5-bp Classical Satellite DNA Loci from Chromosome-1 Instability in Cervical Neoplasia Detected by DNA Breakage Detection/Fluorescence in Situ Hybridization (DBD-FISH)
Abstract
:1. Introduction
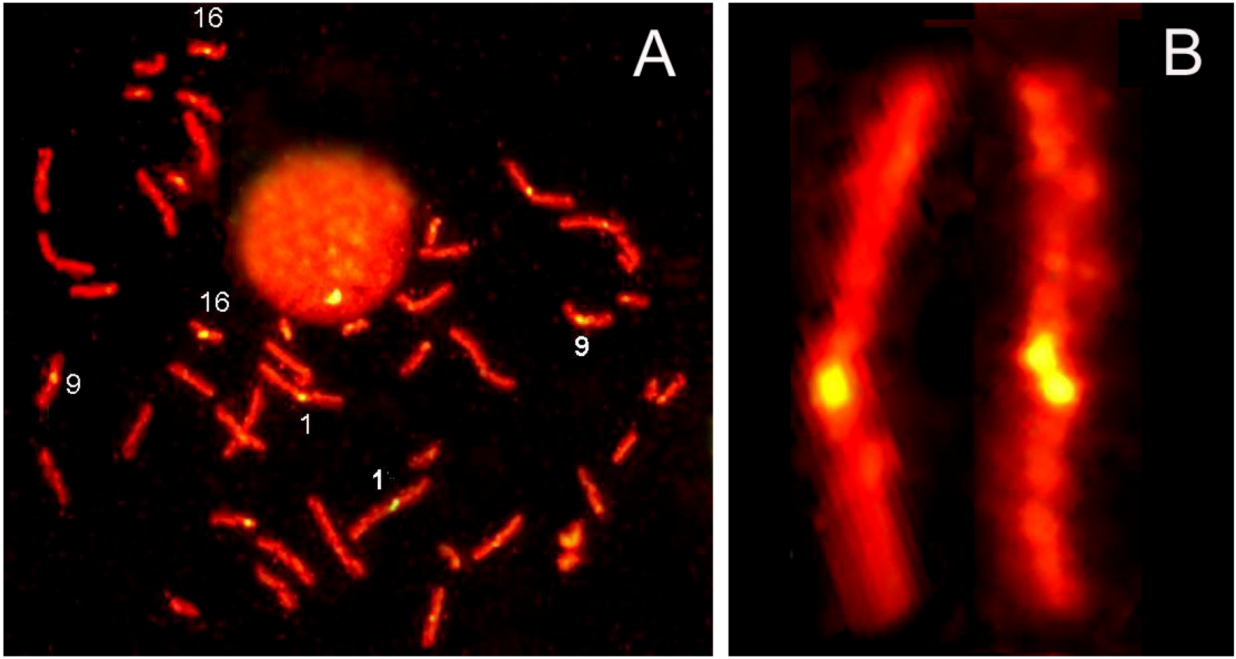
2. Results
| Diagnosis | Aneusomy | Ploidy | ||||
|---|---|---|---|---|---|---|
| Monosomy * | Trisomy ** | Total | Triploidy | Tetraploidy | Total | |
| Cervical Epithelium: | ||||||
| Control | 2 | 2 | 4 | 1 | - | 1 |
| LG-SIL | 3 | 4 | 7 | 1 | 1 | 2 |
| HG-SIL | 3 | 5 | 8 | 3 | 6 | 9 |
| Lymphocytes: | ||||||
| Control | - | - | - | - | - | - |
| LG-SIL | - | 2 | 2 | - | 1 | 1 |
| HG-SIL | 3 | 2 | 5 | - | 2 | 2 |


| Tissue Type | Control (n = 10) | LG-SIL (n = 10) | HG-SIL (n = 10) | Kruskal Wallis | p | |||
|---|---|---|---|---|---|---|---|---|
| Median | Mean Rank | Median | Mean Rank | Median | Mean Rank | |||
| Cervical Epitelium: | ||||||||
| ID-WG | 1.14 × 108 | 5.80 | 6.12 × 109 | 15.70 | 4.69 × 109 | 25.00 | 23.79 | 0.000 |
| Total NC | 4600 | 13.25 | 4600 | 14.70 | 4600 | 18.55 | 3.525 | 0.172 |
| ID Chromosome-1 | 1.65 × 104 | 8.20 | 3.01 × 104 | 15.50 | 8.68 × 104 | 22.80 | 13.75 | 0.001 |
| NC-1 | 200 | 14.50 | 200 | 14.70 | 200 | 17.30 | 1.04 | 0.594 |
| Lymphocytes: | ||||||||
| ID-WG | 3.58 × 104 | 5.70 | 20.79 × 104 | 15.90 | 165.99 × 104 | 24.90 | 23.81 | 0.000 |
| Total NC | 4600 | 14.50 | 4600 | 15.95 | 4600 | 16.05 | 1.04 | 0.595 |
| ID Chromosome-1 | 1.19 × 104 | 5.50 | 9.89 × 104 | 18.10 | 25.88 × 104 | 22.90 | 20.84 | 0.000 |
| NC-1 | 200 | 15.00 | 200 | 16.45 | 200 | 15.05 | 0.645 | 0.724 |
| HPV GENOTYPING | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | 16 | 18 | 31 | 33 | 39 | 44 | 51 | 52 | 54 | 58 | 68 | 70 | 82 |
| LSIL | 4 | 1 | 4 | 3 | 2 | 4 | 4 | 6 | 4 | 1 | - | - | 2 |
| HSIL | 6 | 2 | 2 | - | 4 | 2 | 4 | 3 | 3 | 1 | 1 | 1 | 2 |
| TOTAL | 10 | 3 | 6 | 3 | 6 | 6 | 8 | 9 | 7 | 2 | 1 | 1 | 4 |
3. Experimental Section
3.1. Study Population
3.2. Detection and Genotyping of HPV
3.3. Cell Preparation
3.4. Slide Preparation
3.5. DBD-FISH
3.6. Image Analysis
3.7. Chromosome-Orientation Fluorescence in Situ Hybridization (CO-FISH)
3.8. Statistical Analysis
4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Mullokandov, M.R.; Kholodilov, N.G.; Atkin, N.B.; Burk, R.D.; Johnson, A.B.; Klinger, H.P. Genomic alterations in cervical carcinoma: Losses of chromosome heterozygosity and human papilloma virus tumor status. Cancer Res. 1996, 56, 197–205. [Google Scholar]
- Atkin, N. Cytogenetics of carcinoma of the cervix uteri: A review. Cancer Genet. Cytogenet. 1997, 95, 33–39. [Google Scholar] [CrossRef]
- Wilting, S.M.; Steenbergen, R.D.; Tijssen, M.; van Wieringen, W.N.; Helmerhorst, T.J.; van Kemenade, F.J.; Bleeker, M.C.; van de Wiel, M.A.; Carvalho, B.; Meijer, G.A.; et al. Chromosomal signatures of a subset of high-grade premalignant cervical lesions closely resemble invasive carcinomas. Cancer Res. 2009, 15, 647–655. [Google Scholar]
- Bulten, J.; Melchers, W.J.; Kooy-Smits, M.M.; de Wilde, P.C.; Poddighe, P.J.; Robben, J.C.; Macville, M.V.; Massuger, L.F.; Bakkers, J.M.; Hanselaar, A.G. Numerical aberrations of chromosome 1 in cervical intraepithelial neoplasia are strongly associated with infection with high-risk human papillomavirus types. J. Pathol. 2002, 198, 300–309. [Google Scholar] [CrossRef]
- Cortés-Gutiérrez, E.I.; Dávila-Rodríguez, M.I.; Muraira-Rodríguez, M.; Said-Fernández, S.; Cerda-Flores, R.M. Association between the stages of cervical cancer and chromosome 1 aneusomy. Cancer Genet. Cytogenet. 2005, 159, 44–47. [Google Scholar] [CrossRef]
- Fernández, J.L.; Goyanes, V.; Ramiro-Diaz, J.; Gosálvez, J. Application of FISH for in situ detection and quantification of DNA breakage. Cytogenet. Cell Genet. 1998, 82, 251–256. [Google Scholar] [CrossRef]
- Fernández, J.L.; Vázquez-Gundín, F.; Rivero, M.T.; Genescá, A.; Gosálvez, J.; Goyanes, V. DBD-FISH on neutral comets: Simultaneous analysis of DNA single- and double-strand breaks in individual cells. Exp. Cell Res. 2001, 270, 102–109. [Google Scholar] [CrossRef]
- Fernández, J.L.; Gosálvez, J. Application of FISH to Detect DNA Damage: DNA Breakage Detection-FISH (DBD-FISH). In Methods in Molecular Biology; Didenko, V., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2002. [Google Scholar]
- Cortés-Gutiérrez, E.I.; Dávila-Rodríguez, M.I.; López-Fernández, C.; Fernández, J.L.; Gosálvez, J. Alkali-labile sites in sperm cells from Sus and Ovis species. Int. J. Androl. 2008, 31, 354–363. [Google Scholar] [CrossRef]
- Meyne, J.; Goodwin, E.H.; Moyzis, R.K. Chromosome localization and orientation of the simple sequence repeat of human satellite I DNA.8. Chromosoma 1994, 103, 99–103. [Google Scholar] [CrossRef]
- Fernández, J.L.; Vazquez-Gundin, F.; Rivero, M.T.; Goyanes, V.; Gosálvez, J. Evidence of abundant constitutive alkali-labile sites in human 5 bp classical satellite DNA loci by DBD-FISH. Mutation Res. 2001, 473, 163–168. [Google Scholar] [CrossRef]
- Leal-Garza, C.H.; Cerda-Flores, R.M.; Leal-Elizondo, E.; Cortés-Gutiérrez, E.I. Micronuclei in cervical smears and peripheral blood lymphocytes from women with and without cervical uterine cancer. Mutation Res. 2002, 515, 57–62. [Google Scholar] [CrossRef]
- Cortés-Gutiérrez, E.I.; Cerda-Flores, R.M.; Leal-Garza, C.H. Sister chromatid exchanges in peripheral lymphocytes from women with carcinoma of the uterine cervix. Cancer Genet. Cytogenet. 2000, 122, 121–123. [Google Scholar] [CrossRef]
- Udumudi, A.; Jaiswal, M.; Rajeswari, N.; Desai, N.; Jain, S.; Balakrishna, N.; Rao, K.V.; Ahuja, Y.R. Risk assessment in cervical dysplasia patients by single cell gel electrophoresis assay: A study of DNA damage and repair. Mutat. Res. 1998, 412, 195–205. [Google Scholar] [CrossRef]
- Cortés-Gutiérrez, E.I.; Dávila-Rodríguez, M.I.; Zamudio-González, E.A.; Aguado-Barrera, M.E.; Vargas-Villarreal, J.; Cerda-Flores, R.M. DNA damage in Mexican women with cervical dysplasia evaluated by comet assay. Anal. Quant. Cytol. Histol. 2010, 32, 207–213. [Google Scholar]
- Cortés-Gutiérrez, E.I.; Dávila-Rodríguez, M.I.; Fernandez, J.L.; López-Fernández, C.; Gosálvez, J. DNA damage in women with cervical neoplasia evaluated by DNA breakage detection-fluorescence in situ hybridization. Anal. Quant. Cytol. Histol. 2011, 33, 175–181. [Google Scholar]
- Creasman, W. New gynecologic cancer staining. Obtet. Gynecol. 1990, 75, 287–288. [Google Scholar]
- Eastmond, D.A.; Rupa, D.S.; Hasegawa, L.S. Detection of hyperdiploidy and chromosome breakage in interphase human lymphocytes following exposure to the benzene metabolite hydroquinone using multicolor fluorescence in situ hybridization with DNA probes. Mutat. Res. 1994, 322, 9–20. [Google Scholar] [CrossRef]
- Goodwin, E.; Meyne, J. Strand-specific FISH reveals orientation of chromosome 18 alphoid DNA. Cytogenet. Cell Genet. 1993, 63, 126–127. [Google Scholar] [CrossRef]
- Kurtycz, D.; Nunez, M.; Arts, T.; Bauman, C.; Harris, C.; Inhorn, S.; Meisner, L. Use of fluorescent in situ hybridization to detect aneuploidy in cervical dysplasia. Diagn. Cytopathol. 1996, 15, 46–51. [Google Scholar] [CrossRef]
- Heselmeyer, K.; Macville, M.; Schrock, E.; Blegen, H.; Hellström, A.C.; Shah, K.; Auer, G.; Ried, T. Advanced-stage cervical carcinomas are defined by a recurrent pattern of chromosomal aberrations revealing high genetic instability and a consistent gain of chromosome arm 3q. Genes Chromosomes Cancer 1997, 19, 233–240. [Google Scholar] [CrossRef]
- Adhikary, S.; Eilers, M. Transcriptional regulation and transformation by Myc proteins. Nat. Rev. Mol. Cell Biol. 2005, 6, 635–645. [Google Scholar] [CrossRef]
- Heselmeyer, K.; Schröck, E.; du Manoir, S.; Blegen, H.; Shah, K.; Steinbeck, R.; Auer, G.; Ried, T. Gain of chromosome 3q defines the transition from severe dysplasia to invasive carcinoma of the uterine cervix. Proc. Natl. Acad. Sci. USA 1996, 93, 479–484. [Google Scholar]
- Carone, D.M.; Lawrence, J.B. Heterochromatin instability in cancer: From the barr body to satellites and the nuclear periphery. Semin. Cancer. Biol. 2012, in press. [Google Scholar]
- Cheung, T.H.; Chung, T.K.; Poon, C.S.; Hampton, G.M.; Wang, V.W.; Wong, Y.F. Allelic loss on chromosome 1 is associated with tumor progression of cervical carcinoma. Cancer 1999, 86, 1294–1298. [Google Scholar] [CrossRef]
- Werkmeister, J.; Zaunders, J.; McCarthy, W.; Hersey, P. Characterization of an inhibitor of cell division released in tumour cell cultures. Clin. Exp. Immunol. 1980, 41, 487–496. [Google Scholar]
- Kim, Y.I.; Giuliano, A.; Hatch, K.D.; Schneider, A.; Nour, M.A.; Dallal, G.E.; Selhub, J.; Mason, J.B. Global DNA hypomethylation increases progressively in cervical dysplasia and carcinoma. Cancer 1994, 74, 893–899. [Google Scholar] [CrossRef]
- Dhillon, V.S.; Kler, R.S.; Dhillon, I.K. Chromosome instability and sister chromatid exchange (SCE) studies in patients with carcinoma os cervixuteri. Cancer Genet. Cytogenet. 1996, 86, 54–57. [Google Scholar] [CrossRef]
- Capalash, N.; Sobti, R.C. Spontaneous genomic fragility and cell cycle pregression in lymphocytes of patients with cervical carcinoma. Cancer Genet. Cytogenet. 1996, 88, 30–44. [Google Scholar] [CrossRef]
- Murgia, E.; Ballardin, M.; Bonassi, S.; Barale, R. Validation of micronuclei frequency in peripheral blood lymphocytes as early cancer risk biomarker in a nested case-control study. Mutat. Res. 2008, 639, 27–34. [Google Scholar] [CrossRef]
- Bonassi, S.; Ugolini, D.; Kirsch-Volders, M.; Strömberg, U.; Vermeulen, R.; Tucker, J.D. Human population studies with cytogenetic biomarkers: Review of the literature and future prospective. Environ. Mol. Mutagen. 2005, 45, 258–270. [Google Scholar] [CrossRef]
- Miloseviv-Djordjevic, O.; Grujicic, D.; Bankovic, D.; Arsenijevic, S. Cervical precancerous lesions-chromosomal instability in peripheral blood lymphocytes in relation to lesion stage, age and smoking habits. Acta Obstet. Gynecol. Scand. 2011, 90, 1082–1087. [Google Scholar] [CrossRef]
- Alvarez-Rosero, R.E.; Rodríguez-Argote, J.; Arboleda-Moreno, Y.Y.; Muñoz-Benítez, S.L.; Sierra-Torres, C.H. Chromosome aberrations in peripheral blood lymphocytes of high-risk HPV-infected women with HG-SIL. Environ. Mol. Mutagen. 2008, 49, 688–694. [Google Scholar] [CrossRef]
- Wei, Y.C.; Chou, Y.S.; Chu, T.Y. Detection and typing of minimal human papillomavirus DNA in plasma. Gynaecol. Obstet. 2007, 96, 112–116. [Google Scholar]
- Ho, C.M.; Yang, S.S.; Chien, T.Y.; Huang, S.H.; Jeng, C.J.; Chang, S.F. Detection and quantitation of human papillomavirus type 16, 18 and 52 DNA in the peripheral bllod of cervical cancer patients. Gynecol. Oncol. 2005, 99, 615–621. [Google Scholar] [CrossRef]
- Cortés-Gutiérrez, E.I.; Dávila-Rodríguez, M.I.; Vargas-Villarreal, J.; Hernández-Garza, F.; Cerda-Flores, R.M. Association between human papilloma virus-type infections with micronuclei frequencies. Prague Med. Rep. 2010, 111, 35–41. [Google Scholar]
- Van Wijnen, A.J.; Bidwell, J.P.; Fey, E.G.; Penman, S.; Lian, J.B.; Stein, J.L.; Stein, G.S. Nuclear matrix association of multiple sequence-specific DNA binding activities related to SP-1, ATF, CCAAT, C/EBP, OCT-1, and AP-1. Biochemistry 1993, 32, 8397–8402. [Google Scholar]
- Forment, J.V.; Kaidi, A.; Jackson, S.P. Chromothripsis and cancer: Causers and consequences of chromosome shattering. Nat. Rev. Cancer 2012, 12, 663–670. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cortés-Gutiérrez, E.I.; Ortíz-Hernández, B.L.; Dávila-Rodríguez, M.I.; Cerda-Flores, R.M.; Fernández, J.L.; López-Fernández, C.; Gosálvez, J. 5-bp Classical Satellite DNA Loci from Chromosome-1 Instability in Cervical Neoplasia Detected by DNA Breakage Detection/Fluorescence in Situ Hybridization (DBD-FISH). Int. J. Mol. Sci. 2013, 14, 4135-4147. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms14024135
Cortés-Gutiérrez EI, Ortíz-Hernández BL, Dávila-Rodríguez MI, Cerda-Flores RM, Fernández JL, López-Fernández C, Gosálvez J. 5-bp Classical Satellite DNA Loci from Chromosome-1 Instability in Cervical Neoplasia Detected by DNA Breakage Detection/Fluorescence in Situ Hybridization (DBD-FISH). International Journal of Molecular Sciences. 2013; 14(2):4135-4147. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms14024135
Chicago/Turabian StyleCortés-Gutiérrez, Elva I., Brenda L. Ortíz-Hernández, Martha I. Dávila-Rodríguez, Ricardo M. Cerda-Flores, José Luis Fernández, Carmen López-Fernández, and Jaime Gosálvez. 2013. "5-bp Classical Satellite DNA Loci from Chromosome-1 Instability in Cervical Neoplasia Detected by DNA Breakage Detection/Fluorescence in Situ Hybridization (DBD-FISH)" International Journal of Molecular Sciences 14, no. 2: 4135-4147. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms14024135




