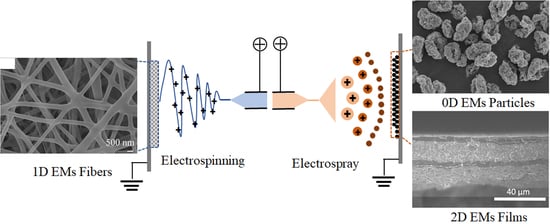
Progress in Electrohydrodynamic Atomization Preparation of Energetic Materials with Controlled Microstructures
Abstract
:1. Introduction
2. Principles of Electrohydrodynamic Atomization
2.1. Process of Electrohydrodynamic Atomization
2.1.1. Electrospray
2.1.2. Electrospinning
2.2. Transition between Electrospinning and Electrospray
2.3. Morphology

3. Particles (0D EMs)
3.1. Recrystallization and Cocrystallization of Organic High Explosives

| Authors | Energetic Materials | Solvent | Operation Parameters Needle Diameter; Flow Rate; Distance; Applied Voltage | Feature |
|---|---|---|---|---|
| Radacsi [4] | RDX 20.8 mg/mL | DMK | 0.15~0.58 mm; 1~5 mL/h; 10~35 cm; 3.8~4.8 kV | 200 nm~600 nm |
| Radacsi [29] | RDX HMX | 200~600 nm RDX spheres; 200~500 nm HMX spheres; 1 μm HMX donut particles | ||
| Reus [25] | TNT 42~840 mg/mL RDX 54~60 mg/mL | DMK | 0.61 mm; 0.5~1.5 mL/h; 3~7 cm; −3.5~−7 kV | submicron RDX (core)/TNT (shell) |
| Huang [26] | LLM-105 0.7%wt | DMF+ NMP (v/v = 6/1) | 19G~27G; 0.025~0.075 mm/min; 25 cm; −15 kV to 7~9 kV. | 200~500 nm spheres stacked with 50 nm nanoparticles |
| Huang [28] | CL-20/TNT, CL-20/DNB CL-20/TNB 100 mg/mL | DMK; EAC; MEK; BAC | 27G; 0.05 mm/min; 20 cm; 5~8 kV to −10 kV. | 1~2 μm CL-20/TNT partial cocrystal, 100~500 nm CL-20/DNB cocrystal, 200~600 nm CL-20/TNB cocrystal |
| Yan [27] | CL-20 20 mg/mL | EAC; DMK | 0.21~0.86 mm; /; 5–12 cm; 4~8 kV; | ~2.8 μm hollow sphere (ethyl acetate); 320~610 nm nanoparticles (acetone) |
3.2. Assembled Particles of Composite Energetic Materials

| Authors | Energetic Materials | Solvent | Operation Parameters Needle Diameter; Flow Rate; Distance; Applied Voltage | Size |
|---|---|---|---|---|
| Wang [33] | Al/NC 173 mg/mL | NC 17 mg/mL; EA + DEE (v/v = 3:1) | coaxial needle 17G/22G; 0.5 mL/h; 10 cm; 19 kV | 2~16 μm |
| Yang [38] | Al/PVDF 100 mg/mL | PVDF 15 mg/mL; DMK/DMF (v/v = 2:1) | 0.51 mm; 3 mL/h; 10 cm; 18 kV | 1~5 μm spheres |
| Yan [39] | Al/NC Al/GAP | NC; GAP 5wt%; DMK + EAC (v/v = 4:1) | 0.8 mm; 0.5~1.0 mL/h; 10~15 cm; 24 kV | 1~6 μm |
| Cheng [36] | Al/B/PVDF 100 mg/mL | PVDF 10 mg/mL; DMK + DMF (v/v = 5:1) | 0.51 mm; 1.5 mL/h; 10 cm; 18 kV | 1~5 μm spheres |
| Wang [31] | Al/AP/NC | NC DMK + MT+ EA+ DEE (v/v = 2:10:3:1) | 0.20 mm; 0.2~1.0 mL/h; 2.5 cm; 18 kV | 0.2~4 μm |
| Zuo [37] | AP/Si/NC | NC; DMK + DMF (v/v = 4:1) | 22G; /; 9 cm; 18 kV to −2 kV | ~10 μm spheres |
| Yao [51] | RDX/polymer 14.3~20 mg/mL | PVAc, PVB, F2604, DOS, 0.7~1.0 mg/mL; EAC; DMK | /; 1.0 mL/h; 10 cm; 19 kV | 1~4 μm spheres |
| Han [52] | RDX+CeO 40.8 mg/mL | DMK | /; 4.5 mL/h; 10 cm; 19 kV | 2 μm spheres |
| Wang [33] | Al/CuO/NC 210 mg/mL | NC~21 mg/mL; EA + DEE (v/v = 3:1) | 0.43 mm; 4.5 mL/h; 10 cm; 10 kV to -9 kV | 2~16 μm |
| Zhao [53] | Al/Ti/I2O5/NC 100 mg/mL | NC 5 mg/mL; EA + DEE (v/v = 3:1) | /; 2.0 mL/h; 15 cm; 20 kV | 5~10 μm |
| Wang [43] | Al/NC/Bi(IO3)3; Al/NC/Cu(IO3)2; Al/NC/Fe(IO3)3 116 mg/mL | NC 6 mg/mL; EA+ DEE(v/v = 19:1) | 0.43 mm; 4.5 mL/h; 10 cm; 8 kV | 3~5 μm; 2~4 μm; 5~7 μm |
| Dai [50] | Al/Bi2O3/NC 133 mg/mL | NC 1.3~13.3 mg/mL; EA + DEE (v/v = 3.5:1) | /; 3.0 mL/h; 10 cm; 18 kV | |
| Song [54] | Al/MnO2/co(PVDF-HFP) | co(PVDF-HFP); EA+ DMF | 0.43 mm; 4.0 mL/h; 10 cm; 14 kV | |
| Song [55] | Al/MnO2 25 mg/mL Al/MnO2/KClO4~30 mg/mL | EA + DI (v/v = 3:1) | 0.43 mm; 4.0 mL/h; 15 cm; 13 kV | |
| Chen [56] | Al/MoO3/PVDF | PVDF; DMF + CYH | 0.42 mm; 4.0 mL/h; 10 cm; 13.5 kV | evenly distribution of Al/MoO3/ PVDF |
| Mei [57] | Al/Mn(IO3)2/NC 95 mg/mL | NC 4.5 mg/mL; EA + DEE (v/v = 3:1) | 0.43 mm; 2.0 mL/h; 10 cm; 19 kV | 2~4 μm |
| Yi [47] | Al/CuSO4·5H2O/NC | NC 4 wt %; IPA | /; 4.5 mL/h; 10 cm; 19 kV | CuSO4·5H2O(1 μm) covered with nano-Al |
| Ghildiyal [40] | Al/Si/Ca(IO3)2/PVDF | PVDF 16.7 mg/mL; DMK + DMF (v/v = 3:1) | 0.43 mm; 2.0 mL/h; 10 cm; 19 kV | 3~5 μm |
| Huang [49] | Al/CL-20/NC; Al/CL-20/F2314 102.5 mg/mL | NC; F2314 2.5 mg/mL; EAC | 19G; 0.25 mm/min; 20 cm; 6.5 kV to −10 kV | 8~16 μm(NC) 8~18 μm(F2314) |
| Yan [58] | Al/Viton/RDX | Viton; DMF + EAC (v/v = 10:3) | coaxial needle 1.45 mm/ 0.57 mm; 0.4~0.5 mL/h; 15 cm; 15.5 kV | 450~750 nm hollow spheres |
| Yan [59] | Al/NC(shell)/RDX(core) | NC 5~15 wt%; DMK + EA; DMK+ EAC | coaxial needle 1.45 mm / 0.57 mm; 1.0 mL/h; 10~15 cm; 12~26 kV | 500~2000 nm |
| Yang [60] | Al/Fe2O3/RDX/NC 115~125 mg/mL | NC 5.0 mg/mL; DMK | 0.8 mm; 3.0 mL/h; 6 cm; 18 kV | |
| Chen [61] | Al/CuO/NC/CL-20 125 mg/mL | NC 6.3 mg/mL; DMK, EAC, EA + DEE, NPA + DEE | 0.43 mm; 1.75 mL/h; 15 cm; 17 kV to −3 kV | 3~6 μm clay-like or granular particles |
| Xiao [41] | Al/CuO/PVDF/RDX, 200 mg/mL | PVDF 10 mg/mL; DMK + DMF(v/v = 4:1) | 23G; 0.14 mm/min; 10 cm; 19 kV | 2~4 μm |
4. Energetic Fibers (1D EMs)

| Authors | Energetic Materials | Binders and Solvents | Operation Parameters Needle Diameter; Flow Rate; Distance; Applied Voltage | Average Fiber Diameter |
|---|---|---|---|---|
| Xie [77] | Al/NC 6~12 wt% | NC 5~10 wt%; DI + DMK (wt/wt = 1/10) | 0.50 mm; 4.0 mL/h; 20 cm; 28~35 kV | 83~98 nm |
| Xie [70] | CuCl2/NC 12 wt% | NC 10 wt%; DI + DMK (wt/wt = 1/10) | /; /; 20 cm; 25 kV | CuCl2/NC 300 nm CuO 100 nm |
| Xu [64] | RDX/NC 200 mg/mL | NC 100 mg/mL; DMK + DMF (v/v = 2:1) | 0.56 mm; 1.8 mL/h; 25 cm; 27 kV | 120 ± 20 nm |
| Clayton [76] | Al/PFPE/PS | PS 30 wt%; DMF | 17G~27G; 0.5~1.25 mL/h; 7.6~10 cm; 12~15 kV | 1.1~5.4 μm |
| Li [78] | B/NC 9 wt% | NC 7.5 wt %; DI + DMK (wt/wt = 1/20) | 0.80 mm; /; /; 20 kV | 200~520 nm |
| Yan [75] | Al/CuO/NC | NC; EA + DEE | 0.80 mm; 4.5 mL/h; 6 cm; 18 kV | 0.3~1.0 μm |
| Lyu [66] | Al/CuO/PVDF/GO ~200 mg/mL | PVP 140 mg/mL; DMK + DMF (v/v = 3:7) | 0.60 mm; 0.07 mm/min; 15 cm; 0.65 kV/cm | 200 nm~4 μm |
| Zhang [68] | Si/PVDF 150 mg/mL | PVDF DMK + DMF (v/v = 1:1) | /; /; 10 cm; 14 kV | 200~300 nm |
| Li [79] | Cu(OCH3CO2)2/Al/ PVP 164 mg/mL | PVP 88 mg/mL DMF + EA (v/v = 5:1) | 0.31 mm; 0.6 mL/h; 18 cm; +13 kV/−3 kV | ~150 nm |
| Li [65] | Al/Fe2O3/NC | NC: 10 wt%; DMK + DMF (v/v = 2:1) | 0.90 mm; 8.0 mL/h; 20 cm; 25 kV | 320 nm |
| Wang [69] | Fe(NO3)3·9H2O/Al/ PVP 231.5 mg/mL Fe2O3/Al/PVP 168.4 mg/mL | PVP 105 mg/mL; DMF + IPA (v/v = 1.3:1) | /; /; 15 cm; 15 kV | ~1 μm |
| Wang [80] | Al/NC/RDX | NC 10% wt; EA + DMK (v/v = 1:1) | /; 0.02 mm/s; 22 cm; +18 kV/−2 kV | 1 μm |
| Pourmortazavi [81] | Al/Fe2O3/NC/DAF | NC MEK | 0.90 mm; 15.0 mL/h; 10~20 cm; 18 kV | 80~232 nm |
| Luo [62] | NC/GAP/LLM-105 12 wt% | GAP + NC 80.2 mg/mL; DMK | 0.80 mm; 3~5 mL/h; 12 cm; 12~18 kV | 758 nm. |
| Luo [82] | NC/GAP/TATB: 12 wt% | NC/GAP 9 wt% DMK | 0.80mm; 4.0~6.0 mL/h; 12cm; 12~18kV | 1036 nm |
| Song [63] | F2602/GAP/CL-20 20 wt% | F2602 + GAP 2~6 wt%; DMK | /; 5 mL/h; 12 cm; 10~20 kV | 377~481 nm |
| Wang [83] | NC/GAP/HNS 12 wt% | NC/GAP 9 wt% DMK | 0.80 mm; 3.0~5.0 mL/h; 12 cm; 12~18 kV | 1074 nm |
| Wang [67] | PVDF(shell)/Al/GAP/NC 120 mg/mL(shell) | GAP/NC; DMF/THF | coaxial needle 17G/ 22G; 0.6 mL/h (shell) + 0.06 mL/h (core); 18 cm; +15 kV/−2 kV | 578 nm |
| Yan [72] | Lead Acetate/PVA 20 wt% | PVA 20 wt%; DI + AA (v/v = 6:1) | 0.40 mm; 1.0 mL/h; /; /; | ~1 μm |
| Wang [71] | Cu-MOF(HKUST)/PAN | PAN; DMF | 0.60 mm; 1.0 mL/h; /; /; | |
| Li [74] | PAN | PAN 87 mg/mL; DMF | /; 4.0 mL/h; 15 cm; 25 kV | 500 nm |
5. Energetic Films (2D EMs)

| Authors | Energetic Materials | Binders and Solvents | Operation Parameters Needle Diameter; Flow Rate; Distance; Applied Voltage | Film Thickness | Combustion Speed |
|---|---|---|---|---|---|
| Huang [84] | Al/PVDF 100 mg/mL | PVDF 50~83.3 mg/mL DMF | 0.43 mm; 5 cm; 1.5 mL/h; +10 kV (nozzle)/−10 kV (substrate) | 170 μm | 23 cm/s (air) 11 cm/s (argon) |
| Li [88] | Al/CuO/PVDF 206.5 mg/mL | PVDF DMF | 0.023 mm; 6 cm; 2.0 mL/h; 2~3 kV/cm. | Laminated ~111 μm | 16.7 cm/s (argon) |
| Li [89] | Al/CuO/PVDF (film) PVDF (fiber) | PVDF 7.7~10.4 wt% DMF (film) DMF + DMK (fiber) | 0.023 mm; 6 cm (fiber), 10 cm (film); 0.5~1.5 mL/h; 2~3 kV/cm | Fiber reinforced film | ~12 cm/s (argon) |
| Hu [90] | AgIO3/CB /NC | NC DMF | 0.43 mm; 4.5 cm; 2.0 mL/h; 18 kV | 65 μm | 4.5 cm/s (air) |
| Hu [86] | Al/Bi(IO3)3/ PVDF 113.4mg/mL | PVDF 50 mg/mL DMF | 0.43 mm; 4.5 cm; 2.0 mL/h; 18 kV | 23 cm/s (air) 5.5 cm/s (argon) | |
| DeLisio [85] | Al/PVDF | PVDF 50 mg/mL DMF | 0.43 mm; 4.0 cm; 2.0 mL/h; 18 kV | 50~100 μm | 5.5 cm/s (argon) |
| Wang [87] | Al/PVDF+ Al/PVDF/I2 67.4 mg/mL + 404.4 mg/mL | PVDF 50 mg/mL DMF | 0.43 mm; 2 cm; 2.0 mL/h; 3.3~5.0 kV/cm | Laminated film 32~124 μm | ~35 cm/s (argon) |
| Wang [91] | Al/PVDF/SiO2 ~71 mg/mL | PVDF 50 mg/mL DMF | 20~124 μm | ~11 cm/s (argon) | |
| Wang [92] (electrospray) | Al/PVDF 152 mg/mL Al/AP/PVDF 193 mg/mL | PVDF 90 mg/mL DMF | / | ~600 μm ~310 μm | 25 cm/s (air/argon), 5 cm/s (water); 9 cm/s (argon) |
6. Perspectives
6.1. Potential 3D Structures
6.2. Development Challenges
6.2.1. Mass and Continuous Production
6.2.2. Processing Safety
6.2.3. Binder
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | acetic acid |
| AP | ammonium perchlorate |
| BAC | n-butyl acetate |
| CYH | cyclohexane |
| CL-20 | 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane |
| DAF | 3,4-diaminofurazan |
| DEE | diethyl ether |
| DI | distilled water |
| DMF | N, N-dimethylformamide |
| DMK | acetone |
| DNB | 1,3-dinitrobenzene |
| DOC | dioctyl sebacate |
| EA | ethanol |
| EAC | ethyl acetate |
| F2314 | one of fluoropolymer |
| F2602 | one of fluororubber |
| F2604 | copolymer of vinylidene fluoride and hexafluropropylene |
| GAP | glycidyl azidepolymer |
| HMX | octogen |
| HNS | 2,2′,4,4′,6,6′-Hexanitrostilbene |
| IPA | isopropanol |
| LLM-105 | 2,6-diamino-3,5-dinitropyrazine-1-oxide |
| MEK | methyl ethyl ketone |
| MT | methanol |
| MOF | metal–organic framework |
| NC | nitrocellulose |
| NMP | N-methyl pyrrolidone |
| NPA | n-Propyl Alcohol (1-Propanol) |
| PAN | polyacrylonitrile |
| PFPE | perfluoropolyether |
| PS | polystyrene |
| PTFE/Teflon | polytetrafluoroethylene |
| PVAc | polyvinyl acetate |
| PVB | polyvinyl butyral |
| PVP | polyvinyl pyridine |
| PVDF | polyvinylidene fluoride |
| RDX | hexogen |
| TATB | tramino-trinitrobenzene |
| TNB | 1,3,5-trinitrobenzene |
| TNT | 2,4,6-trinitrotoluene |
| Viton | dipolymers of hexafluoropropylene and vinylidene fluoride |
References
- Ma, X.; Li, Y.; Hussain, I.; Shen, R.; Yang, G.; Zhang, K. Core–Shell Structured Nanoenergetic Materials: Preparation and Fundamental Properties. Adv. Mater. 2020, 32, 2001291. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Torabi, M.; Lu, J.; Shen, R.; Zhang, K. Nanostructured Energetic Composites: Synthesis, Ignition/Combustion Modeling, and Applications. ACS Appl. Mater. Interfaces 2014, 6, 3058–3074. [Google Scholar] [CrossRef] [PubMed]
- Sovizi, M.R.; Hajimirsadeghi, S.S.; Naderizadeh, B. Effect of particle size on thermal decomposition of nitrocellulose. J. Hazard. Mater. 2009, 168, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Radacsi, N.; Stankiewicz, A.I.; Creyghton, Y.L.M.; van der Heijden, A.E.D.M.; ter Horst, J.H. Electrospray Crystallization for High-Quality Submicron-Sized Crystals. Chem. Eng. Technol. 2011, 34, 624–630. [Google Scholar] [CrossRef]
- He, W.; Liu, P.-J.; He, G.-Q.; Gozin, M.; Yan, Q.-L. Highly Reactive Metastable Intermixed Composites (MICs): Preparation and Characterization. Adv. Mater. 2018, 30, 1706293. [Google Scholar] [CrossRef]
- Senthil Muthu Kumar, T.; Senthil Kumar, K.; Rajini, N.; Siengchin, S.; Ayrilmis, N.; Varada Rajulu, A. A comprehensive review of electrospun nanofibers: Food and packaging perspective. Compos. Part B Eng. 2019, 175, 107074. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Jaworek, A. Electrospray droplet sources for thin film deposition. J. Mater. Sci. 2007, 42, 266–297. [Google Scholar] [CrossRef]
- Jaworek, A.; Sobczyk, A. Electrospraying route to nanotechnology: An overview. J. Electrost. 2008, 66, 197–219. [Google Scholar] [CrossRef]
- Kavadiya, S.; Biswas, P. Electrospray deposition of biomolecules: Applications, challenges, and recommendations. J. Aerosol Sci. 2018, 125, 182–207. [Google Scholar] [CrossRef]
- Kelder, E.M.; Marijnissen, J.C.M.; Karuga, S.W. EDHA for energy production, storage and conversion devices. J. Aerosol Sci. 2018, 125, 119–147. [Google Scholar] [CrossRef]
- Wang, J.; Xu, H.; Huo, Y.; Wang, Y.; Dong, M. Progress of electrospray and electrospinning in energy applications. Nanotechnology 2020, 31, 132001. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijden, A.E.D.M. Developments and challenges in the manufacturing, characterization and scale-up of energetic nanomaterials—A review. Chem. Eng. J. 2018, 350, 939–948. [Google Scholar] [CrossRef]
- Comet, M.; Martin, C.; Schnell, F.; Spitzer, D. Nanothermites: A short Review. Factsheet for Experimenters, Present and Future Challenges. Propellants Explos. Pyrotech. 2019, 44, 18–36. [Google Scholar] [CrossRef] [Green Version]
- Muravyev, N.V.; Monogarov, K.A.; Schaller, U.; Fomenkov, I.V.; Pivkina, A.N. Progress in Additive Manufacturing of Energetic Materials: Creating the Reactive Microstructures with High Potential of Applications. Propellants Explos. Pyrotech. 2019, 44, 941–969. [Google Scholar] [CrossRef]
- Jaworek, A.; Sobczyk, A.T.; Krupa, A. Electrospray application to powder production and surface coating. J. Aerosol Sci. 2018, 125, 57–92. [Google Scholar] [CrossRef]
- Rayleigh, L. On the equilibrium of liquid conducting masses charged with electricity. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1882, 14, 184–186. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Jiang, J.; Davoodi, P.; Srinivasan, M.P.; Wang, C.-H. Electrohydrodynamic atomization: A two-decade effort to produce and process micro-/nanoparticulate materials. Chem. Eng. Sci. 2015, 125, 32–57. [Google Scholar] [CrossRef] [Green Version]
- Fridrikh, S.V.; Yu, J.H.; Brenner, M.P.; Rutledge, G.C. Controlling the fiber diameter during electrospinning. Phys. Rev. Lett. 2003, 90, 144502–144505. [Google Scholar] [CrossRef] [Green Version]
- McKee, M.G.; Wilkes, G.L.; Colby, R.H.; Long, T.E. Correlations of Solution Rheology with Electrospun Fiber Formation of Linear and Branched Polyesters. Macromolecules 2004, 37, 1760–1767. [Google Scholar] [CrossRef]
- Gupta, P.; Elkins, C.; Long, T.E.; Wilkes, G.L. Electrospinning of linear homopolymers of poly(methyl methacrylate): Exploring relationships between fiber formation, viscosity, molecular weight and concentration in a good solvent. Polymer 2005, 46, 4799–4810. [Google Scholar] [CrossRef]
- Bodnar, E.; Grifoll, J.; Rosell-Llompart, J. Polymer solution electrospraying: A tool for engineering particles and films with controlled morphology. J. Aerosol Sci. 2018, 125, 93–118. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Borra, J.-P. Review on water electro-sprays and applications of charged drops with focus on the corona-assisted cone-jet mode for High Efficiency Air Filtration by wet electro-scrubbing of aerosols. J. Aerosol Sci. 2018, 125, 208–236. [Google Scholar] [CrossRef]
- Reus, M.A.; Hoetmer, G.; van der Heijden, A.E.D.M.; ter Horst, J.H. Concomitant crystallization for in situ encapsulation of organic materials. Chem. Eng. Processing Process Intensif. 2014, 80, 11–20. [Google Scholar] [CrossRef]
- Huang, C.; Liu, J.; Ding, L.; Wang, D.; Yang, Z.; Nie, F. Facile Fabrication of Nanoparticles Stacked 2,6-diamino-3,5-dinitropyrazine-1-oxide (LLM-105) Sub-microspheres via Electrospray Deposition. Propellants Explos. Pyrotech. 2018, 43, 188–193. [Google Scholar] [CrossRef]
- Yan, S.; Li, M.; Sun, L.; Jiao, Q.; Huang, R. Fabrication of Nano- and Micron- Sized Spheres of CL-20 by Electrospray. Cent. Eur. J. Energetic Mater. 2018, 15, 572–589. [Google Scholar] [CrossRef]
- Huang, C.; Xu, J.; Tian, X.; Liu, J.; Pan, L.; Yang, Z.; Nie, F. High-Yielding and Continuous Fabrication of Nanosized CL-20-Based Energetic Cocrystals via Electrospraying Deposition. Cryst. Growth Des. 2018, 18, 2121–2128. [Google Scholar] [CrossRef]
- Radacsi, N.; Bouma, R.H.B.; Krabbendam-la Haye, E.L.M.; ter Horst, J.H.; Stankiewicz, A.I.; van der Heijden, A.E.D.M. On the Reliability of Sensitivity Test Methods for Submicrometer-Sized RDX and HMX Particles. Propellants Explos. Pyrotech. 2013, 38, 761–769. [Google Scholar] [CrossRef]
- Young, G.; Wilson, D.P.; Kessler, M.; DeLisio, J.B.; Zachariah, M.R. Ignition and Combustion Characteristics of Al/RDX/NC Nanostructured Microparticles. Combust. Sci. Technol. 2020, 193, 2259–2275. [Google Scholar] [CrossRef]
- Wang, H.; Jacob, R.J.; DeLisio, J.B.; Zachariah, M.R. Assembly and encapsulation of aluminum NP’s within AP/NC matrix and their reactive properties. Combust. Flame 2017, 180, 175–183. [Google Scholar] [CrossRef]
- Egan, G.C.; Sullivan, K.T.; LaGrange, T.; Reed, B.W.; Zachariah, M.R. In situ imaging of ultra-fast loss of nanostructure in nanoparticle aggregates. J. Appl. Phys. 2014, 115, 084903–084908. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Jian, G.; Yan, S.; DeLisio, J.B.; Huang, C.; Zachariah, M.R. Electrospray Formation of Gelled Nano-Aluminum Microspheres with Superior Reactivity. Acs Appl. Mater. Interfaces 2013, 5, 6797–6801. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jian, G.; Egan, G.C.; Zachariah, M.R. Assembly and reactive properties of Al/CuO based nanothermite microparticles. Combust. Flame 2014, 161, 2203–2208. [Google Scholar] [CrossRef]
- Jacob, R.J.; Wei, B.; Zachariah, M.R. Quantifying the enhanced combustion characteristics of electrospray assembled aluminum mesoparticles. Combust. Flame 2016, 167, 472–480. [Google Scholar] [CrossRef]
- Cheng, L.; Huang, C.; Yang, Y.; Li, Y.; Meng, Y.; Li, Y.; Chen, H.; Song, D.; Artiaga, R. Preparation and Combustion Performance of B/PVDF/Al Composite Microspheres. Propellants Explos. Pyrotech. 2020, 45, 657–664. [Google Scholar] [CrossRef]
- Zuo, B.; Zhang, J.; Chen, S.; Liang, Q.; Qiao, X.; Zhao, F.; Liu, P.-J.; Yan, Q.-L. Fabrication of Si@AP/NC metastable intermixed nanocomposites (MICs) by electrospray method and their thermal reactivity. Adv. Compos. Hybrid Mater. 2019, 2, 361–372. [Google Scholar] [CrossRef]
- Yang, H.; Huang, C.; Chen, H. Tuning reactivity of nanoaluminum with fluoropolymer via electrospray deposition. J. Therm. Anal. Calorim. 2017, 127, 2293–2299. [Google Scholar] [CrossRef]
- Yan, T.; Ren, H.; Li, Y.; Wang, H.; Jiao, Q. Tailoring Structural Energetics for Enhanced Reactivity of Nano-Aluminum Particles Based Microspheres. Adv. Eng. Mater. 2019, 21, 1900176. [Google Scholar] [CrossRef]
- Ghildiyal, P.; Ke, X.; Biswas, P.; Nava, G.; Schwan, J.; Xu, F.; Kline, D.J.; Wang, H.; Mangolini, L.; Zachariah, M.R. Silicon Nanoparticles for the Reactivity and Energetic Density Enhancement of Energetic-Biocidal Mesoparticle Composites. ACS Appl. Mater. Interfaces 2020, 13, 458–467. [Google Scholar] [CrossRef]
- Xiao, L.; Zhao, L.; Ke, X.; Zhang, T.; Hao, G.; Hu, Y.; Zhang, G.; Guo, H.; Jiang, W. Energetic metastable Al/CuO/PVDF/RDX microspheres with enhanced combustion performance. Chem. Eng. Sci. 2020, 231, 116302–116311. [Google Scholar] [CrossRef]
- Monk, I.; Schoenitz, M.; Jacob, R.J.; Dreizin, E.L.; Zachariah, M.R. Combustion Characteristics of Stoichiometric Al-CuO Nanocomposite Thermites Prepared by Different Methods. Combust. Sci. Technol. 2017, 189, 555–574. [Google Scholar] [CrossRef]
- Wang, H.; Jian, G.; Zhou, W.; DeLisio, J.B.; Lee, V.T.; Zachariah, M.R. Metal lodate-Based Energetic Composites and Their Combustion and Biocidal Performance. ACS Appl. Mater. Interfaces 2015, 7, 17363–17370. [Google Scholar] [CrossRef] [PubMed]
- Young, G.; Wang, H.; Zachariah, M.R. Application of Nano-Aluminum/Nitrocellulose Mesoparticles in Composite Solid Rocket Propellants. Propellants Explos. Pyrotech. 2015, 40, 413–418. [Google Scholar] [CrossRef]
- Dai, J.; Wang, F.; Ru, C.; Xu, J.; Wang, C.; Zhang, W.; Ye, Y.; Shen, R. Ammonium Perchlorate as an Effective Additive for Enhancing the Combustion and Propulsion Performance of Al/CuO Nanothermites. J. Phys. Chem. C 2018, 122, 10240–10247. [Google Scholar] [CrossRef]
- Ru, C.; Wang, F.; Xu, J.; Dai, J.; Shen, Y.; Ye, Y.; Zhu, P.; Shen, R. Superior performance of a MEMS-based solid propellant microthruster (SPM) array with nanothermites. Microsyst. Technol. 2017, 23, 3161–3174. [Google Scholar] [CrossRef]
- Yi, Z.; Ang, Q.; Li, N.; Shan, C.; Li, Y.; Zhang, L.; Zhu, S. Sulfate-Based Nanothermite: A Green Substitute of Primary Explosive Containing Lead. ACS Sustain. Chem. Eng. 2018, 6, 8584–8590. [Google Scholar] [CrossRef]
- Mei, X.; Zhong, G.; Cheng, Y. The ignition and combustion characteristics of Al/Ni(IO3)2·4H2O nanothermites. J. Energetic Mater. 2019, 37, 378–386. [Google Scholar] [CrossRef]
- Huang, C.; Yang, Z.; Li, Y.; Zheng, B.; Yan, Q.; Guan, L.; Luo, G.; Li, S.; Nie, F. Incorporation of high explosives into nano-aluminum based microspheres to improve reactivity. Chem. Eng. J. 2020, 383, 123110–123117. [Google Scholar] [CrossRef]
- Dai, J.; Xu, J.; Wang, F.; Tai, Y.; Shen, Y.; Shen, R.; Ye, Y. Facile formation of nitrocellulose-coated Al/Bi2O3 nanothermites with excellent energy output and improved electrostatic discharge safety. Mater. Des. 2018, 143, 93–103. [Google Scholar] [CrossRef]
- Yao, J.; Li, B.; Xie, L.; Peng, J. Electrospray preparation and thermal properties of the composites based on RDX. J. Therm. Anal. Calorim. 2017, 130, 835–842. [Google Scholar] [CrossRef]
- Han, Z.; Wang, D.; Wang, H.; Henkes, C. Electrospray formation of RDX/ceria mixture and its thermal decomposition performance. J. Therm. Anal. Calorim. 2016, 123, 449–455. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, X.; Wang, H.; Wu, T.; Kline, D.J.; Rehwoldt, M.; Ren, H.; Zachariah, M.R. Titanium enhanced ignition and combustion of Al/I2O5 mesoparticle composites. Combust. Flame 2020, 212, 245–251. [Google Scholar] [CrossRef]
- Song, J.; Guo, T.; Yao, M.; Chen, J.-L.; Ding, W.; Bei, F.-L.; Zhang, X.-N.; Yin, Q.; Huang, J.-Y.; Li, C.-H. Thermal and combustion behavior of Al-MnO2 nanothermite with poly(vinylidene fluoride -co- hexafluoropropylene) energetic binder. Def. Technol. 2020, 17, 1289–1295. [Google Scholar] [CrossRef]
- Song, J.; Guo, T.; Yao, M.; Ding, W.; Zhang, X.; Bei, F.; Tang, J.; Huang, J.; Yu, Z. Thermal behavior and combustion of Al nanoparticles/ MnO2-nanorods nanothermites with addition of potassium perchlorate. RSC Adv. 2019, 9, 41319–41325. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Tao, G.; Miao, Y.; Jiaxing, S.; Wen, D.; Yiming, M.; Shi, L.; Rui, Z. Thermal behavior and combustion performance of Al/MoO3 nanothermites with addition of poly (vinylidene fluorine) using electrospraying. Mater. Res. Express 2020, 7, 115009. [Google Scholar] [CrossRef]
- Mei, X.; Zhong, G.; Cheng, Y. Ignition and combustion characteristics of aluminum/manganese iodate/nitrocellulose biocidal nanothermites. J. Therm. Anal. Calorim. 2019, 138, 425–432. [Google Scholar] [CrossRef]
- Yan, T.; Ren, H.; Liu, J.; Jiao, Q. Facile preparation and synergetic energy releasing of nano-Al@RDX@Viton hollow microspheres. Chem. Eng. J. 2020, 379, 122333. [Google Scholar] [CrossRef]
- Yan, T.; Ren, H.; Jiao, Q.; Yu, L.; Li, Y. Assembly and morphological properties of energetic polymer microspheres. Integr. Ferroelectr. 2018, 191, 116–125. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Huang, H.; Zhao, Y.; Song, K.; Wang, H.; Xie, W.; Cheng, Y.; Fan, X. Preparation and characterization of the Al/Fe2O3/RDX/NC nanocomposites by electrospray. J. Therm. Anal. Calorim. 2019, 137, 1615–1620. [Google Scholar] [CrossRef]
- Chen, L.; Ru, C.; Zhang, H.; Zhang, Y.; Chi, Z.; Wang, H.; Li, G. Assembling Hybrid Energetic Materials with Controllable Interfacial Microstructures by Electrospray. ACS Omega 2021, 6, 16816–16825. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Wang, Y.; Huang, H.; Shang, F.; Song, X. An Electrospun Preparation of the NC/GAP/Nano-LLM-105 Nanofiber and Its Properties. Nanomaterials 2019, 9, 854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, X.; Guo, K.; Wang, Y.; Li, F. Characterization and Properties of F2602/GAP/CL-20 Energetic Fibers with High Energy and Low Sensitivity Prepared by the Electrospinning Method. ACS Omega 2020, 5, 11106–11114. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, R.; Jiang, X.; Shen, J.; Huang, T.; Yang, G.; Nie, F.; Pei, C. Preparation and Properties of Nano-composite Fiber RDX/NC. Chin. J. Explos. Propellants 2012, 35, 28–31. [Google Scholar]
- Li, R.; Xu, H.; Hu, H.; Yang, G.; Wang, J.; Shen, J. Microstructured Al/Fe2O3/Nitrocellulose Energetic Fibers Realized by Electrospinning. J. Energetic Mater. 2014, 32, 50–59. [Google Scholar] [CrossRef]
- Lyu, J.-Y.; Chen, S.; He, W.; Zhang, X.-X.; Tang, D.-Y.; Liu, P.-J.; Yan, Q.-L. Fabrication of high-performance graphene oxide doped PVDF/CuO/Al nanocomposites via electrospinning. Chem. Eng. J. 2019, 368, 129–137. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Shen, Y.; Wang, C.-a.; Zhang, Z.; Li, F.; Cheng, J.; Ye, Y.; Shen, R. Fabrication of energetic aluminum core/hydrophobic shell nanofibers via coaxial electrospinning. Chem. Eng. J. 2022, 427, 132001. [Google Scholar] [CrossRef]
- Zhang, C.; Mao, H.; Cui, R.; Zhang, X.; Yang, J.; Ji, J.; Zhou, X. Electrospinning preparation, energetic characteristics and reaction mechanism of corrosion-resistant Si@PVDF nanostructured energetic films. Combust. Flame 2022, 237, 111887. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, T.-F.; Ge, Z.; Luo, Y.-J. Morphology-controlled synthesis of Al/Fe2O3 nano-composites via electrospinning. Chin. Chem. Lett. 2015, 26, 1535–1537. [Google Scholar] [CrossRef]
- Xie, L.; Li, Z.; Li, X.; Wenlong, W.; Hanjiang, Y. Electrospun copper oxide nanofibers and catalysis for combustion of ammonium perchlorate. Ferroelectrics 2019, 549, 23–28. [Google Scholar] [CrossRef]
- Wang, Q.; Han, J.; Zhang, Y.; Yan, Z.; Velasco, E.; Yang, L.; Wang, B.; Zang, S.-Q. Fabrication of Copper Azide Film through Metal–Organic Framework for Micro-Initiator Applications. ACS Appl. Mater. Interfaces 2019, 11, 8081–8088. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yang, L.; Han, J.-M.; Li, H.; Huo, J. Fabrication of a nanoscale homogeneous lead azide@carbon fiber film with low electrostatic sensitivity by in situ synthesis. N. J. Chem. 2021, 45, 11780–11785. [Google Scholar] [CrossRef]
- He, W.; Li, Z.-H.; Chen, S.; Yang, G.; Yang, Z.; Liu, P.-J.; Yan, Q.-L. Energetic metastable n-Al@PVDF/EMOF composite nanofibers with improved combustion performances. Chem. Eng. J. 2020, 383, 123146. [Google Scholar] [CrossRef]
- Li, Y.; Ren, H.; Jiao, Q. Preparation of New Structure Energetic Composite of HNIW Implanted into Macroporous Fibosa. Chin. J. Energetic Mater. 2017, 25, 309–314. [Google Scholar] [CrossRef]
- Yan, S.; Jian, G.; Zachariah, M.R. Electrospun nanofiber-based thermite textiles and their reactive properties. ACS Appl. Mater. Interfaces 2012, 4, 6432–6435. [Google Scholar] [CrossRef]
- Clayton, N.A.; Kappagantula, K.S.; Pantoya, M.L.; Kettwich, S.C.; Iacono, S.T. Fabrication, Characterization, and Energetic Properties of Metallized Fibers. ACS Appl. Mater. Interfaces 2014, 6, 6049–6053. [Google Scholar] [CrossRef]
- Xie, L.; Shao, Z.; Wang, W.; Wang, F. Preparation of AlNPs/NC Composite Nanofibers by Electrospinning. Integr. Ferroelectr. 2011, 127, 184–192. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.; Hong, Y.; Yang, Y.; Cheng, Y.; Chen, H. Electrospun nanofiber-based nanoboron/nitrocellulose composite and their reactive properties. J. Therm. Anal. Calorim. 2017, 130, 1063–1068. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.-T.; Wang, C.-A.; Shen, Y.; Zhang, Z.-H.; Cheng, J.; Wu, S.-Z.; Ye, Y.-H.; Shen, R.-Q. Positive effects of PVP in MIC: Preparation and characterization of Al-Core heterojunction fibers. Def. Technol. 2021. [Google Scholar] [CrossRef]
- Wang, W.; Li, H.; Yang, Y.; Zhao, F.; Li, H.; Xu, K. Enhanced thermal decomposition, laser ignition and combustion properties of NC/Al/RDX composite fibers fabricated by electrospinning. Cellulose 2021, 28, 6089–6105. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Kohsari, I.; Zandavar, H.; Foroutan Koudehi, M.; Mirsadeghi, S. Electrospinning and thermal characterization of nitrocellulose nanofibers containing a composite of diaminofurazan, aluminum nano-powder and iron oxide nanoparticles. Cellulose 2019, 26, 4405–4415. [Google Scholar] [CrossRef]
- Luo, T.; Wang, Y.; Liu, L.; Song, X. Characterization and Thermochemical Properties of NC/GAP/nano-TATB Electrospinning Composite Fibers with 3D Network Structure. Chin. J. Energetic Mater. 2020, 28, 925–935. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, T.; Song, X.; Li, F. Electrospinning Preparation of NC/GAP/Submicron-HNS Energetic Composite Fiber and its Properties. ACS Omega 2019, 4, 14261–14271. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Jian, G.; DeLisio, J.B.; Wang, H.; Zachariah, M.R. Electrospray Deposition of Energetic Polymer Nanocomposites with High Mass Particle Loadings: A Prelude to 3D Printing of Rocket Motors. Adv. Eng. Mater. 2015, 17, 95–101. [Google Scholar] [CrossRef]
- DeLisio, J.B.; Hu, X.; Wu, T.; Egan, G.C.; Young, G.; Zachariah, M.R. Probing the Reaction Mechanism of Aluminum/Poly(vinylidene fluoride) Composites. J. Phys. Chem. B 2016, 120, 5534–5542. [Google Scholar] [CrossRef]
- Hu, X.; DeLisio, J.B.; Li, X.; Zhou, W.; Zachariah, M.R. Direct Deposit of Highly Reactive Bi(IO3)3- Polyvinylidene Fluoride Biocidal Energetic Composite and its Reactive Properties. Adv. Eng. Mater. 2017, 19, 1500532. [Google Scholar] [CrossRef]
- Wang, H.; Holdren, S.; Zachariah, M.R. Preparation and combustion of laminated iodine containing aluminum/polyvinylidene fluoride composites. Combust. Flame 2018, 197, 120–126. [Google Scholar] [CrossRef]
- Li, X.; Guerieri, P.; Zhou, W.; Huang, C.; Zachariah, M.R. Direct Deposit Laminate Nanocomposites with Enhanced Propellent Properties. Acs Appl. Mater. Interfaces 2015, 7, 9103–9109. [Google Scholar] [CrossRef]
- Li, X.; Zachariah, M.R. Direct Deposit of Fiber Reinforced Energetic NanoComposites. Propellants Explos. Pyrotech. 2017, 42, 1079–1084. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, W.; Wang, X.; Wu, T.; Delisio, J.B.; Zachariah, M.R. On-the-fly green generation and dispersion of AgI nanoparticles for cloud seeding nuclei. J. Nanopart. Res. 2016, 18, 214. [Google Scholar] [CrossRef]
- Wang, H.; DeLisio, J.B.; Holdren, S.; Wu, T.; Yang, Y.; Hu, J.; Zachariah, M.R. Mesoporous Silica Spheres Incorporated Aluminum/Poly (Vinylidene Fluoride) for Enhanced Burning Propellants. Adv. Eng. Mater. 2018, 20, 1700547. [Google Scholar] [CrossRef]
- Wang, H.; Kline, D.J.; Rehwoldt, M.; Wu, T.; Zhao, W.; Wang, X.; Zachariah, M.R. Architecture Can Significantly Alter the Energy Release Rate from Nanocomposite Energetics. ACS Appl. Polym. Mater. 2019, 1, 982–989. [Google Scholar] [CrossRef]
- Sevely, F.; Liu, X.; Wu, T.; Mesnilgrente, F.; Franc, B.; Assie-Souleille, S.; Dollat, X.; Rossi, C. Effect of Process Parameters on the Properties of Direct Written Gas-Generating Reactive Layers. ACS Appl. Polym. Mater. 2021, 3, 3972–3980. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Zhang, W.; Zheng, Z.; Yu, C.; Wang, J.; Song, C.; Chen, J.; Lei, X.; Ma, K. Direct ink writing of nAl/pCuO/HPMC with outstanding combustion performance and ignition performance. Combust. Flame 2022, 236, 111747. [Google Scholar] [CrossRef]
- Cloupeau, M.; Prunet-Foch, B. Electrohydrodynamic spraying functioning modes: A critical review. J. Aerosol Sci. 1994, 25, 1021–1036. [Google Scholar] [CrossRef]
- An, B.W.; Kim, K.; Lee, H.; Kim, S.; Shim, Y.; Lee, D.; Song, J.Y.; Park, J. High-Resolution Printing of 3D Structures Using an Electrohydrodynamic Inkjet with Multiple Functional Inks. Adv. Mater. 2015, 27, 4322–4328. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zheng, G.; Zhu, P.; Liu, J.; Liu, Y.; Wang, X.; Li, W.; Guo, S. Controlling of Electrospray Deposition for Micropatterns. Micromachines 2018, 9, 72. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Wang, D.; Wang, Q.; Song, K.; Liang, J.; Sun, Y.; Madoua, M. Thermally Assisted Electrohydrodynamic Jet High-Resolution Printing of High-Molecular Weight Biopolymer 3D Structures. Macromol. Mater. Eng. 2018, 303, 1800345. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Ru, C.; Zhang, H.; Zhang, Y.; Wang, H.; Hu, X.; Li, G. Progress in Electrohydrodynamic Atomization Preparation of Energetic Materials with Controlled Microstructures. Molecules 2022, 27, 2374. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27072374
Chen L, Ru C, Zhang H, Zhang Y, Wang H, Hu X, Li G. Progress in Electrohydrodynamic Atomization Preparation of Energetic Materials with Controlled Microstructures. Molecules. 2022; 27(7):2374. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27072374
Chicago/Turabian StyleChen, Lihong, Chengbo Ru, Hongguo Zhang, Yanchun Zhang, Hongxing Wang, Xiuli Hu, and Gang Li. 2022. "Progress in Electrohydrodynamic Atomization Preparation of Energetic Materials with Controlled Microstructures" Molecules 27, no. 7: 2374. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27072374