Covalent Organic Frameworks for Chemical and Biological Sensing
Abstract
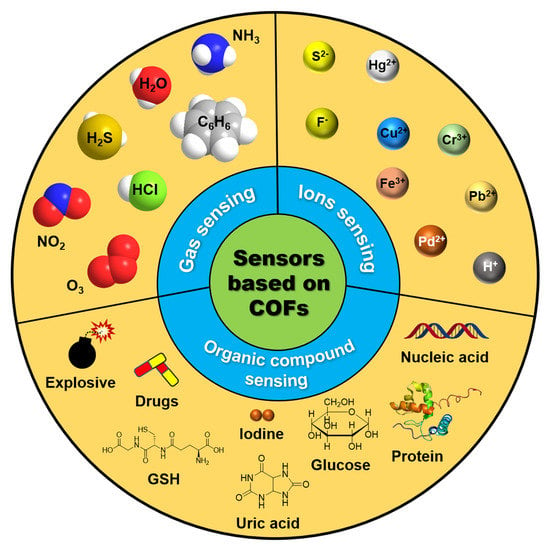
:1. Introduction
2. Basic Principles of COF-Based Sensing
2.1. Designing a COF or COF-Based Hybird Material for Selective Adsorption
2.2. Signals Produced by the Adsorption of the Analyte
2.2.1. Fluorescence
2.2.2. Chromism
2.2.3. Capacitance and Conductivity
2.2.4. Electrochemical or Photoelectrochemical Signals
3. Application of COFs for Sensing Various Analytes
3.1. Gas Sensing
3.1.1. Acidic and Alkaline Gases
| Analyte | Year | COF Names | Specific Binding Site | Type of Detectable Signal | Detection Range | LOD | Reference |
|---|---|---|---|---|---|---|---|
| NH3 | 2016 | TPE−Ph−COF | Boronate | Fluorescence (turn off) | - | sub ppm level | [60] |
| 2018 | HMP−TAPB−1HMP−TAPB−1 | Heptazine | conductivity | 1–200 ppm | 1 ppm | [61] | |
| 2018 | COP−COP−1 | Triazine | Fluorescence (turn on) | - | 5.8925 × 10−4 mL/mL | [62] | |
| 2019 | Ph−An−COF | Boronate | Fluorescence (turn off) | - | - | [63] | |
| 2019 | COF−DC−8 | - | Conductivity | 2–80 ppm | 56.8–70 ppb | [64] | |
| 2021 | TAPB−BPDA COF | Imine | Conductivity | 5–100 ppm | 10 ppb | [65] | |
| TFA | 2019 | Per−N COF | Imine | Chromism | 0.035–110 mg L−1 | 35 μg L−1 | [47] |
| HCl | 2018 | COP−COP−1 | Triazine | Fluorescence (turn off) | 1.0967 × 10−4 mL/mL | [62] | |
| 2019 | PBHP−TAPT COF | Triazine | Chromism, conductivity | 20–3000 ppm | 20 ppm | [57] | |
| 2019 | COF−ETBA−DAB | Imine | Fluorescence | 4.7 ppm | [66] | ||
| 2020 | BCTB−BCTA COF | Imine | Fluorescence (turn off) | 1–25 mM | 10 nM | [48] | |
| H2O | 2013 | TAPP−DHNDA−COF | Iminol | Chromism | 20–100% RH | [54] | |
| 2017 | COF−TXDBA | Boronate | Conductivity | 11–98% RH | [56] | ||
| 2018 | Py−TT COF | Chromism | 0.64–0.98 p/p0 | [53] | |||
| 2020 | TAPB−PDA−OH COF | Iminol | Chromism | [55] | |||
| 2021 | DUT−175 | Imine | Chromism | 33–94% RH | [67] | ||
| Benzene | 2020 | BTA−TAPT-COF | Aromatic group | Capacitance | 500 ppb–100 ppm | 340 ppb | [58] |
| NO2 | 2019 | COF−DC−8 | Conductivity | 2–40 ppm | 1–16 ppb | [64] | |
| 2020 | CON−10 | Conductivity | 2.242 ppb | [68] | |||
| 2020 | T−2DP | Conductivity | 0.15–5 ppm | 2.2 ppb | [69] | ||
| 2021 | NiPc−CoTAA | Conductivity | 1–40 ppm | [70] | |||
| NO | 2019 | COF−DC−8 | Conductivity | 0.02–40 ppm | 1–5 ppb | [64] | |
| H2S | 2017 | PNT−1 | Triazine, pyridine | Fluorescence (turn off) | 53 ppb | [71] | |
| 2019 | COF−DC−8 | Conductivity | 2–80 ppm | 121 ppb | [64] | ||
| O3 | 2021 | P−COFTPB−DMTP−COF | imine | Chromism | 0.1 ppm | [72] |
3.1.2. Water Vapor (Humidity) Sensing
3.1.3. Harmful Gases Sensing
3.2. Inorganic Ions Sensing
3.2.1. Metal Ions Sensing
3.2.2. pH Sensing (H+ Sensing)
3.2.3. Inorganic Anions Sensing
3.3. Molecular Sensing
3.3.1. Explosive Sensing
3.3.2. Iodine Sensing
3.3.3. Drug Sensing
3.3.4. Small Biomolecules Sensing
3.3.5. Other Small Molecules Sensing
3.3.6. Biomacromolecule Sensing
| Analyte | Year | COF/COF Hybrid Names | Specific Binding Site | Type of Detectable Signal | Detection Range | LOD | Reference |
|---|---|---|---|---|---|---|---|
| Cardiac Troponin I | 2018 | TB−Au−COFs−Ab2 | Antibody | Chromism | 0.5 pg/mL–10.0 ng/mL | 0.17 pg/mL | [165] |
| 2021 | HRP−Ab2−Au−COF | Antibody | Chromism | 5 pg/mL–10 ng/mL | 1.7 pg/mL | [166] | |
| Heat shock protein 90α | 2019 | Fe3O4@TpBD−DSS−Ab−MEG | Antibody | MS | 50 pg/mL | [167] | |
| C-reactive protein | 2018 | AuNPs @COF−TPPa-1 | Antibody | Electrochemical signal (EIS, CV) | 0.017 ng/mL | 0.05–80 ng/mL | [168] |
| 2018 | COF−LZU8 | Antibody | Electrochemical signal (DPV) | 0.016 ng/mL | 0.05–150 ng/mL | [169] | |
| 2019 | p−COF | Aptamer | Photoelectrochemical signal | 0.1 ng/mL | 0.5–100 ng/mL | [174] | |
| DNA | 2017 | TpTta | DNA hybridization | Fluorescence, “turn on” | 10–100 nM | 3.7 nM | [171] |
| 2017 | TPA−COF | DNA hybridization | Fluorescence, “turn on” | 0.02–5 nM | 20 pM | [172] | |
| 2018 | EB-TFP iCOF | DNA hybridization | Fluorescence, “turn on” | 0–32 mM | - | [173] | |
| 2021 | Cu−MOF@CuPc−TA−COF | DNA hybridization | Electrochemical signal | 1 fM–1 nM | 0.18 fM | [28] | |
| Photoelectrochemical signal | 0.07 fM |
4. Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diamond, D.; Coyle, S.; Scarmagnani, S.; Hayes, J. Wireless Sensor Networks and Chemo-/Biosensing. Chem. Rev. 2008, 108, 652–679. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.; Brown, R.B. Chemical Sensors with Integrated Electronics. Chem. Rev. 2008, 108, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Aksyuk, V.A. Sensing without Power. Nat. Nanotechnol. 2017, 12, 940–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, Z.; Wang, L.; Shen, G. Recent Advances in Smart Wearable Sensing Systems. Adv. Mater. Technol. 2018, 3, 1800444. [Google Scholar] [CrossRef] [Green Version]
- Mondal, S.; Zehra, N.; Choudhury, A.; Iyer, P.K. Wearable Sensing Devices for Point of Care Diagnostics. ACS Appl. Bio Mater. 2021, 4, 47–70. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, X.; Fang, X.; Kong, J. Wearable Chem-biosensing Devices: From Basic Research to Commercial Market. Lab Chip 2021, 21, 4285–4310. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Lin, G.; Feng, Y. Recent Progress in the Fabrication of Flexible Materials for Wearable Sensors. Biomater. Sci. 2022, 10, 614–632. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, X.; Chen, P. Biological and Chemical Sensors Based on Graphene Materials. Chem. Soc. Rev. 2012, 41, 2283–2307. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Zhou, X.; Zhang, Y. Review on the Graphene Based Optical Fiber Chemical and Biological Sensors. Sensors Actuat. B-Chem. 2016, 231, 324–340. [Google Scholar] [CrossRef]
- Angizi, S.; Khalaj, M.; Alem, S.A.A.; Pakdel, A.; Willander, M.; Hatamie, A.; Simchi, A. Towards the Two-dimensional Hexagonal Boron Nitride (2D h-BN) Electrochemical Sensing Platforms. J. Electrochem. Soc. 2020, 167, 126513. [Google Scholar] [CrossRef]
- Gan, X.; Zhao, H.; Quan, X. Two-dimensional MoS2: A Promising Building Block for Biosensors. Biosens. Bioelectron. 2017, 89, 56–71. [Google Scholar] [CrossRef]
- Pumera, M.; Loo, A.H. Layered Transition-metal Dichalcogenides (MoS2 and WS2) for Sensing and Biosensing. TrAC-Trend. Anal. Chem. 2014, 61, 49–53. [Google Scholar] [CrossRef]
- Sinha, A.; Dhanjai; Zhao, H.M.; Huang, Y.J.; Lu, X.B.; Chen, J.P.; Jain, R. MXene: An Emerging Material for Sensing and Biosensing. TrAC-Trend. Anal. Chem. 2018, 105, 424–435. [Google Scholar] [CrossRef]
- Wales, D.J.; Grand, J.; Ting, V.P.; Burke, R.D.; Edler, K.J.; Bowen, C.R.; Mintova, S.; Burrows, A.D. Gas Sensing Using Porous Materials for Automotive Applications. Chem. Soc. Rev. 2015, 44, 4290–4321. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.-B.; Liu, S.-Y.; Ye, J.-W.; Li, X.-Y.; Zhang, J.-P. Photoluminescent Metal-Organic Frameworks for Gas Sensing. Adv. Sci. 2016, 3, 1500434. [Google Scholar] [CrossRef]
- Koo, W.T.; Jang, J.S.; Kim, I.D. Metal-Organic Frameworks for Chemiresistive Sensors. Chem 2019, 5, 1938–1963. [Google Scholar] [CrossRef]
- Teo, W.L.; Liu, J.; Zhou, W.; Zhao, Y. Facile Preparation of Antibacterial MOF-fabric Systems for Functional Protective Wearables. SmartMat 2021, 2, 567–578. [Google Scholar] [CrossRef]
- Meng, Z.; Mirica, K.A. Covalent Organic Frameworks as Multifunctional Materials for Chemical Detection. Chem. Soc. Rev. 2021, 50, 13498–13558. [Google Scholar] [CrossRef]
- Liu, X.; Huang, D.; Lai, C.; Zeng, G.; Qin, L.; Wang, H.; Yi, H.; Li, B.; Liu, S.; Zhang, M.; et al. Recent Advances in Covalent Organic Frameworks (COFs) as a Smart Sensing Material. Chem. Soc. Rev. 2019, 48, 5266. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Dong, R.; Feng, X.; Kaskel, S.; Matoga, D.; Stavila, V. Electronic Devices Using Open Framework Materials. Chem. Rev. 2020, 120, 8581–8640. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Liu, R.; Tan, K.; Gong, Y.; Chen, Y.; Li, Z.; Xie, S.; He, T.; Lu, Z.; Yang, H.; Jiang, D. Covalent Organic Frameworks: An Ideal Platform for Designing Ordered Materials and Advanced Applications. Chem. Soc. Rev. 2021, 50, 120–242. [Google Scholar] [CrossRef]
- Jiang, D. Covalent Organic Frameworks: An Amazing Chemistry Platform for Designing Polymers. Chem 2020, 6, 2461–2483. [Google Scholar] [CrossRef]
- Li, Z.; He, T.; Gong, Y.; Jiang, D. Covalent Organic Frameworks: Pore Design and Interface Engineering. Acc. Chem. Res. 2020, 53, 1672–1685. [Google Scholar] [CrossRef]
- Li, Y.; Chen, W.; Xing, G.; Jiang, D.; Chen, L. New Synthetic Strategies Towards Covalent Organic Frameworks. Chem. Soc. Rev. 2020, 49, 2852–2868. [Google Scholar] [CrossRef]
- Jin, P.; Niu, X.; Zhang, F.; Dong, K.; Dai, H.; Zhang, H.; Wang, W.; Chen, H.; Chen, X. Stable and Reusable Light-Responsive Reduced Covalent Organic Framework (COF−300−AR) as an Oxidase-Mimicking Catalyst for GSH Detection in Cell Lysate. ACS Appl. Mater. Interfaces 2020, 12, 20414–20422. [Google Scholar] [CrossRef]
- Li, G.; Ma, W.; Yang, Y.; Zhong, C.; Huang, H.; Ouyang, D.; He, Y.; Tian, W.; Lin, J.; Lin, Z. Nanoscale Covalent Organic Frameworks with Donor−Acceptor Structures as Highly Efficient Light-Responsive Oxidase-like Mimics for Colorimetric Detection of Glutathione. ACS Appl. Mater. Interfaces 2021, 13, 49482–49489. [Google Scholar] [CrossRef]
- Xu, M.; Chen, K.; Zhu, L.; Zhang, S.; Wang, M.; He, L.; Zhang, Z.; Du, M. MOF@COF Heterostructure Hybrid for Dual-Mode Photoelectrochemical−Electrochemical HIV-1 DNA Sensing. Langmuir 2021, 37, 13479–13492. [Google Scholar] [CrossRef]
- Cui, W.-R.; Zhang, C.-R.; Jiang, W.; Liang, R.-P.; Wen, S.-H.; Peng, D.; Qiu, J.-D. Covalent Organic Framework Nanosheet-Based Ultrasensitive and Selective Colorimetric Sensor for Trace Hg2+ Detection. ACS Sustain. Chem. Eng. 2019, 7, 9408–9415. [Google Scholar] [CrossRef]
- Côtè, A.P.; Benin, A.I.; Ockwig, N.W.; O’keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, H.; Yaghi, O.M. Storage of Hydrogen, Methane, and Carbon Dioxide in Highly Porous Covalent Organic Frameworks for Clean Energy Applications. J. Am. Chem. Soc. 2009, 131, 8875–8883. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Jiang, D. Covalent Organic Frameworks for Energy Conversions: Current Status, Challenges, and Perspectives. CCS Chem. 2021, 3, 2003–2024. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Shao, P.; Han, Y.; Gao, X.; Ma, L.; Yuan, S.; Ma, X.; Zhou, J.; Feng, X.; et al. Exfoliation of Covalent Organic Frameworks into Few-Layer Redox-Active Nanosheets as Cathode Materials for Lithium-Ion Batteries. J. Am. Chem. Soc. 2017, 139, 4258–4261. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Furukawa, H.; Yaghi, O.M.; Goddard, W.A., III. Covalent Organic Frameworks as Exceptional Hydrogen Storage Materials. J. Am. Chem. Soc. 2008, 130, 11580–11581. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, S.; Chen, Y.; Zhang, Z.; Ma, S. Covalent Organic Frameworks for Separation Applications. Chem. Soc. Rev. 2020, 49, 708–735. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Li, X.; Zhu, J.; Zhang, G.; Van Puyvelde, P.; Van der Bruggen, B. Covalent Organic Frameworks for Membrane Separation. Chem. Soc. Rev. 2019, 48, 2665–2681. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Wang, H.-Y.; Zheng, H.; Zhang, W.; Cao, R. Porphyrin-based Frameworks for Oxygen Electrocatalysis and Catalytic Reduction of Carbon Dioxide. Chem. Soc. Rev. 2021, 50, 2540–2581. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Yadav, P.; Yadav, M.; Gupta, R.; Rana, P.; Srivastava, A.; Zbořil, R.; Varma, R.S.; Antonietti, M.; Gawande, M.B. Recent Development of Covalent Organic Frameworks (COFs): Synthesis and Catalytic (Organic-Electro-Photo) Applications. Mater. Horiz. 2020, 7, 411–454. [Google Scholar] [CrossRef]
- Liu, J.G.; Wang, N.; Ma, L.L. Recent Advances in Covalent Organic Frameworks for Catalysis. Chem-Asian J. 2020, 15, 338–351. [Google Scholar] [CrossRef]
- Yusran, Y.; Li, H.; Guan, X.; Fang, Q.; Qiu, S. Covalent Organic Frameworks for Catalysis. EnergyChem 2020, 2, 100035. [Google Scholar] [CrossRef]
- Ren, X.; Li, C.; Kang, W.; Li, H.; Ta, N.; Ye, S.; Hu, L.; Wang, X.; Li, C.; Yang, Q. Enormous Promotion of Photocatalytic Activity through the Use of Near-Single Layer Covalent Organic Frameworks. CCS Chem. 2021, 3, 2453–2463. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Dong, M.; Wang, Y.-W.; Chen, Y.-T.; Wang, H.-Z.; Su, C.-Y.; Wang, W. Thioether-Based Fluorescent Covalent Organic Framework for Selective Detection and Facile Removal of Mercury(II). J. Am. Chem. Soc. 2016, 138, 3031–3037. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Covalent Organic Frameworks (COFs) for Environmental Applications. Coord. Chem. Rev. 2019, 400, 213046. [Google Scholar] [CrossRef]
- Li, C.; Liu, L.; Kang, J.; Xiao, Y.; Feng, Y.; Cao, F.-F.; Zhang, H. Pristine MOF and COF Materials for Advanced Batteries. Energy Stor. Mater. 2020, 31, 115–134. [Google Scholar] [CrossRef]
- Feng, L.; Qian, C.; Zhao, Y. Recent Advances in Covalent Organic Framework-Based Nanosystems for Bioimaging and Therapeutic Applications. ACS Mater. Lett. 2020, 2, 1074–1092. [Google Scholar] [CrossRef]
- Bhambri, H.; Khullar, S.; Sakshi; Mandal, S.K. Nitrogen-rich Covalent Organic Frameworks: A Promising Class of Sensory Materials. Mater. Adv. 2022, 3, 19–124. [Google Scholar] [CrossRef]
- Ascherl, L.; Evans, E.W.; Gorman, J.; Orsborne, S.; Bessinger, D.; Bein, T.; Friend, R.H.; Auras, F. Perylene-Based Covalent Organic Frameworks for Acid Vapor Sensing. J. Am. Chem. Soc. 2019, 141, 15693–15699. [Google Scholar] [CrossRef]
- EL-Mahdy, A.F.M.; Laia, M.-Y.; Kuo, S.-W. A Highly Fluorescent Covalent Organic Framework as A Hydrogen Chloride Sensor: Roles of Schiff Base Bonding and π-Stacking. J. Mater. Chem. C 2020, 8, 9520–9528. [Google Scholar] [CrossRef]
- Wang, M.; Hu, M.; Liu, J.; Guo, C.; Peng, D.; Jia, Q.; He, L.; Zhang, Z.; Du, M. Covalent Organic Framework-based ElectroChemical Aptasensors for the Ultrasensitive Detection of Antibiotics. Biosens. Bioelectron. 2019, 132, 8–16. [Google Scholar] [CrossRef]
- Xiong, Y.; Su, L.; He, X.; Duan, Z.; Zhang, Z.; Chen, Z.; Xie, W.; Zhu, D.; Luo, Y. Colorimetric Determination of Copper Ions Based on Regulation of the Enzyme-Mimicking Activity of Covalent Triazine Frameworks. Sens. Actuators B 2017, 253, 384–391. [Google Scholar] [CrossRef]
- Skorjanc, T.; Shetty, D.; Valant, M. Covalent Organic Polymers and Frameworks for Fluorescence-Based Sensors. ACS Sens. 2021, 6, 1461–1481. [Google Scholar] [CrossRef]
- Guo, L.; Yang, L.; Li, M.; Kuang, L.; Song, Y.; Wang, L. Covalent Organic Frameworks for Fluorescent Sensing: Recent Developments and Future Challenges. Coord. Chem. Rev. 2021, 440, 213957. [Google Scholar] [CrossRef]
- Ascherl, L.; Evans, E.W.; Hennemann, M.; Nuzzo, D.D.; Hufnagel, A.G.; Beetz, M.; Friend, R.H.; Clark, T.; Bein, T.; Auras, F. Solvatochromic Covalent Organic Frameworks. Nat. Commun. 2018, 9, 3802. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Jiang, Y.; Li, X.; Li, X.; Wang, J.; Wu, Q.; Liu, X. Solvothermal Synthesis of Microporous, Crystalline Covalent Organic Framework Nanofibers and Their Colorimetric Nanohybrid Structures. ACS Appl. Mater. Interfaces 2013, 5, 8845–8849. [Google Scholar] [CrossRef]
- Jhulki, S.; Evans, A.M.; Hao, X.L.; Cooper, M.W.; Feriante, C.H.; Leisen, J.; Li, H.; Lam, D.; Hersam, M.C.; Barlow, S.; et al. Humidity Sensing through Reversible Isomerization of a Covalent Organic Framework. J. Am. Chem. Soc. 2020, 142, 783–791. [Google Scholar] [CrossRef]
- Singh, H.; Tomer, V.K.; Jena, N.; Bala, I.; Sharma, N.; Nepak, D.; Sarkar, A.D.; Kailasam, K.; Pal, S.K. A Porous, Crystalline Truxene-Based Covalent Organic Framework and Its Application in Humidity Sensing. J. Mater. Chem. A 2017, 5, 21820–21827. [Google Scholar] [CrossRef]
- Kulkarni, R.; Noda, Y.; Barange, D.K.; Kochergin, Y.S.; Lyu, P.; Balcarova, B.; Nachtigall, P.; Bojdys, M.J. Real-Time Optical and Electronic Sensing with a β-Amino Enone Linked, Triazine-Containing 2D Covalent Organic Framework. Nat. Commun. 2019, 10, 3228. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Li, N.; Linghu, J.; Dong, J.; Wang, Y.; Karmakar, A.; Yuan, J.; Li, M.; Buenconsejo, P.J.S.; Liu, G.; et al. Chip-Level Integration of Covalent Organic Frameworks for Trace Benzene Sensing. ACS Sens. 2020, 5, 1474–1481. [Google Scholar] [CrossRef]
- Doonan, C.J.; Tranchemontagne, D.J.; Glover, T.G.; Hunt, J.R.; Yaghi, O.M. Exceptional Ammonia Uptake by a Covalent Organic Framework. Nat. Chem. 2010, 2, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Dalapati, S.; Jin, E.; Addicoat, M.; Heine, T.; Jiang, D. Highly Emissive Covalent Organic Frameworks. J. Am. Chem. Soc. 2016, 138, 5797–5800. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sharma, N.; Srinivasan, P.; Kumar, S.; Rayappan, J.B.B.; Kailasam, K. Heptazine Based Organic Framework as a Chemiresistive Sensor for Ammonia Detection at Room Temperature. J. Mater. Chem. A 2018, 6, 18389–18395. [Google Scholar] [CrossRef]
- Xu, N.; Wang, R.-L.; Li, D.-P.; Zhou, Z.-Y.; Zhang, T.; Xie, Y.-Z.; Su, Z.-M. Continuous Detection of HCl and NH3 Gases with a High-Performance Fluorescent Polymer Sensor. New J. Chem. 2018, 42, 13367–13374. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Z.; Yan, Y.; Li, G.; Hao, C. A Mechanism of the Luminescent Covalent Organic Framework for the Detection of NH3. New J. Chem. 2019, 43, 9274–9279. [Google Scholar] [CrossRef]
- Meng, Z.; Stolz, R.M.; Mirica, K.A. Two-Dimensional Chemiresistive Covalent Organic Framework with High Intrinsic Conductivity. J. Am. Chem. Soc. 2019, 141, 11929–11937. [Google Scholar] [CrossRef]
- Niu, F.; Shao, Z.-W.; Zhu, J.-L.; Tao, L.-M.; Ding, Y. Structural Evolution of Imine-linked Covalent Organic Frameworks and Their NH3 Sensing Performance. J. Mater. Chem. C 2021, 9, 8562–8569. [Google Scholar] [CrossRef]
- Cui, F.-Z.; Xie, J.-J.; Jiang, S.-Y.; Gan, S.-X.; Ma, D.-L.; Liang, R.-R.; Jiang, G.-F.; Zhao, X. A Gaseous Hydrogen Chloride Chemosensor Based on a 2D Covalent Organic Framework. Chem. Commun. 2019, 55, 4550–4553. [Google Scholar] [CrossRef]
- Gilmanova, L.; Bon, V.; Shupletsov, L.; Pohl, D.; Rauche, M.; Brunner, E.; Kaskel, S. Chemically Stable Carbazole-Based Imine Covalent Organic Frameworks with Acidochromic Response for Humidity Control Applications. J. Am. Chem. Soc. 2021, 143, 18368–18373. [Google Scholar] [CrossRef]
- Ko, W.C.; Kim, M.-S.; Kwon, Y.J.; Jeong, J.; Kim, W.R.; Choi, H.; Park, J.K.; Jeong, Y.K. Two-Dimensional Semiconducting Covalent Organic Nanosheets for Highly Sensitive and Stable NO2 Sensing Under Humid Conditions. J. Mater. Chem. A 2020, 8, 19246–19253. [Google Scholar] [CrossRef]
- Yang, K.; Yuan, W.; Hua, Z.; Tang, Y.; Yin, F.; Xia, D. Triazine-Based Two-Dimensional Organic Polymer for Selective NO2 Sensing with Excellent Performance. ACS Appl. Mater. Interfaces 2020, 12, 3919–3927. [Google Scholar] [CrossRef]
- Yue, Y.; Cai, P.; Xu, X.; Li, H.; Chen, H.; Zhou, H.-C.; Huang, N. Conductive Metallophthalocyanine Framework Films with High Carrier Mobility as Efficient Chemiresistors. Angew. Chem. Int. Ed. 2021, 60, 10806–10813. [Google Scholar] [CrossRef]
- Guo, L.; Wang, M.; Zeng, X.; Cao, D. Luminescent Porous Organic Polymer Nanotubes for Highly Selective Sensing of H2S. Mater. Chem. Front. 2017, 1, 2643–2650. [Google Scholar]
- Yan, D.; Wang, Z.; Cheng, P.; Chen, Y.; Zhang, Z. Rational Fabrication of Crystalline Smart Materials for Rapid Detection and Efficient Removal of Ozone. Angew. Chem. Int. Ed. 2021, 60, 6055–6060. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.-R.; Jiang, W.; Zhang, C.-R.; Liang, R.-P.; Liu, J.; Qiu, J.-D. Regenerable Carbohydrazide-Linked Fluorescent Covalent Organic Frameworks for Ultrasensitive Detection and Removal of Mercury. ACS Sustain. Chem. Eng. 2020, 8, 445–451. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, G. Application of Synthesized 2,5-bis(allyloxy)terephthalohydrazide Functionalized Covalent Organic Framework Material as a Fluorescence Probe for Selective Detection of Mercury (II). Mater. Today Commun. 2021, 27, 102440. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Xi, H.; Mu, Y.; Liu, X. A Robust and Luminescent Covalent Organic Framework as A Highly Sensitive and Selective Sensor for the Detection of Cu2+ Ions. Chem. Commun. 2016, 52, 6613–6616. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, Y.; Liu, J.; Du, J.; Yu, Y.; Wang, S.; Liang, Z.; Yu, J. Multifunctional Porous Tröger’s Base Polymers with TetraPhenylethene Units: CO2 Adsorption, Luminescence and Sensing Properties. Polym. Chem. 2017, 8, 4842–4848. [Google Scholar] [CrossRef]
- Jin, E.; Li, J.; Geng, K.; Jiang, Q.; Xu, H.; Xu, Q.; Jiang, D. Designed Synthesis of Stable Light-Emitting Two-Dimensional sp2 Carbon-Conjugated Covalent Organic Frameworks. Nat. Commun. 2018, 9, 4143. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Jiang, Y.; Feng, L.; Hua, Y.; Liu, H.; Fan, C.; Yin, M.; Li, S.; Lv, X.; Wang, H. Q-graphene-scaffolded Covalent Organic Frameworks as Fluorescent Probes and Sorbents for the Fluorimetry and Removal of Copper Ions. Anal. Chim. Acta 2019, 1057, 88–97. [Google Scholar] [CrossRef]
- Wang, T.; Xue, R.; Chen, H.; Shi, P.; Lei, X.; Wei, Y.; Guo, H.; Yang, W. Preparation of Two New Polyimide Bond Linked Porous Covalent Organic Frameworks and Their Fluorescence Sensing Application for Sensitive and Selective Determination of Fe3+. New J. Chem. 2017, 41, 14272–14278. [Google Scholar] [CrossRef]
- Li, M.; Cui, Z.; Pang, S.; Meng, L.; Ma, D.; Li, Y.; Shi, Z.; Feng, S. Luminescent Covalent Organic Framework as a Recyclable Turn-off Fluorescent Sensor for Cations and Anions in Aqueous Solution. J. Mater. Chem. C 2019, 7, 11919–11925. [Google Scholar] [CrossRef]
- Wu, X.; Wang, B.; Yang, Z.; Chen, L. Novel Imine-linked Covalent Organic Frameworks: Preparation, Characterization and Application. J. Mater. Chem. A 2019, 7, 5650–5655. [Google Scholar] [CrossRef]
- Chen, G.; Lan, H.-H.; Cai, S.-L.; Sun, B.; Li, X.-L.; He, Z.-H.; Zheng, S.-R.; Fan, J.; Liu, Y.; Zhang, W.-G. Stable Hydrazone-Linked Covalent Organic Frameworks Containing O,N,O′-Chelating Sites for Fe(III) Detection in Water. ACS Appl. Mater. Interfaces 2019, 11, 12830–12837. [Google Scholar] [CrossRef] [Green Version]
- Cui, D.; Ding, X.; Xie, W.; Xu, G.; Su, Z.; Xu, Y.; Xie, Y. A Tetraphenylethylene-based Covalent Organic Framework for Waste Gas Adsorption and Highly Selective Detection of Fe3+. CrystEngComm 2021, 23, 5569–5574. [Google Scholar] [CrossRef]
- Li, D.-M.; Zhang, S.-Y.; Wan, J.-Y.; Zhang, W.-Q.; Yan, Y.-L.; Tang, X.-H.; Zheng, S.-R.; Cai, S.-L.; Zhang, W.-G. A New Hydrazone-linked Covalent Organic Framework for Fe(III) Detection by Fluorescence and QCM Technologies. CrystEngComm 2021, 23, 3594–3601. [Google Scholar] [CrossRef]
- Liang, X.; Ni, Z.; Zhao, L.; Ge, B.; Zhao, H.; Li, W. Multifunctional Triphenylbenzene-based Polyimide Covalent Organic Framework with Absolute Eclipsed Stacking Models for Fluorescence Detecting of Fe3+ and Electrochemical Detecting of Pb2+. Microchem. J. 2021, 170, 106663. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, C.; Huang, W.; Chen, Y.; Wang, Y.; Wang, J. Covalent Organic Framework as A Novel Electrochemical Platform for Highly Sensitive and Stable Detection of Lead. Talanta 2018, 188, 578–583. [Google Scholar] [CrossRef]
- Wang, R.; Ji, W.; Huang, L.; Guo, L.; Wang, X. Electrochemical Determination of Lead(II) in Environmental Waters Using a Sulfydryl Modified Covalent Organic Framework by Square Wave Anodic Stripping Voltammetry (SWASV). Anal. Lett. 2019, 52, 1757–1770. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, L.; Wang, Q.; Zhang, L.; Zhu, P.; Yu, J.; Zhang, Y. Porphyrin-Based Covalent Organic Framework Thin Films as Cathodic Materials for “On−Off−On” Photoelectrochemical Sensing of Lead Ions. ACS Appl. Mater. Interfaces 2021, 13, 20397–20404. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhong, W.; Cui, K.; Zhuang, Z.; Li, L.; Li, L.; Bi, J.; Yu, Y. A Covalent Organic Framework Bearing Thioether Pendant Arms for Selective Detection and Recovery of Au from Ultra-low Concentration Aqueous Solution. Chem. Commun. 2018, 54, 9977–9980. [Google Scholar] [CrossRef]
- Cui, W.-R.; Zhang, C.-R.; Jiang, W.; Li, F.-F.; Liang, R.-P.; Liu, J.; Qiu, J.-D. Regenerable and Stable sp2 Carbon-conjugated Covalent Organic Frameworks for Selective Detection and Extraction of Uranium. Nat. Commun. 2020, 11, 436. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yang, G.-P.; Xiao, S.-J.; Tan, Q.-G.; Zheng, Q.-Q.; Liang, R.-P.; Qiu, J.-D. Facile Construction of Covalent Organic Framework Nanozyme for Colorimetric Detection of Uranium. Small 2021, 17, 2102944. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, R.; Zhu, W.; Zhang, Z.; Chen, Y.; Wang, S.; Deng, Q. Desirability of Position 2, 2′-bipyridine Group into COFs for the Fluorescence Sensing of Ni(II). Sens. Actuators B Chem. 2021, 344, 130216. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, K.; Zhu, L.; Xu, M.; Song, Y.; Zhang, Z.; Du, M. Direct Growth of Two-dimensional Phthalocyanine-based COF on Cu-MOF to Construct a Photoelectrochemical-Electrochemical Dual-mode Biosensing Platform for High-efficiency Determination of Cr(III). Dalton Trans. 2021, 50, 14285–14295. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, Y.; Zhao, Y.; Xia, M.; Liu, X.; Shen, T.; Feng, L.; Yuan, N.; Chen, Q. Fluorescent Test Paper via the In Situ Growth of COFs for Rapid and Convenient Detection of Pd(II) Ions. ACS Appl. Mater. Interfaces 2021, 13, 1644–1650. [Google Scholar] [CrossRef]
- Yue, J.-Y.; Ding, X.-L.; Wang, Y.-T.; Wen, Y.-X.; Yang, P.; Ma, Y.; Tang, B. Dual Functional sp2 Carbon-conjugated Covalent Organic Frameworks for Fluorescence Sensing and Effective Removal and Recovery of Pd2+ Ions. J. Mater. Chem. A 2021, 9, 26861–26866. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, Q.; Ma, R.; Wang, Z.; Yang, Y.; Sha, H.; Ma, X.; Ruan, X.; Zou, X.; Yuan, Y.; et al. Constructing an Ion Pathway for Uranium Extraction from Seawater. Chem 2020, 6, 1683–1691. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, Y.; Ma, X.; Meng, Q.; Wang, L.; Zhao, S.; Zhu, G. Molecularly Imprinted Porous Aromatic Frameworks and Their Composite Components for Selective Extraction of Uranium Ions. Adv. Mater. 2018, 30, 1706507. [Google Scholar] [CrossRef]
- Li, Z.; Meng, Q.; Yang, Y.; Zou, X.; Yuan, Y.; Zhu, G. Constructing Amidoxime-modified Porous Adsorbents with Open Architecture for Cost-effective and Efficient Uranium Extraction. Chem. Sci. 2020, 11, 4747–4752. [Google Scholar] [CrossRef] [Green Version]
- Cui, W.-R.; Li, F.; Xu, R.-H.; Zhang, C.-R.; Chen, X.-R.; Yan, R.-H.; Liang, R.-P.; Qiu, J.-D. Regenerable Covalent Organic Frameworks for Photo-enhanced Uranium Adsorption from Seawater. Angew. Chem. Int. Ed. 2020, 59, 17684–17690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, X.; Feng, X.; Xia, H.; Mu, Y.; Liu, X. Covalent Organic Frameworks as pH Responsive Signaling Scaffolds. Chem. Commun. 2016, 52, 11088–11091. [Google Scholar] [CrossRef]
- Chen, L.; He, L.; Ma, F.; Liu, W.; Wang, Y.; Silver, M.A.; Chen, L.; Zhu, L.; Gui, D.; Diwu, J.; et al. Covalent Organic Framework Functionalized with 8-Hydroxyquinoline as a Dual-Mode Fluorescent and Colorimetric pH Sensor. ACS Appl. Mater. Interfaces 2018, 10, 15364–15368. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, L.; Xie, Y.; Song, Y.; Wang, L. Ratiometric Electrochemical Sensing and Biosensing Based on Multiple Redox-Active State COFDHTA-TTA. Sens. Actuators B Chem. 2019, 281, 1009–1015. [Google Scholar] [CrossRef]
- Yuan, F.; Kong, Y.; You, J.; Zhang, C.; Xian, Y. Rational Synthesis of Imine-Linked Fluorescent Covalent Organic Frameworks with Different pKa for pH Sensing In Vitro and In Vivo. ACS Appl. Mater. Interfaces 2021, 13, 51351–51361. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, K.; Hu, X.; Shi, P.; Guo, Z.; Zhan, H. Novel One-Dimensional Covalent Organic Framework as a H+ Fluorescent Sensor in Acidic Aqueous Solution. ACS Appl. Mater. Interfaces 2021, 13, 1145–1151. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Xia, H.; Ding, X.; Luo, X.; Liu, X.; Mu, Y. Triarylboron-Linked Conjugated Microporous Polymers: Sensing and Removal of Fluoride Ions. Chem. Eur. J. 2015, 21, 17355–17362. [Google Scholar] [CrossRef]
- Li, Z.; Huang, N.; Lee, K.H.; Feng, Y.; Tao, S.; Jiang, Q.; Nagao, Y.; Irle, S.; Jiang, D. Light-Emitting Covalent Organic Frameworks: Fluorescence Improving via Pinpoint Surgery and Selective Switch-On Sensing of Anions. J. Am. Chem. Soc. 2018, 140, 12374–12377. [Google Scholar] [CrossRef]
- Su, L.; Zhang, Z.; Xiong, Y. Water Dispersed Two-dimensional Ultrathin Fe(III)-Modified Covalent Triazine Framework Nanosheets: Peroxidase Like Activity and Colorimetric Biosensing Applications. Nanoscale 2018, 10, 20120–20125. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, F.; Zhang, C.; Yin, S.-Y.; Teng, L.; Chen, L.; Hu, X.-X.; Liu, H.-W.; Yin, X.; Zhang, X.-B. Ultrathin Two-dimensional Covalent Organic Framework Nanoprobe for Interference-Resistant Two-Photon Fluorescence Bioimaging. Chem. Sci. 2018, 9, 8402–8408. [Google Scholar] [CrossRef] [Green Version]
- Paul, B.D.; Snyder, S.H. H2S Signalling Through Protein Sulfhydration and Beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 499–507. [Google Scholar] [CrossRef]
- Gadalla, M.M.; Snyder, S.H. Hydrogen sulfide as a gasotransmitter. J. Neurochem. 2010, 113, 14–26. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Qiu, L.-G.; Yuan, Y.-P.; Xie, A.-J.; Shen, Y.-H.; Zhu, J.-F. Microwave-assisted Synthesis of Highly Fluorescent Nanoparticles of a Melamine-based Porous Covalent Organic Framework for Trace-level Detection of Nitroaromatic Explosives. J. Hazard. Matter. 2012, 221–222, 147–154. [Google Scholar] [CrossRef]
- Sang, N.; Zhan, C.; Cao, D. Highly Sensitive and Selective Detection of 2,4,6-trinitrophenol Using Covalent-Organic Polymer Luminescent Probes. J. Mater. Chem. A 2015, 3, 92–96. [Google Scholar] [CrossRef]
- Guo, L.; Cao, D.; Yun, J.; Zeng, X. Highly Selective Detection of Picric Acid from Multicomponent Mixtures of Nitro Explosives by Using COP Luminescent Probe. Sens. Actuators B Chem. 2017, 243, 753–760. [Google Scholar] [CrossRef]
- Jiang, S.; Meng, L.; Ma, W.; Qia, Q.; Zhang, W.; Xua, B.; Liu, L.; Tian, W. Morphology Controllable Conjugated Network Polymers Based on AIE-active Building Block for TNP Detection. Chin. Chem. Lett. 2021, 32, 1037–1040. [Google Scholar] [CrossRef]
- Rao, M.R.; Fang, Y.; Feyter, S.D.; Perepichka, D.F. Conjugated Covalent Organic Frameworks via Michael Addition-Elimination. J. Am. Chem. Soc. 2017, 139, 2421–2427. [Google Scholar] [CrossRef]
- Dalapati, S.; Jin, S.; Gao, J.; Xu, Y.; Nagai, A.; Jiang, D. An Azine-Linked Covalent Organic Framework. J. Am. Chem. Soc. 2013, 135, 17310–17313. [Google Scholar] [CrossRef]
- Xiang, Z.; Cao, D. Synthesis of Luminescent Covalent–Organic Polymers for Detecting Nitroaromatic Explosives and Small Organic Molecules. Macromol. Rapid Commun. 2012, 33, 1184–1190. [Google Scholar] [CrossRef]
- Kaleeswaran, D.; Vishnoi, P.; Murugavel, R. [3+3] Imine and β-ketoenamine Tethered Fluorescent Covalent-Organic FrameWorks for CO2 Uptake and Nitroaromatic Sensing. J. Mater. Chem. C 2015, 3, 7159–7171. [Google Scholar] [CrossRef]
- Das, G.; Biswal, B.P.; Kandambeth, S.; Venkatesh, V.; Kaur, G.; Addicoat, M.; Heine, T.; Verma, S.; Banerjee, R. Chemical Sensing in Two Dimensional Porous Covalent Organic Nanosheets. Chem. Sci. 2015, 6, 3931–3939. [Google Scholar] [CrossRef]
- Gomes, R.; Bhaumik, A. A New Triazine Functionalized Luminescent Covalent Organic Framework for Nitroaromatic Sensing and CO2 Storage. RSC Adv. 2016, 6, 28047–28054. [Google Scholar] [CrossRef]
- Lin, G.; Ding, H.; Yuan, D.; Wang, B.; Wang, C. A Pyrene-Based, Fluorescent Three-Dimensional Covalent Organic Framework. J. Am. Chem. Soc. 2016, 138, 3302–3305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, S.; Yan, Y.; Xia, F.; Huang, A.; Xian, Y. Highly Fluorescent Polyimide Covalent Organic Nanosheets as Sensing Probes for the Detection of 2,4,6-Trinitrophenol. ACS Appl. Mater. Interfaces 2017, 9, 13415–13421. [Google Scholar] [CrossRef] [PubMed]
- Kaleeswaran, D.; Murugavel, R. Picric Acid Sensing and CO2 Capture by a Sterically Encumbered Azo-linked Fluorescent Triphenylbenzene Based Covalent Organic Polymer. J. Chem. Sci. 2018, 130, 1. [Google Scholar] [CrossRef] [Green Version]
- Das, P.; Mandal, S.K. A Dual-functionalized, Luminescent and Highly Crystalline Covalent Organic Framework: Molecular Decoding Strategies for VOCs and Ultrafast TNP Sensing. J. Mater. Chem. A 2018, 6, 16246–16256. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Yu, Y.; Du, J.; Cui, Y.; Song, X.; Liang, Z. Conjugated Microporous Polymers Based on Biphenylene for CO2 Adsorption and Luminescence Detection of Nitroaromatic Compounds. New J. Chem. 2018, 42, 9482–9487. [Google Scholar] [CrossRef]
- Gao, Q.; Li, X.; Ning, G.-H.; Leng, K.; Tian, B.; Liu, C.; Tang, W.; Xu, H.-S.; Loh, K.P. Highly Photoluminescent Two-dimensional Iminebased Covalent Organic Frameworks for Chemical Sensing. Chem. Commun. 2018, 54, 2349–2352. [Google Scholar] [CrossRef]
- Faheem, M.; Aziz, S.; Jing, X.; Ma, T.; Du, J.; Sun, F.; Tian, Y.; Zhu, G. Dual Luminescent Covalent Organic Frameworks for Nitro-explosive Detection. J. Mater. Chem. A 2019, 7, 27148–27155. [Google Scholar] [CrossRef]
- Pan, L.; Liu, Z.; Tian, M.; Schroeder, B.C.; Aliev, A.E.; Faul, C.F.J. Luminescent and Swellable Conjugated Microporous Polymers for Detecting Nitroaromatic Explosives and Removing Harmful Organic Vapors. ACS Appl. Mater. Interfaces 2019, 11, 48352–48362. [Google Scholar] [CrossRef]
- Das, P.; Chakraborty, G.; Mandal, S.K. Comprehensive Structural and Microscopic Characterization of an Azine−Triazine-Functionalized Highly Crystalline Covalent Organic Framework and Its Selective Detection of Dichloran and 4-Nitroaniline. ACS Appl. Mater. Interfaces 2020, 12, 10224–10232. [Google Scholar] [CrossRef]
- Chen, J.-Q.; Zheng, Q.-Q.; Xiao, S.-J.; Zhang, L.; Liang, R.-P.; Ouyang, G.; Qiu, J.-D. Construction of Two-Dimensional Fluorescent Covalent Organic Framework Nanosheets for the Detection and Removal of Nitrophenols. Anal. Chem. 2022, 94, 2517–2526. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Li, Y.; Xia, M.; Xia, T.; Sun, H.; Sui, Z.; Hu, X.-M.; Chen, Q. Ultra-Stable Fluorescent 2D Covalent Organic Framework for Rapid Adsorption and Selective Detection of Radioiodine. Microporous Mesoporous Mater. 2021, 319, 111046. [Google Scholar] [CrossRef]
- Wang, P.; Xu, Q.; Li, Z.; Jiang, W.; Jiang, Q.; Jiang, D. Exceptional Iodine Capture in 2D Covalent Organic Frameworks. Adv. Mater. 2018, 30, 1801991. [Google Scholar] [CrossRef]
- Geng, T.; Zhang, W.; Zhu, Z.; Kai, X. Triazine-based conjugated microporous polymers constructing triphenylamine and its derivatives with nitrogen as core for iodine adsorption and flfluorescence sensing I2. Microporous Mesoporous Mater. 2019, 273, 163–170. [Google Scholar] [CrossRef]
- Geng, T.; Ma, L.; Chen, G.; Zhang, C.; Zhang, W.; Xia, H.; Zhu, H. Poly[1,3,6,8-tetra(2-thiophenyl)pyrene] and poly[1,3,6,8-tetra(3-thiophenyl)pyrene] conjugated microporous polymers for reversible adsorbing and fluorescent sensing iodine. J. Polym. Res. 2019, 26, 113. [Google Scholar] [CrossRef]
- Geng, T.; Liu, M.; Zhang, C.; Hu, C.; Xu, H. Synthesis of secondary amine-based fluorescent porous organic polymers via Friedel–Crafts polymerization reaction for adsorbing and fluorescent sensing iodine. J. Appl. Polym. Sci. 2020, 137, e49255. [Google Scholar] [CrossRef]
- Geng, T.; Zhang, C.; Chen, G.; Ma, L.; Zhang, W.; Xia, H. Synthesis of tetraphenylethylene-based fluorescent conjugated microporous polymers for fluorescent sensing and adsorbing iodine. Microporous Mesoporous Mater. 2019, 284, 468–475. [Google Scholar] [CrossRef]
- Geng, T.; Chen, G.; Ma, L.; Zhang, C.; Zhang, W.; Xu, H. The spirobifluorene-based fluorescent conjugated microporous polymers for reversible adsorbing iodine, fluorescent sensing iodine and nitroaromatic compounds. Eur. Polym. J. 2019, 115, 37–44. [Google Scholar] [CrossRef]
- Geng, T.; Ma, L.; Chen, G.; Zhang, C.; Zhang, W.; Niu, Q. Fluorescent conjugated microporous polymers containing pyrazine moieties for adsorbing and fluorescent sensing of iodine. Environ. Sci. Pollut. Res. 2020, 27, 20235–20245. [Google Scholar] [CrossRef]
- Geng, T.; Zhang, C.; Hu, C.; Liu, M.; Fei, Y.; Xia, H. Synthesis of 1,6-disubstituted pyrene-based conjugated microporous polymers for reversible adsorption and fluorescence sensing of iodine. New J. Chem. 2020, 44, 2312–2320. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Huang, W.; Wang, Y.; Hu, X. A Novel AuNPs−doped COFs Composite as Electrochemical Probe for Chlorogenic Acid Detection with Enhanced Sensitivity and Stability. Sens. Actuators B Chem. 2018, 276, 362–369. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Liu, H. A Novel Fluorescence and SPE Adsorption Nanomaterials of Molecularly Imprinted Polymers Based on Quantum Dot-Grafted Covalent Organic Frameworks for the High Selectivity and Sensitivity Detection of Ferulic Acid. Nanomaterials 2019, 9, 305. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Xua, L.; Waterhouse, G.I.N.; Wang, M.; Qiao, X.; Xu, Z. Novel Three-dimensional Electrochemical Sensor with Dual Signal Amplification Based on MoS2 Nanosheets and High-conductive NH2MWCNT@COF for Sulfamerazine Determination. Sens. Actuators B Chem. 2019, 281, 107–114. [Google Scholar] [CrossRef]
- Zhou, N.; Ma, Y.; Hu, B.; He, L.; Wang, S.; Zhang, Z.; Lu, S. Construction of Ce−MOF@COF Hybrid Nanostructure: Label-free Aptasensor for the Ultrasensitive Detection of Oxytetracycline Residues in Aqueous Solution Environments. Biosens. Bioelectron. 2019, 127, 92–100. [Google Scholar] [CrossRef]
- Ma, X.; Pang, C.; Li, S.; Xiong, Y.; Li, J.; Luo, J.; Yang, Y. Synthesis of Zr-coordinated Amide Porphyrin-based Two-dimensional Covalent Organic Framework at Liquid-liquid Interface for Electrochemical Sensing of Tetracycline. Biosens. Bioelectron. 2019, 146, 111734. [Google Scholar] [CrossRef]
- Wang, J.-M.; Lian, X.; Yan, B. Eu3+-Functionalized Covalent Organic Framework Hybrid Material as a Sensitive Turn-On Fluorescent Switch for Levofloxacin Monitoring in Serum and Urine. Inorg. Chem. 2019, 58, 9956–9963. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, H.; Geng, W.; Wang, Y. A Dual-function Molecularly Imprinted Optopolymer Based on Quantum Dotsgrafted Covalent-Organic Frameworks for the Sensitive Detection of Tyramine in Fermented Meat Products. Food Chem. 2019, 277, 639–645. [Google Scholar] [CrossRef]
- Dong, J.; Li, X.; Peh, S.B.; Yuan, Y.D.; Wang, Y.; Ji, D.; Peng, S.; Liu, G.; Ying, S.; Yuan, D.; et al. Restriction of Molecular Rotors in Ultrathin Two-Dimensional Covalent Organic Framework Nanosheets for Sensing Signal Amplification. Chem. Mater. 2019, 31, 146–160. [Google Scholar] [CrossRef]
- Gua, Y.; Wang, J.; Shia, H.; Pana, M.; Liu, B.; Fang, G.; Wang, S. Electrochemiluminescence sensor based on upconversion nanoparticles and oligoaniline-crosslinked gold nanoparticles imprinting recognition sites for the determination of dopamine. Biosens. Bioelectron. 2019, 128, 129–136. [Google Scholar] [CrossRef]
- Yao, X.; Shen, J.; Liu, Q.; Fa, H.; Yang, M.; Hou, C. A Novel Electrochemical Aptasensor for the Sensitive Detection of Kanamycin Based on UiO-66NH2/MCA/MWCNT@rGONR Nanocomposites. Anal. Methods 2020, 12, 4967–4976. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, L.; Yan, B. Indicator Displacement Assay Inside Dye-Functionalized Covalent Organic Frameworks for Ultrasensitive Monitoring of Sialic Acid, an Ovarian Cancer Biomarker. ACS Appl. Mater. Interfaces 2020, 12, 12990–12997. [Google Scholar] [CrossRef]
- Han, Z.-Y.; Zhang, H.; Li, H.-K.; Zhu, Q.-Q.; He, H. Ingenious Construction of an Electrochemical Aptasensor based on a Au@COF/GO−NH2 Composite with Excellent Detection Performance. J. Mater. Chem. C 2021, 9, 4576. [Google Scholar] [CrossRef]
- Yang, M.; Mao, Y.; Wang, B.; Lin, L.; Wang, Y.; Zhang, L.; Jiang, Y.; Zhao, M.; Chen, H.; Zhang, Y. Heterometallic Mg@Fe−MIL−101/TpPa−1−COF Grown on Stainless Steel Mesh: Enhancing Photo-degradation, Fluorescent Detection and Toxicity Assessment for Tetracycline Hydrochloride. Colloid Surf. A 2021, 631, 127725. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, J.; Yang, F.; Lv, S.; Wang, J.; Wang, S. Easy Green Construction of a Universal Sensing Platform Based on Crystalline Polyimide Covalent Organic Frameworks with Sensitive Fluorescence Response to Metal Ions and Antibiotics. ACS Appl. Bio Mater. 2021, 4, 995–1002. [Google Scholar] [CrossRef]
- Wang, J.; Qu, X.; Zhao, L.; Yan, B. Fabricating Nanosheets and Ratiometric Detection of 5-Fluorouracil by Covalent Organic Framework Hybrid Material. Anal. Chem. 2021, 93, 4308–4316. [Google Scholar] [CrossRef]
- Song, Y.; Ma, R.; Hao, L.; Yang, X.; Wang, C.; Wu, Q.; Wang, Z. Application of Covalent Organic Framework as the Adsorbent for Solid-phase Extraction of Trace Levels of Pesticide Residues Prior to High-performance Liquid Chromatography-Ultraviolet Detection. J. Chromatogr. A 2018, 1572, 20–26. [Google Scholar] [CrossRef]
- Ji, W.-H.; Guo, Y.-S.; Wang, X.; Lu, X.-F.; Guo, D.-S. Amino-modified Covalent Organic Framework as Solid Phase Extraction Absorbent for Determination of Carboxylic Acid Pesticides in Environmental Water Samples. J. Chromatogr. A 2019, 1595, 11–18. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Zhang, D.; Zheng, F.; Huang, M.; Sun, J.; Sun, X.; Li, H.; Wang, J.; Sun, B. A Fluorescent Nanoprobe for 4-ethylguaiacol Based on the Use of A Molecularly Imprinted Polymer Doped with A Covalent Organic Framework Grafted onto Carbon Nanodots. Microchim. Acta 2019, 186, 182. [Google Scholar] [CrossRef]
- Kandambeth, S.; Mallick, A.; Lukose, B.; Mane, M.V.; Heine, T.; Banerjee, R. Construction of Crystalline 2D Covalent Organic Frameworks with Remarkable Chemical (Acid/Base) Stability via a Combined Reversible and Irreversible Route. J. Am. Chem. Soc. 2012, 134, 19524–19527. [Google Scholar] [CrossRef]
- Liu, X.; Chen, H.; Wang, R.; Zang, S.-Q.; Mak, T.C.W. Cationic Covalent-Organic Framework as Efficient Redox Motor for High-Performance Lithium–Sulfur Batteries. Small 2020, 16, 2002932. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Wei, T.; Bao, J.; Zhu, Q.; Dai, Z. Fe-Porphyrin-Based Covalent Organic Framework As a Novel Peroxidase Mimic for a One-Pot Glucose Colorimetric Assay. ACS Appl. Bio Mater. 2018, 1, 382–388. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, X.; Ma, Y.; Tan, H.; Li, Y. A novel Peroxidase/oxidase Mimetic Fe-porphyrin Covalent Organic Framework Enhanced the Luminol Chemiluminescence Reaction and Its Application in Glucose Sensing. Luminescence 2020, 35, 1366–1372. [Google Scholar] [CrossRef]
- Yue, J.-Y.; Ding, X.-L.; Wang, L.; Yang, R.; Bi, J.-S.; Song, Y.-W.; Yang, P.; Ma, Y.; Tang, B. Novel Enzyme-Functionalized Covalent Organic Frameworks for the Colorimetric Sensing of Glucose in Body Fluids and Drinks. Mater. Chem. Front. 2021, 5, 3859–3866. [Google Scholar] [CrossRef]
- He, Y.; Lin, X.; Tang, Y.; Ye, L. A Selective Sensing Platform for the Simultaneous Detection of Ascorbic acid, Dopamine, and Uric Acid Based on AuNPs/carboxylated COFs/Poly(fuchsin basic) Film. Anal. Methods 2021, 13, 4503–4514. [Google Scholar] [CrossRef]
- Wu, X.; Han, X.; Xu, Q.; Liu, Y.; Yuan, C.; Yang, S.; Liu, Y.; Jiang, J.; Cui, Y. Chiral BINOL-Based Covalent Organic Frameworks for Enantioselective Sensing. J. Am. Chem. Soc. 2019, 141, 7081–7089. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, N.; Ali, A.; Wei, Q.; Wu, D.; Ren, X. Electrochemical Ultrasensitive Detection of Cardiac Troponin I Using Covalent Organic Frameworks for Signal Amplification. Biosens. Bioelectron. 2018, 119, 176–181. [Google Scholar] [CrossRef]
- Feng, S.; Yan, M.; Xue, Y.; Huang, J.; Yang, X. Electrochemical Immunosensor for Cardiac Troponin I Detection Based on Covalent Organic Framework and Enzyme-Catalyzed Signal Amplification. Anal. Chem. 2021, 93, 13572–13579. [Google Scholar] [CrossRef]
- Zhai, R.; Gong, X.Y.; Xie, J.; Yuan, Y.; Xu, F.; Jiang, Y.; Huang, Z.; Dai, X.; Zhang, Y.; Qian, X.; et al. Ultrasensitive Analysis of Heat Shock Protein 90α with Antibodies Orderly Arrayed on a Novel Type of Immunoprobe Based on Magnetic COFs. Talanta 2019, 191, 553–560. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, M.; Deng, Y.; Bai, R.; Zhang, X.; Li, D.; Zhang, K.; Hu, R.; Yang, Y. The Preparation of C-Reactive Protein Immunosensor Based on Nano-Mimetic Enzyme Co3O4. J. Biomed. Nanotechnol. 2018, 14, 1169–1177. [Google Scholar] [CrossRef]
- Deng, Y.; Du, X.; Ma, Y.; Zhang, K.; Zhang, X.; Li, D.-L.; Bai, R.; Hu, R.; Yang, Y.-H. Development of C-Reactive Protein Immunosensor Using Thionine/Au Nanoparticles-Covalent Organic Framework-LZU8 as Label. Nanosci. Nanotechnol. Lett. 2018, 10, 520–527. [Google Scholar] [CrossRef]
- Liu, T.-Z.; Hu, R.; Zhang, X.; Zhang, K.-L.; Liu, Y.; Zhang, X.-B.; Bai, R.-Y.; Li, D.; Yang, Y.-H. Metal–Organic Framework Nanomaterials as Novel Signal Probes for Electron Transfer Mediated Ultrasensitive Electrochemical Immunoassay. Anal. Chem. 2016, 88, 12516–12523. [Google Scholar] [CrossRef]
- Li, W.; Yang, C.-X.; Yan, X.-P. A Versatile Covalent Organic Framework-based Platform for Sensing Biomolecules. Chem. Commun. 2017, 53, 11469–11471. [Google Scholar] [CrossRef]
- Peng, Y.; Huang, Y.; Zhu, Y.; Chen, B.; Wang, L.; Lai, Z.; Zhang, Z.; Zhao, M.; Tan, C.; Yang, N.; et al. Ultrathin Two-Dimensional Covalent Organic Framework Nanosheets: Preparation and Application in Highly Sensitive and Selective DNA Detection. J. Am. Chem. Soc. 2017, 139, 8698–8704. [Google Scholar] [CrossRef] [Green Version]
- Mal, A.; Mishra, R.K.; Praveen, V.K.; Khayum, M.A.; Banerjee, R.; Ajayaghosh, A. Supramolecular Reassembly of Self-Exfoliated Ionic Covalent Organic Nanosheets for Label-Free Detection of Double-Stranded DNA. Angew. Chem. Int. Ed. 2018, 57, 8443–8447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chi, K.-N.; Li, D.-L.; Deng, Y.; Ma, Y.-C.; Xu, Q.-Q.; Hu, R.; Yang, Y.-H. 2D-porphrinic Covalent Organic Framework-Based Aptasensor with Enhanced Photoelectrochemical Response for the Detection of C-reactive Protein. Biosens. Bioelectron. 2019, 129, 64–71. [Google Scholar] [CrossRef]













| Name | Monomer 1 | Monomer 2 | Linker |
|---|---|---|---|
| Boronate |  |  |  |
| Imine |  |  |  |
| Hydrazone |  |  |  |
| Maleimide |  |  |  |
| Phenazine |  |  |  |
| Acrylonitrile |  |  |  |
| Triazine |  |  | |
| Borazine |  |  | |
| Benzimidazole |  |  |  |
| Benzobisoxazole |  |  |  |
| Analyte | Year | COF Names | Specific Binding Site | Type of Detectable Signal | Detection Range | LOD | Reference |
|---|---|---|---|---|---|---|---|
| Hg2+ | 2016 | COF−LZU8 | Thioether | Fluorescence, “turn off” | - | 25.0 ppb | [42] |
| 2019 | Tp−Bpy NSs | AuNPs | Chromism | - | 0.33 nM | [29] | |
| 2020 | TFPPy−CHYD | Carbohydrazide | Fluorescence, “turn off” | 0.05 μM–4 μM | 17 nM. | [73] | |
| 2021 | BATHz−Bt | Carbon-carbon double bonds | Fluorescence, “turn off” | 0–27.5 mM | 26 nM | [74] | |
| Cu2+ | 2016 | COF−JLU3 | Hydroxyl and azine | Fluorescence, “turn off” | 0–0.4 mM | 0.31 mM | [75] |
| 2017 | CTF | Triazine | Chromism | 1.0 g/L-80.0 g/L | 0.05 g/L | [50] | |
| 2017 | LMOP−15LMOP−15 | Tertiary amino group | Fluorescence, “turn off” | 5.1 × 10−8 M | [76] | ||
| 2018 | sp2c-COFs | Cyano groups | Fluorescence, “turn off” | 88 ppb | [77] | ||
| 2019 | QG−scaffolded COF | N atoms and hydroxyl groups | Fluorescence, “turn off” | 0.0010~10.0 μM | 0.50 nM | [78] | |
| Fe3+ | 2017 | PI−COF 201 | Amino groups | Fluorescence, “turn off” | 5.0–400 μM | 0.13 μM | [79] |
| PI−COF 202 | 5.0–300 μM | 0.22 μM | |||||
| 2019 | COF−TT | Amino groups | Fluorescence, “turn off” | 0–1.2 mM | 0.369 mM | [80] | |
| 2019 | TaDAP TaDA | Imine | Fluorescence, “turn off” | 0.02–0.2 mM | 18 μM | [81] | |
| 2019 | Bth−Dha, Bth−Dma | O,N,O′-chelating sites | Fluorescence, “turn off” | - | 0.17 μM | [82] | |
| 2021 | TTPE−COF | Fluorescence “turn off” | 10−8–10−2 M | 3.07 μM | [83] | ||
| 2021 | Tfpa−Mth COF | Hydrazide and phenol ether | Fluorescence, “turn off” and QCM | - | 64 nM | [84] | |
| 2021 | PMDA−TAPB | Carbonyl group | Fluorescence, “turn off” | - | - | [85] | |
| Pb2+ | 2018 | TAPB-DMTP−COF | Amino groups | Electrochemical signals | 0.0050–2.0 μM | 0.0019 μmol/L | [86] |
| 2019 | Sulfhydryl modified TAPB−DMTTAPB−DMTTAPB−DMTP−COF | Sulfhydryl | Electrochemical signals | 0.05–20 ng·mL−1 | 0.015 ng·mL−1 | [87] | |
| 2021 | TAPP−COF | Photoelectrochemical signal | 0.05–1000 nM | 0.012 nM | [88] | ||
| 2021 | PMDA−TAPB | Amino groups | Electrochemical signals | 5–9000 nM | 1.22 nM | [85] | |
| Au+ | 2018 | TTB−COF | Thioether | Fluorescence, “turn on” | 1.0–10.0 mM | 1.39 mM | [89] |
| UO22+ | 2020 | TFPT−BTAN−AO | Carbon-carbon double bonds | Fluorescence, “turn off” | - | 6.7 nM | [90] |
| 2021 | Tph−BDP | Imines of the CT complex | Chromism | 0.18–75 μM | 0.05 μM | [91] | |
| Ni2+ | 2021 | BPD−COFs | N atoms | Fluorescence, “turn off” | 0.420–1.26 × 103 pM | 68.0 pM | [92] |
| Cr3+ | 2021 | CoPc−PT−COF@Cu−MOF | Bipyridine | Electrochemical signals | 10−1–105 pM | 0.0229 pM | [93] |
| Pd2+ | 2021 | XB−COFs | Carbon-carbon double bonds | Fluorescence, “turn off” | - | 0.29 μM | [94] |
| 2021 | PY−SE−COF | Selenodiazole | Fluorescence, “turn off” | 20–450 mM | 0.45 mM | [95] |
| Analyte | Year | COF/COF Hybrid Names | Specific Binding Site | Type of Detectable Signal | Detection Range | Reference |
|---|---|---|---|---|---|---|
| H+ | 2016 | COF−JLU4 | Amine | Fluorescence “turn off” | pH 0.9–13.0 | [100] |
| 2018 | COF−HQ | Quinoline | Fluorescence “turn off” | pH 1.0–5.0 | [101] | |
| 2019 | COFDHTA-TTA | - | Electrochemical signals | pH 3.0–11.0 | [102] | |
| 2021 | COF2 | Imine or triazine | Fluorescence “turn on” | pH 5.0–8.0 | [103] | |
| 2021 | COF−TP | Amine | Fluorescence “turn off” | pH 0–6.0 | [104] |
| Analyte | Year | COF Names | Specific Binding Site | Type of Detectable Signal | Detection Range | LOD | Reference |
| CrO42−(Cr2O72−) | 2019 | COF−TT | Fluorescence, “turn off” | 0.343 mM | [80] | ||
| MnO4− | 2019 | COF−TT | Fluorescence, “turn off” | 0.320 mM | [80] | ||
| F− | 2015 | BCMP−3 | Boron sites | Fluorescence, “turn off” | [105] | ||
| 2018 | TFPPy−DETHz−COF | Amine | Fluorescence, “turn off” | - | 50.5 ppb | [106] | |
| 2018 | 2D−Fe−CTF | Triazine | Chromism | 10–100 μM | 0.56 μM | [107] | |
| S2− | 2018 | TpASH | Azide | Fluorescence, “turn on” | 1 μM–5 mM | 0.12 μM | [108] |
| COF Names | Year | Analyte | Type of Detectable Signal | Detection Range | LOD | Reference |
|---|---|---|---|---|---|---|
| SNW−1 | 2012 | picric acid | Fluorescence, “turn off” | 0.2–52.4 μM | 0.05 μM | [111] |
| COP−2 COP−3 COP−4 | 2012 | picric acid | Fluorescence, “turn off” | - | ~1 ppm | [117] |
| TNT | ~1 ppm | |||||
| Py−Azine COF | 2013 | picric acid | Fluorescence, “turn off” | 0–70 ppm | - | [116] |
| COF−301 COF−401 | 2015 | picric acid | Fluorescence, “turn off” | - | 1 ppm | [112] |
| iPrTAPB−TFP | 2015 | picric acid | Fluorescence, “turn off” | - | 1 ppm | [118] |
| TfpBDH−CONs | 2015 | picric acid | Fluorescence, “turn on/off” | - | 1 × 10−3 M | [119] |
| TRIPTA | 2016 | picric acid | Fluorescence, “turn off” | - | 51.96 nM | [120] |
| 3D−Py−COF | 2016 | picric acid | Fluorescence, “turn off” | 0−20 ppm | - | [121] |
| 3′PD | 2017 | picric acid | Fluorescence, “turn off” | [115] | ||
| triacetone triperoxide | ||||||
| PI−CONs | 2017 | picric acid | Fluorescence, “turn off” | 0.5–10 μM | 0.25 μM | [122] |
| COP−612 COP−616 | 2017 | picric acid | Fluorescence, “turn off” | - | 15 ppm | [113] |
| LMOP−15 | 2017 | picric acid | Fluorescence, “turn off” | - | 0.33 μM | [76] |
| iPrTAPB−Azo−COP | 2018 | picric acid | Fluorescence, “turn off” | - | 13 ppm | [123] |
| 1 | 2018 | picric acid | Fluorescence, “turn off” | - | 68 ppb | [124] |
| CMP−LS1 CMP−LS2 | 2018 | picric acid | Fluorescence, “turn off” | - | - | [125] |
| Py−TPE−COF | 2018 | picric acid | Fluorescence, “turn off” | - | 10 ppm | [126] |
| DL−COF | 2019 | picric acid | Fluorescence, “turn off” | - | 13.10 ppb | [127] |
| 2,4-dinitrophenol | 8.56 ppb | |||||
| 2,4-dinitrotoluene | 10.40 ppb | |||||
| 4-nitrophenol | 5.15 ppb | |||||
| 4-nitrotoluene | 6.92 ppb | |||||
| LPCMP1−4 | 2019 | TNT | Fluorescence, “turn off” | 0–100 ppm | - | [128] |
| ANCOF | 2020 | Dichloran | Fluorescence, “turn off” | - | 142 ppb | [129] |
| 4-nitroaniline | - | 89 ppb | ||||
| A−COF | 2021 | picric acid | Fluorescence, “turn off” | - | 0.09 μM | [114] |
| TFPB−TTA COF | 2022 | DNP | Fluorescence, “turn off” | 50 nM–10 μM | 18 nM | [130] |
| picric acid | 50 nM–12.5 μM | 16 nM |
| Year | COF/COF Hybrid Names | Analyte | Specific Binding Site | Type of Detectable Signal | Detection Range | LOD | Reference |
|---|---|---|---|---|---|---|---|
| 2018 | TAPB−DMTTAPB−DMTP−COFs/AuNPs | Chlorogenic acid | - | Electrochemical signals (CV) | 1.0 × 10−8–4.0 × 10−5 mol L−1 | 9.5 × 10−9 mol L−1 | [140] |
| 2018 | 2D Fe−CTFs | Sarcosine | Sarcosine oxidase | Chromism | 10–100 μM | 0.56μM | [107] |
| ochratoxin A | Aptamer | 0.2–0.8 μM | - | ||||
| 2019 | Py−M−COF | Enrofloxacin | Aptamer | Electrochemical signals (EIS) | 0.01 pg mL−1–2 ng mL−1 | 6.07 fg mL−1 | [49] |
| Ampicillin | 0.001–1000 pg mL−1 | 0.04 fg mL−1 | |||||
| 2019 | QD−grafted COFs | Ferulic Acid | Molecular imprinting, amino groups | Fluorescence, “turn on” | 0.03–60 mg kg−1 | 5 μg kg−1 | [141] |
| 2019 | MIP/MoS2/NH2−MWCNT@COF | Sulfamerazine | Molecular imprinting | Electrochemical signals | 0.30–2.0 × 102 μM | 0.11 μM | [142] |
| 2019 | Ce−MOF@MCA | Oxytetracycline | Aptamer | Electrochemical signal (EIS) | 0.1–0.5 ng mL−1 | 17.4 fg/mL | [143] |
| 2019 | Zr−amide−Por-based 2D COF | Tetracycline | Molecular imprinting | Electrochemical signals | 5–60 pM | 2.3 pM | [144] |
| 2019 | Eu@TpPa−1 | Levofloxacin | Europium ions | Fluorescence, “turn off” | 10−6–10−2 M | 0.2 μM | [145] |
| 2019 | MIOP based on QDs−grafted COFs | Tyramine | H-bond, shape selectivity | Fluorescence, “turn on” | 35–35,000 µg/kg | 7.0 µg/kg | [146] |
| SPE–HPLC | 20–2000 µg/kg | 5.0 µg/kg | |||||
| 2019 | NUS−30 | L-dopa | Azine | Fluorescence, “turn off” | [147] | ||
| 2019 | PATP@AuNPs−crosslinked MIP | Dopamine | Molecular imprinting | electrochemiluminescence | 10–14−10−6 M | 2 × 10−15 M | [148] |
| 2020 | UiO−66−NH2/MCA/MWCNT@rGONR | Kanamycin | Aptamer | Electrochemical signals | 25–900 nM | 13 nM | [149] |
| 2020 | TpPa−1@Dye | Sialic acid | Cr3+ | Fluorescence, “turn on” | 10−8–10−2 M | 7.08 × 10−9 M | [150] |
| 2021 | Au@COF/GO−NH2 | Chloramphenicol | Aptamer | Electrochemical signal (EIS) | 0.0001–1 ng mL-1 | 16.13 fg mL−1 | [151] |
| 2021 | Mg@Fe−MIL−101/TpPa−1−COF | Tetracycline | Mg2+ | Fluorescence, “turn off” | - | - | [152] |
| 2021 | COF−1 or COF−2 | Tetracycline | - | Fluorescence, “turn off” | 0.005−0.0625 mM | 0.002 mM | [153] |
| ofloxacin | 0.025−0.25 mM | 0.0065 mM | |||||
| 2021 | Eu@TpPa−1 | 5-Fluorouracil | π−π stacking interactions | Fluorescence | 10−7–10−3 M | 6.45 × 10−8 M | [154] |
| 2018 | DAAQ−TFP | Diflubenzuron | Amino and carbonyl group (H bonding) π−π stacking interactions | HPLC | 0.2–160.0 ng mL−1 | 0.02 ng mL−1 | [155] |
| Triflumuron | 0.2–160.0 ng mL−1 | 0.02 ng mL−1 | |||||
| Hexaflumuron | 0.2–160.0 ng mL−1 | 0.05 ng mL−1 | |||||
| Teflubenzuron | 0.2–160.0 ng mL−1 | 0.04 ng mL−1 | |||||
| 2019 | NH2@COF | Carboxylic acid pesticides | Amino group | HPLC-DAD | 0.2–100 ng mL−1 | 0.04–0.20 ng mL−1 | [156] |
| 2019 | CNs−grafted COFs@MIP | 4-ethylguaiacol | The surface of the silica matrix by acid–base pairing interactions | Fluorescence, “turn off” | 0.025–1μg ml−1 | 17 ng mL−1 | [157] |
| Analyte | Year | COF/COF Hybrid Names | Specific Binding Site | Type of Detectable Signal | Detection Range | LOD | Reference |
|---|---|---|---|---|---|---|---|
| Glucose | 2018 | Fe−COF | Glucose oxidase | Chromism | 5–350 μM | 1.1 μM | [160] |
| 2019 | COFDHTA-TTA | Glucose oxidase | Electrochemical signal | 0.60 μM–6.0 mM | 0.38 μM | [102] | |
| 2020 | Fe−PorCOF | Glucose oxidase | Chemiluminescence | 0.01–10 μM | 5.3 nM | [161] | |
| 2021 | COFHD–GOX | Glucose oxidase | Chromism | 5–2000 μM | 0.54 μM | [162] | |
| Uric acid | 2021 | COF−DC−8 | Hydroxyl, triazine | Electrochemical signals | 5.0–25 μM, 25–250 μM | 0.77 μM | [163] |
| Ascorbic acid | 2021 | COF−DC−8 | Hydroxyl, triazine | Electrochemical signals | 30–180 μM, 0.18–1.5 μM | 12.0 μM | [163] |
| Dopamine | 2021 | COF−DC−8 | Hydroxyl, triazine | Electrochemical signals | 1.0–6.0 μM, 8.0–50 μM | 0.25 μM | [163] |
| GSH | 2020 | COF−300−AR | Chromism | 1–15 μM | 1.0 μM | [26] | |
| 2021 | Py−TT COF | Chromism | 0.4 − 60 μM | 0.225 μM | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Liu, D.; Wang, G. Covalent Organic Frameworks for Chemical and Biological Sensing. Molecules 2022, 27, 2586. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27082586
Zhang S, Liu D, Wang G. Covalent Organic Frameworks for Chemical and Biological Sensing. Molecules. 2022; 27(8):2586. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27082586
Chicago/Turabian StyleZhang, Shiji, Danqing Liu, and Guangtong Wang. 2022. "Covalent Organic Frameworks for Chemical and Biological Sensing" Molecules 27, no. 8: 2586. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27082586