nd(III), Er(III)] complexes. Inorg. Chem. 2005, 44, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Komissar, D.A.; Metlin, M.T.; Ambrozevich, S.A.; Taydakov, I.V.; Tobokhova, A.S.; Varaksina, E.A.; Selyukov, A.S. Luminescence properties of pyrazolic 1,3-diketone Ho3+ complex with 1,10-phenanthroline. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 222, 117229–117238. [Google Scholar] [CrossRef]
- Dang, S.; Yu, J.; Yu, J.; Wang, X.; Sun, L.; Feng, J.; Fan, W.; Zhang, H. Novel holmium (Ho) and praseodymium (Pr) ternary complexes with fluorinated-ligand and 4,5-diazafluoren-9-one. Mater. Lett. 2011, 65, 1642–1644. [Google Scholar] [CrossRef]
- Dang, S.; Sun, L.-N.; Song, S.-Y.; Zhang, H.-J.; Zheng, G.-L.; Bi, Y.-F.; Guo, H.-D.; Guo, Z.-Y.; Feng, J. Syntheses, crystal structures and near-infrared luminescent properties of holmium (Ho) and praseodymium (Pr) ternary complexes. Inorg. Chem. Commun. 2008, 11, 531–534. [Google Scholar] [CrossRef]
- Coban, M.B.; Amjad, A.; Aygun, M.; Kara, H. Sensitization of HoIII and SmIII luminescence by efficient energy transfer from antenna ligands: Magnetic, visible and NIR photoluminescence properties of GdIII, HoIII and SmIII coordination polymers. Inorg. Chim. Acta 2017, 455, 25–33. [Google Scholar]
- Ahmed, Z. Iftikhar, K. Sensitization of visible and NIR emitting lanthanide(III) ions in noncentrosymmetric complexes of hexafluoroacetylacetone and unsubstituted monodentate pyrazole. Phys. Chem. A 2013, 117, 11183–11201. [Google Scholar] [CrossRef] [PubMed]
- Atwood, D.A. (Ed.) The Rare Earth Elements: Fundamentals and Applications; John Wiley & Sons Ltd.: Chichester, UK, 2005. [Google Scholar]











| Compound | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|
| Empirical formula | C42H26F9HoN2O6 | C40H26F9HoN2O6 | C48H42F9HoN2O6 | C54H36F9HoN2O6 | C52H32F9HoN2O6 |
| Formula mass | 990.58 | 966.56 | 1078.76 | 1144.78 | 1116.73 |
| System | Monoclinic | Monoclinic | Triclinic | Orthorhombic | Orthorhombic |
| Space group | P21/c | P21/n | P-1 | Pca21 | Pna21 |
| a (Å) | 9.6058(7) | 11.0408(10) | 12.3569(16) | 20.2138(9) | 20.7013(6) |
| b (Å) | 36.627(2) | 22.6440(18) | 13.6076(18) | 11.7503(5) | 10.9059(3) |
| c (Å) | 10.7464(7) | 15.2463(13) | 14.3853(18) | 19.5852(7) | 42.3027(10) |
| α (°) | 90 | 90 | 92.478(5) | 90 | 90 |
| β (°) | 92.932(3) | 101.972(3) | 99.883(5) | 90 | 90 |
| γ (°) | 90 | 90 | 105.233(5) | 90 | 90 |
| V (Å3) | 3776.0(4) | 3728.8(6) | 2289.3(5) | 4651.8(3) | 9550.5(4) |
| Z | 4 | 4 | 2 | 4 | 8 |
| μ (mm−1) | 2.192 | 2.218 | 1.815 | 1.792 | 1.744 |
| Dcalc (Mg/m3) | 1.742 | 1.722 | 1.565 | 1.635 | 1.553 |
| θ max (°) | 26.420 | 34.495 | 27.171 | 28.998 | 28.000 |
| Data collected | 91277 | 103169 | 50752 | 93718 | 264468 |
| Unique refl./Rint | 7737/0.0836 | 15617/0.0776 | 10082/0.0390 | 12315/0.0812 | 23060/0.0515 |
| Parameters/Restraints | 542/0 | 523/0 | 601/0 | 651/1 | 1262/19 |
| Goodness-of-fit on F2 | 1.120 | 1.050 | 1.131 | 1.012 | 1.165 |
| R1/wR2 (all data) | 0.0615/0.1342 | 0.0466/0.0809 | 0.0445/0.1042 | 0.0374/0.0632 | 0.0466/0.1060 |
| Compound 2 | Compound 3 | Compound 4 | |||
| Ho1-O1 | 2.3111(2) | Ho1-O1 | 2.315(2) | Ho54-O1 | 2.292(3) |
| Ho1-O2 | 2.3051(2) | Ho1-O2 | 2.343(2) | Ho54-O2 | 2.331(3) |
| Ho1-O3 | 2.3064(2) | Ho1-O3 | 2.297(2) | Ho54-O3 | 2.323(3) |
| Ho1-O4 | 2.3139(2) | Ho1-O4 | 2.326(2) | Ho54-O4 | 2.320(3) |
| Ho1-O5 | 2.3647(2) | Ho1-O5 | 2.287(2) | Ho54-O47 | 2.305(3) |
| Ho1-O6 | 2.3225(2) | Ho1-O6 | 2.330(2) | Ho54-O48 | 2.323(3) |
| Ho1-N1 | 2.5477(2) | Ho1-N1 | 2.524(2) | Ho54-N52 | 2.535(4) |
| Ho1-N2 | 2.5549(2) | Ho1-N2 | 2.527(3) | Ho54-N53 | 2.541(4) |
| O1-Ho1-O2 | 72.59(1) | O1-Ho1-O2 | 72.58(7) | O1-Ho54-O2 | 73.50(11) |
| O3-Ho1-O5 | 72.74(1) | O3-Ho1-O4 | 72.69(3) | O3-Ho54-O4 | 73.70(13) |
| O4-Ho1-O6 | 76.21(1) | O5-Ho1-O6 | 73.61(8) | O47-Ho54-O48 | 72.77(11) |
| N1-Ho1-N2 | 64.66(1) | N1-Ho1-N2 | 63.89(8) | N52-Ho54-N53 | 63.80(13) |
| Compound 5 | Compound 6 | ||||
| Ho1-O1 | 2.336(4) | Ho1-O1 | 2.273(6) | Ho2-O7 | 2.342(6) |
| Ho1-O2 | 2.293(4) | Ho1-O2 | 2.323(6) | Ho2-O8 | 2.285(6) |
| Ho1-O3 | 2.321(4) | Ho1-O3 | 2.362(6) | Ho2-O9 | 2.283(6) |
| Ho1-O4 | 2.311(4) | Ho1-O4 | 2.268(6) | Ho2-O10 | 2.319(6) |
| Ho1-O5 | 2.328(4) | Ho1-O5 | 2.320(6) | Ho2-O11 | 2.337(6) |
| Ho1-O6 | 2.313(4) | Ho1-O6 | 2.318(6) | Ho2-O12 | 2.341(6) |
| Ho1-N1 | 2.515(5) | Ho1-N1 | 2.495(7) | Ho2-N3 | 2.511(7) |
| Ho1-N2 | 2.518(4) | Ho1-N2 | 2.530(7) | Ho2-N4 | 2.516(7) |
| O1-Ho1-O2 | 71.77(13) | O1-Ho1-O2 | 73.8(2) | O7-Ho2-O8 | 72.1(2) |
| O3-Ho1-O4 | 72.95(12) | O3-Ho1-O4 | 71.5(2) | O9-Ho2-O10 | 73.5(2) |
| O5-Ho1-O6 | 72.37(13) | O5-Ho1-O6 | 72.7(2) | O11-Ho2-O12 | 72.0(2) |
| N1-Ho1-N2 | 64.38(16) | N1-Ho1-N2 | 64.5(3) | N3-Ho2-N4 | 64.6(2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mautner, F.A.; Bierbaumer, F.; Vicente, R.; Speed, S.; Tubau, Á.; Font-Bardía, M.; Fischer, R.C.; Massoud, S.S. Magnetic and Luminescence Properties of 8-Coordinate Holmium(III) Complexes Containing 4,4,4-Trifluoro-1-Phenyl- and 1-(Naphthalen-2-yl)-1,3-Butanedionates. Molecules 2022, 27, 1129. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27031129
Mautner FA, Bierbaumer F, Vicente R, Speed S, Tubau Á, Font-Bardía M, Fischer RC, Massoud SS. Magnetic and Luminescence Properties of 8-Coordinate Holmium(III) Complexes Containing 4,4,4-Trifluoro-1-Phenyl- and 1-(Naphthalen-2-yl)-1,3-Butanedionates. Molecules. 2022; 27(3):1129. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27031129
Chicago/Turabian StyleMautner, Franz A., Florian Bierbaumer, Ramon Vicente, Saskia Speed, Ánnia Tubau, Mercè Font-Bardía, Roland C. Fischer, and Salah S. Massoud. 2022. "Magnetic and Luminescence Properties of 8-Coordinate Holmium(III) Complexes Containing 4,4,4-Trifluoro-1-Phenyl- and 1-(Naphthalen-2-yl)-1,3-Butanedionates" Molecules 27, no. 3: 1129. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27031129