Comparability of in Vitro Tests for Bioactive Nanoparticles: A Common Assay to Detect Reactive Oxygen Species as an Example
Abstract
:1. Introduction
1.1. Brief Description of the Different Assays and Their Weakness
1.1.1. Fluorescent and Chemiluminescent Assays
1.1.2. Nitric Oxide Assay
1.1.3. Electron Spin Resonance Assay
1.1.4. Glutathione Peroxidase Assay
1.1.5. Lipid Peroxidation Assay
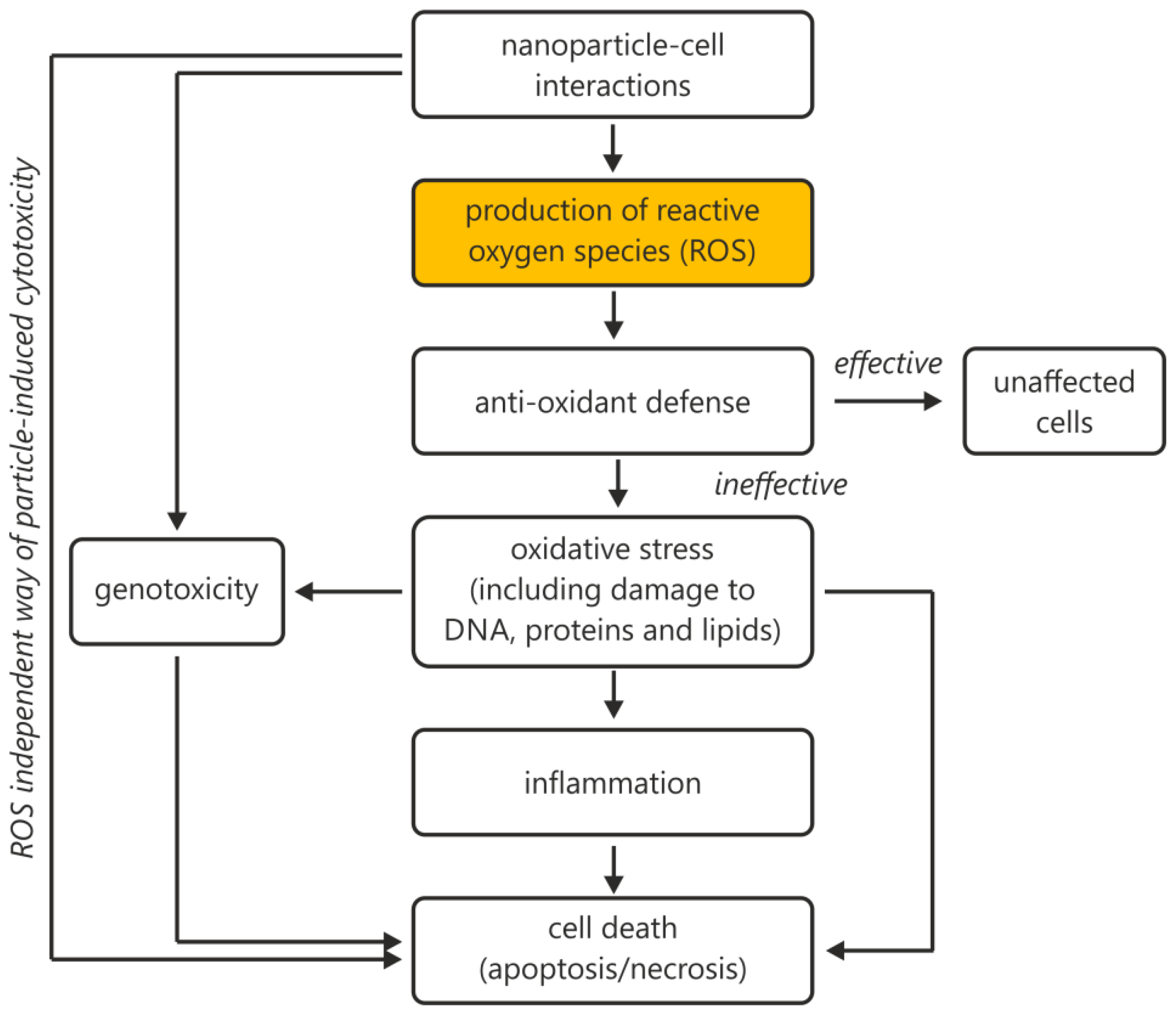
1.2. General Weakness in the Measurement and Quantification of ROS
2. Results and Discussion
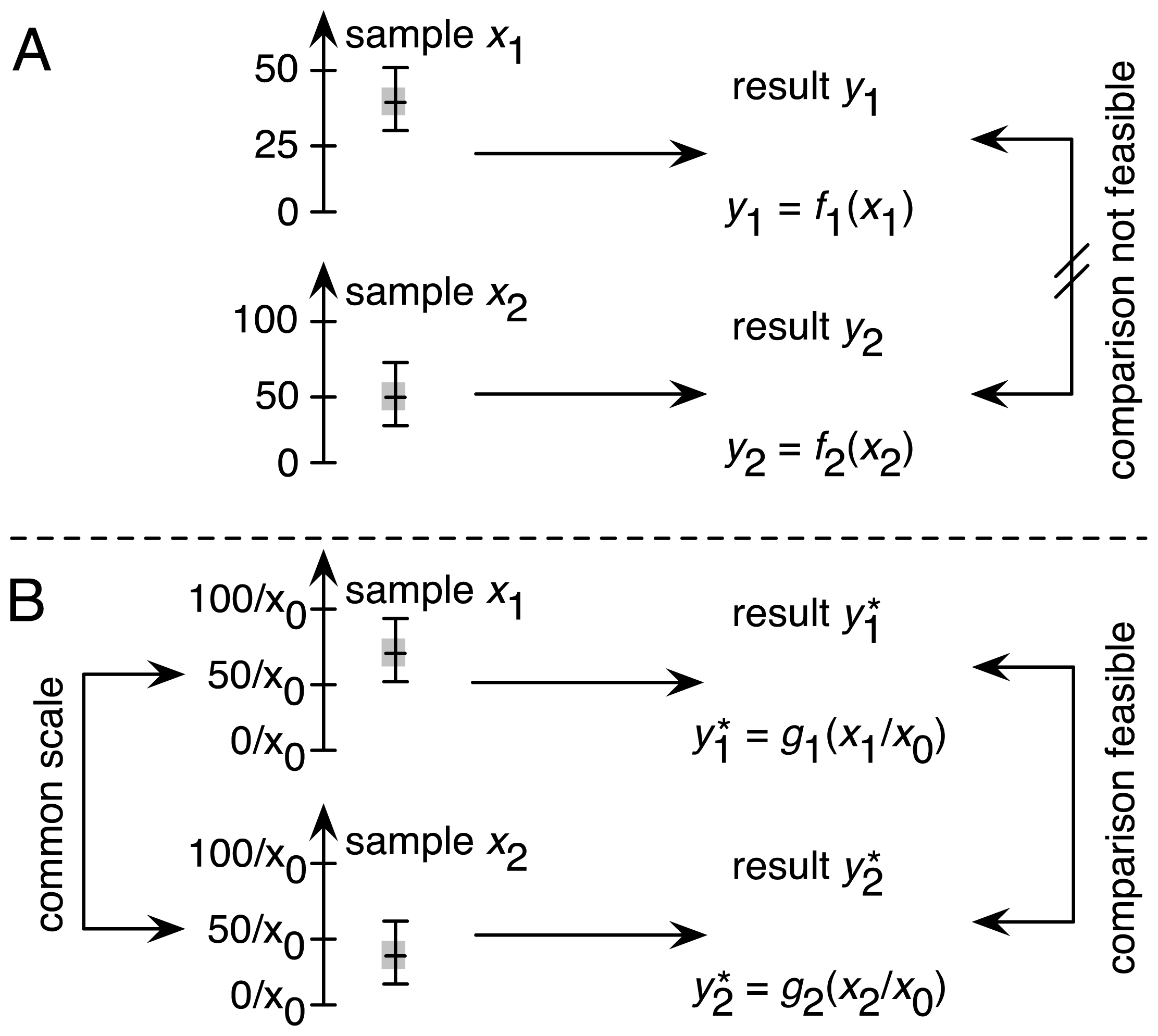
2.1. Traceability as One of the Four Factors in Achieving Comparability
2.1.1. Establishing Traceability for the DCF Assay: Theoretical Considerations
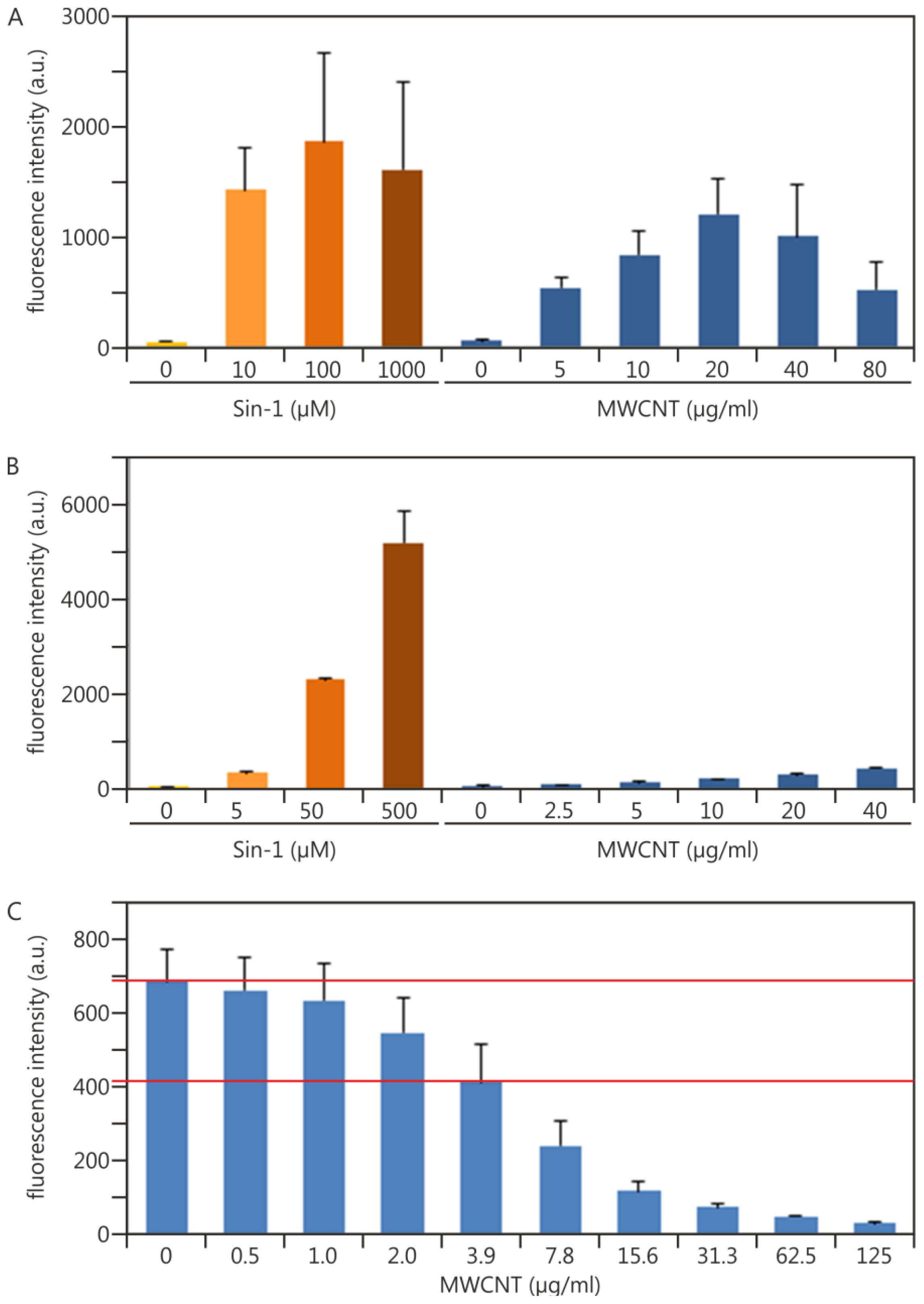
2.1.2. Establishing Traceability for the DCF Assay: Practical Experiments Using Sin-1 as a Potential Chemical Reaction Control
2.1.3. Experimental Data Adding Quality Control Measures in terms of NP Interference: Multiwalled Carbon Nanotubes as an Example
3. Experimental Section
3.1. Nanoparticles
3.2. Cell Culture
3.3. Determination of ROS by DCF Assay
3.3.1. Preparation and Handling of Sin-1
3.3.2. Cell-Based DCF Assay
3.3.3. Cell-Free Interference Controls
3.3.3.1. Intrinsic ROS Activity of NPs
3.3.3.2. Quenching Effect of NPs
3.4. Assessment of Cytotoxicity Using the MTS Cell Viability Assay
3.5. Data Processing (MTS & DCF)
4. Conclusions
- be non-toxic at a radical generating concentration;
- be chemically stable without solvent;
- be dilutable in water (biological fluids);
- generate a stable rate of radical formation in solution/suspension over time (months/years).
Acknowledgments
Conflicts of Interest
References
- Riehemann, K.; Schneider, S.W.; Luger, T.A.; Godin, B.; Ferrari, M.; Fuchs, H. Nanomedicine-challenge and perspectives. Angew. Chem. Int. Ed. Engl 2009, 48, 872–897. [Google Scholar]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar]
- Warheit, D.B. How meaningful are the results of nanotoxicity studies in the absence of adequate material characterization? Toxicol. Sci 2008, 101, 183–185. [Google Scholar]
- Warheit, D.B.; Laurence, B.R.; Reed, K.L.; Roach, D.H.; Reynolds, G.A.; Webb, T.R. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol. Sci 2004, 77, 117–125. [Google Scholar]
- Warheit, D.B.; Webb, T.R.; Reed, K.L.; Frerichs, S.; Sayes, C.M. Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: Differential responses related to surface properties. Toxicology 2007, 230, 90–104. [Google Scholar]
- Bury, N.R.; Wood, C.M. Mechanism of branchial apical silver uptake by rainbow trout is via the proton-coupled Na(+) channel. Am. J. Physiol 1999, 277, 1385–1391. [Google Scholar]
- Fabrega, J.; Luoma, S.N.; Tyler, C.R.; Galloway, T.S.; Lead, J.R. Silver nanoparticles: Behaviour and effects in the aquatic environment. Environ. Int 2011, 37, 517–531. [Google Scholar]
- Aruoja, V.; Dubourguier, H.C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ 2009, 407, 1461–1468. [Google Scholar]
- Ganesh, R.; Smeraldi, J.; Hosseini, T.; Khatib, L.; Olson, B.H.; Rosso, D. Evaluation of nanocopper removal and toxicity in municipal wastewaters. Environ. Sci. Technol 2010, 44, 7808–7813. [Google Scholar]
- Griffitt, R.J.; Luo, J.; Gao, J.; Bonzongo, J.C.; Barber, D.S. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ. Toxicol. Chem 2008, 27, 1972–1978. [Google Scholar]
- Griffitt, R.J.; Weil, R.; Hyndman, K.A.; Denslow, N.D.; Powers, K.; Taylor, D.; Barber, D.S. Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio). Environ. Sci. Technol 2007, 41, 8178–8186. [Google Scholar]
- Heng, B.C.; Zhao, X.; Tan, E.C.; Khamis, N.; Assodani, A.; Xiong, S.; Ruedl, C.; Ng, K.W.; Loo, J.S. Evaluation of the cytotoxic and inflammatory potential of differentially shaped zinc oxide nanoparticles. Arch. Toxicol 2011, 85, 1517–1528. [Google Scholar]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Moller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol 2008, 21, 1726–1732. [Google Scholar]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar]
- Pujalte, I.; Passagne, I.; Brouillaud, B.; Treguer, M.; Durand, E.; Ohayon-Courtes, C.; L’Azou, B. Cytotoxicity and oxidative stress induced by different metallic nanoparticles on human kidney cells. Part. Fibre Toxicol 2011, 8, 10. [Google Scholar]
- Hancock, J.T.; Desikan, R.; Neill, S.J. Role of reactive oxygen species in cell signalling pathways. Biochem. Soc. Trans 2001, 29, 345–350. [Google Scholar]
- Donaldson, K.; Stone, V.; Borm, P.J.; Jimenez, L.A.; Gilmour, P.S.; Schins, R.P.; Knaapen, A.M.; Rahman, I.; Faux, S.P.; Brown, D.M.; et al. Oxidative stress and calcium signaling in the adverse effects of environmental particles (PM10). Free Radic Biol. Med 2003, 34, 1369–1382. [Google Scholar]
- Latella, L.; Sacco, A.; Pajalunga, D.; Tiainen, M.; Macera, D.; D’Angelo, M.; Felici, A.; Sacchi, A.; Crescenzi, M. Reconstitution of cyclin D1-associated kinase activity drives terminally differentiated cells into the cell cycle. Mol. Cell Biol 2001, 21, 5631–5643. [Google Scholar]
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Peters, S.; Hankin, S.; Aschberger, K.; Stone, V. A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: The contribution of physico-chemical characteristics. Nanotoxicology 2010, 4, 207–246. [Google Scholar]
- Donaldson, K.; Brown, D.M.; Mitchell, C.; Dineva, M.; Beswick, P.H.; Gilmour, P.; MacNee, W. Free radical activity of PM10: Iron-mediated generation of hydroxyl radicals. Environ. Health Perspect 1997, 105, 1285–1289. [Google Scholar]
- Held, P. An Introduction to Reactive Oxygen Species; BioTek White Paper: Winooski, VT, USA, 2010. [Google Scholar]
- Ruch, W.; Cooper, P.H.; Baggiolini, M. Assay of H2O2 production by macrophages and neutrophils with homovanillic acid and horse-radish peroxidase. J. Immunol. Methods 1983, 63, 347–357. [Google Scholar]
- Zhou, M.; Diwu, Z.; Panchuk-Voloshina, N.; Haugland, R.P. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: Applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal. Biochem 1997, 253, 162–168. [Google Scholar]
- Halliwell, B.; Whiteman, M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br. J. Pharmacol 2004, 142, 231–255. [Google Scholar]
- Gasser, M.; Wick, P.; Clift, M.J.; Blank, F.; Diener, L.; Yan, B.; Gehr, P.; Krug, H.F.; Rothen-Rutishauser, B. Pulmonary surfactant coating of multi-walled carbon nanotubes (MWCNTs) influences their oxidative and pro-inflammatory potential in vitro. Part. Fibre Toxicol 2012, 9, 17. [Google Scholar]
- Tarpey, M.M.; Wink, D.A.; Grisham, M.B. Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol 2004, 286, 431–444. [Google Scholar]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J., 2nd; Ischiropoulos, H. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic. Biol. Med 2012, 52, 1–6. [Google Scholar]
- Rothe, G.; Valet, G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2′,7′-dichlorofluorescin. J. Leukoc. Biol 1990, 441, 440–448. [Google Scholar]
- Wardman, P. Methods to measure the reactivity of peroxynitrite-derived oxidants toward reduced fluoresceins and rhodamines. Methods Enzymol 2008, 441, 261–282. [Google Scholar]
- Shulaev, V.; Oliver, D.J. Metabolic and proteomic markers for oxidative stress. New tools for reactive oxygen species research. Plant Physiol 2006, 141, 367–372. [Google Scholar]
- Kohno, M. Applications of electron spin resonance spectrometry for reactive oxygen species and reactive nitrogen species research. J. Clin. Biochem. Nutr 2010, 47, 1–11. [Google Scholar]
- Hermans, N.; Cos, P.; Maes, L.; de Bruyne, T.; Vanden Berghe, D.; Vlietinck, A.J.; Pieters, L. Challenges and pitfalls in antioxidant research. Curr. Med. Chem 2007, 14, 417–430. [Google Scholar]
- Hartley, D.P.; Kolaja, K.L.; Reichard, J.; Petersen, D.R. 4-Hydroxynonenal and malondialdehyde hepatic protein adducts in rats treated with carbon tetrachloride: Immunochemical detection and lobular localization. Toxicol. Appl. Pharmacol 1999, 161, 23–33. [Google Scholar]
- van Acker, S.A.; van den Berg, D.J.; Tromp, M.N.; Griffioen, D.H.; van Bennekom, W.P.; van der Vijgh, W.J.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med 1996, 20, 331–342. [Google Scholar]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar]
- Byrdwell, W.C.; Neff, W.E. Dual parallel electrospray ionization and atmospheric pressure chemical ionization mass spectrometry (MS), MS/MS and MS/MS/MS for the analysis of triacylglycerols and triacylglycerol oxidation products. Rapid Commun. Mass Spectrom 2002, 16, 300–319. [Google Scholar]
- Buerki-Thurnherr, T.; Xiao, L.; Diener, L.; Arslan, O.; Hirsch, C.; Maeder-Althaus, X.; Grieder, K.; Wampfler, B.; Mathur, S.; Wick, P.; et al. In vitro mechanistic study towards a better understanding of ZnO nanoparticle toxicity. Nanotoxicology 2013, 7, 402–416. [Google Scholar]
- Keston, A.S.; Brandt, R. The fluorometric analysis of ultramicro quantities of hydrogen peroxide. Anal. Biochem 1965, 11, 1–5. [Google Scholar]
- Bureau International des Poids et Mesures (BIPM), International Vocabulary of Metrology: Basic and General Concepts and Associated Terms (VIPM). In The Joint Committee for Guides in Metrology (JCGM); BIPM: Sevres Cedex, France, 2012; pp. 1–108.
- Hirsch, C.; Roesslein, M.; Krug, H.F.; Wick, P. Nanomaterial cell interactions: Are current in vitro tests reliable? Nanomedicine 2011, 6, 837–847. [Google Scholar]
- Limbach, L.K.; Wick, P.; Manser, P.; Grass, R.N.; Bruinink, A.; Stark, W.J. Exposure of engineered nanoparticles to human lung epithelial cells: Influence of chemical composition and catalytic activity on oxidative stress. Environ. Sci. Technol 2007, 41, 4158–4163. [Google Scholar]
- Lipton, S.A.; Choi, Y.B.; Pan, Z.H.; Lei, S.Z.; Chen, H.S.; Sucher, N.J.; Loscalzo, J.; Singel, D.J.; Stamler, J.S. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature 1993, 364, 626–632. [Google Scholar]
- Piret, J.P.; Jacques, D.; Audinot, J.N.; Mejia, J.; Boilan, E.; Noel, F.; Fransolet, M.; Demazy, C.; Lucas, S.; Saout, C.; et al. Copper(II) oxide nanoparticles penetrate into HepG2 cells, exert cytotoxicity via oxidative stress and induce pro-inflammatory response. Nanoscale 2012, 4, 7168–7184. [Google Scholar]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med 1999, 27, 612–616. [Google Scholar]
- Eurachem CITAC Working Group, Traceability in Chemical Measurement. In A Guide to Achieving Comparable Results in Chemical Measurement, 1st Ed; Ellison, S.L.R.; King, B.; Rösslein, M.; Salit, M.; Williams, A. (Eds.) Eurachem: Uppsala, Sweden, 2003; pp. 1–43.
- Lieber, M.; Smith, B.; Szakal, A.; Nelson-Rees, W.; Todaro, G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int. J. Cancer 1976, 17, 62–70. [Google Scholar]
- Thurnherr, T.; Su, D.; Diener, L.; Weinberg, G.; Masnser, P.; Pfänder, N.; Arrigo, R.; Schuster, M.E.; Wick, P.; Krug, H.F. Comprehensive evaluation of in vitro toxicity of three large-scale produced carbon nanotubes on human Jurkat T cells ans a comparison to crocidolite asbestos. Nanotoxicology 2009, 3, 319–338. [Google Scholar]
- Voelkel, K.; Krug, H.F.; Diabate, S. Formation of reactive oxygen species in rat epithelial cells upon stimulation with fly ash. J. Biosci 2003, 28, 51–55. [Google Scholar]

© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Roesslein, M.; Hirsch, C.; Kaiser, J.-P.; Krug, H.F.; Wick, P. Comparability of in Vitro Tests for Bioactive Nanoparticles: A Common Assay to Detect Reactive Oxygen Species as an Example. Int. J. Mol. Sci. 2013, 14, 24320-24337. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms141224320
Roesslein M, Hirsch C, Kaiser J-P, Krug HF, Wick P. Comparability of in Vitro Tests for Bioactive Nanoparticles: A Common Assay to Detect Reactive Oxygen Species as an Example. International Journal of Molecular Sciences. 2013; 14(12):24320-24337. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms141224320
Chicago/Turabian StyleRoesslein, Matthias, Cordula Hirsch, Jean-Pierre Kaiser, Harald F. Krug, and Peter Wick. 2013. "Comparability of in Vitro Tests for Bioactive Nanoparticles: A Common Assay to Detect Reactive Oxygen Species as an Example" International Journal of Molecular Sciences 14, no. 12: 24320-24337. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms141224320



