Ultra Low-Dose Radiation: Stress Responses and Impacts Using Rice as a Grass Model
Abstract
:1. Introduction
2. Experimental Section
2.1. Soil sample and gamma- and beta- ray measurements
2.2. Test plants and in vitro rice seedling system
2.3. Total RNA extraction and Northern analysis
2.4. Semi-quantitative RT-PCR analysis
2.5. Phytoalexin estimation
3. Results and Discussion
3.1. Radioactive Chernobyl soil triggers defense/stress responses in rice leaves
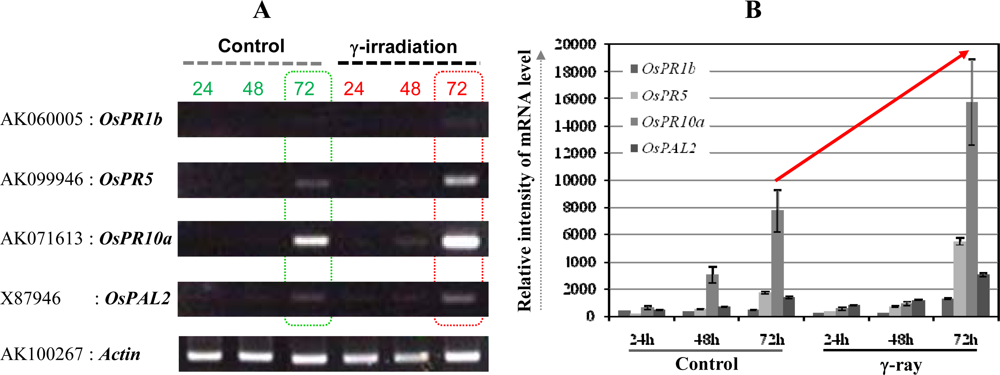
3.2. Ultra low-dose gamma (γ)-radiation also triggers the expression of defense/stress marker genes
3.3. The OsPR10a is a potential candidate marker gene for radiation responses
4. Conclusions
Acknowledgments
References
- Stone, R. Return to the inferno: Chornobyl after 20 years. Science 2006, 312, 180–182. [Google Scholar]
- Kovalchuk, O; Dubrova, YE; Arkhipov, A; Hohn, B; Kovalchuk, I. Wheat mutation rate after Chernobyl. Nature 2000, 407, 583–584. [Google Scholar]
- Jwa, NS; Agrawal, GK; Tamogami, S; Yonekura, M; Han, O; Iwahashi, H; Rakwal, R. Role of defense/stress-related marker genes, proteins and secondary metabolites in defining rice self-defense mechanisms. Plant Physiol. Biochem 2006, 44, 261–273. [Google Scholar]
- Agrawal, GK; Rakwal, R. Rice proteomics: A cornerstone for cereal food crop proteomes. Mass Spec. Rev 2006, 25, 1–53. [Google Scholar]
- Agrawal, GK; Rakwal, R; Jwa, NS; Agrawal, VP. Signaling molecules and blast pathogen attack activates rice OsPR1a and OsPR1b genes: A model illustrating components participating during defense/stress response. Plant Physiol. Biochem 2001, 39, 1095–1103. [Google Scholar]
- Agrawal, GK; Jwa, NS; Iwahashi, H; Rakwal, R. Importance of ascorbate peroxidases OsAPX1 and OsAPX2 in the rice pathogen response pathways and growth and reproduction revealed by their transcriptional profiling. Gene 2003, 322, 93–103. [Google Scholar]
- Kusumi, K; Komori, H; Satoh, H; Iba, K. Characterization of a zebra mutant of rice with increased susceptibility to light stress. Plant Cell Phys 2000, 41, 158–164. [Google Scholar]
- Cho, K; Shibato, J; Agrawal, GK; Jung, YH; Kubo, A; Jwa, NS; Tamogami, S; Satoh, K; Kikuchi, S; Higashi, T; Kimura, S; Saji, H; Tanaka, Y; Iwahashi, H; Masuo, Y; Rakwal, R. Integrated transcriptomics, proteomics and metabolomics analyses to survey ozone responses in the leaves of rice seedling. J. Proteome Res 2008, 7, 2980–2998. [Google Scholar]
- Tamogami, S; Rakwal, R; Kodama, O. Phytoalexin production by amino acid conjugates of jasmonic acid through induction of naringenin-7-O-methyltransferase, a key enzyme on phytoalexin biosynthesis in rice (Oryza sativa L.). FEBS Lett 1997, 401, 239–242. [Google Scholar]
- Tobin, EM; Silverthorne, J. Light regulation of gene expression in higher plants. Annu. Rev. Plant Physiol 1985, 36, 569–593. [Google Scholar]
- Dean, C; Pichersky, E; Dunsmuir, P. Structure, evolution and regulation of RbcS genes in higher plants. Annu. Rev. Plant Physiol 1989, 40, 415–439. [Google Scholar]
- Kimura, S; Shibato, J; Agrawal, GK; Kim, YK; Nahm, BH; Jwa, NS; Iwahashi, H; Rakwal, R. Microarray analysis of rice leaf response to radioactivity from contaminated Chernobyl soil. Rice Gen. Newsl 2008, 24, 52–54. [Google Scholar]
- Annals of the ICRP; ICRP: Oxford, UK, 2007; 103, pp. 1–135.
- Hooker, AM; Bhat, M; Day, TK; Lane, JM; Swinburne, SJ; Morley, AA; Sykes, PJ. The linear no-threshold model does not hold for low dose ionizing radiation. Rad. Res 2004, 162, 447–452. [Google Scholar]


| Radionuclide | Half-life | Energy (keV) [emission ratio] | Radioactivity [Bq/g-dry] |
|---|---|---|---|
| 241Am *1) | 432.2 year | 59.54 (35.9%) | 0.67 ± 0.013 |
| 137 Cs *1) | 30.07year | 661.7 (85.1%) | 67.9 ± 0.024 |
| 134Cs *1) | 2.065 year | 604.7 (97.6%) | 0.16 ± 0.003 |
| 795.8 (85.5%) | 0.18 ± 0.002 | ||
| 90Sr *2) | 28.78 year | 546 (100%) | 3.18 ± 0.33 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/). This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rakwal, R.; Agrawal, G.K.; Shibato, J.; Imanaka, T.; Fukutani, S.; Tamogami, S.; Endo, S.; Sahoo, S.K.S.; Masuo, Y.; Kimura, S. Ultra Low-Dose Radiation: Stress Responses and Impacts Using Rice as a Grass Model. Int. J. Mol. Sci. 2009, 10, 1215-1225. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms10031215
Rakwal R, Agrawal GK, Shibato J, Imanaka T, Fukutani S, Tamogami S, Endo S, Sahoo SKS, Masuo Y, Kimura S. Ultra Low-Dose Radiation: Stress Responses and Impacts Using Rice as a Grass Model. International Journal of Molecular Sciences. 2009; 10(3):1215-1225. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms10031215
Chicago/Turabian StyleRakwal, Randeep, Ganesh Kumar Agrawal, Junko Shibato, Tetsuji Imanaka, Satoshi Fukutani, Shigeru Tamogami, Satoru Endo, Sarata Kumar Sahoo Sahoo, Yoshinori Masuo, and Shinzo Kimura. 2009. "Ultra Low-Dose Radiation: Stress Responses and Impacts Using Rice as a Grass Model" International Journal of Molecular Sciences 10, no. 3: 1215-1225. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms10031215




