Prevention of Tendon Adhesions by ERK2 Small Interfering RNAs
Abstract
:1. Introduction
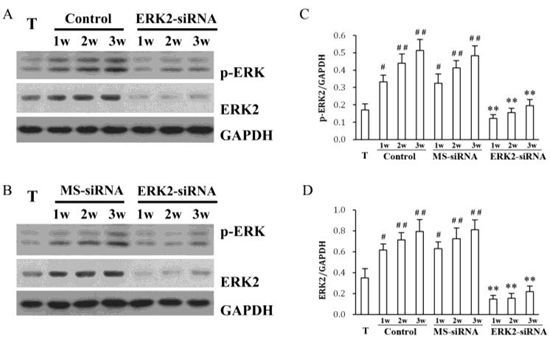
2. Results and Discussion
2.4. Effect of ERK2 siRNA on Biomechanical Properties of Repaired Tendons
3. Experimental Section
3.2. Animal Model
3.3. In Vivo Bioluminescence Assay
3.4. Western Blotting
3.5. Macroscopic Evaluation
3.6. Histological Evaluation of Adhesion Tissues
3.7. Biomechanical Evaluation
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Angermann, P.; Lohmann, M. Injuries to the hand and wrist. A study of 50,272 injuries. J. Hand Surg. Br 1993, 18, 642–644. [Google Scholar]
- Schöffl, V.; Heid, A.; Küpper, T. Tendon injuries of the hand. World J. Orthop 2012, 3, 62–69. [Google Scholar]
- Miller, J.A.; Ferguson, R.L.; Powers, D.L.; Burns, J.W.; Shalaby, S.W. Efficacy of hyaluronic acid/nonsteroidal anti-inflammatory drug systems in preventing postsurgical tendon adhesions. J. Biomed. Mater. Res. 1997, 38, 25–33. [Google Scholar]
- Özgenel, G.Y. The effects of a combination of hyaluronic and amniotic membrane on the formation of peritendinous adhesions after flexor tendon surgery in chickens. J. Bone Joint Surg. Br 2004, 86, 301–307. [Google Scholar]
- Menderes, A.; Mola, F.; Tayfur, V.; Vayvada, H.; Barutcu, A. Prevention of peritendinous adhesions following flexor tendon injury with Seprafilm. Ann. Plast Surg 2004, 53, 560–564. [Google Scholar]
- Hanff, G.; Hagberg, L. Prevention of restrictive adhesions with expanded polytetrafluoroethylene diffusible membrane following flexor tendon repair, an experimental study in rabbits. J. Hand Surg. Am 1998, 23, 658–664. [Google Scholar]
- Moran, S.L.; Ryan, C.K.; Orlando, G.S.; Pratt, C.E.; Michalko, K.B. Effects of 5-fluorouracil on flexor tendon repair. J. Hand Surg. Am 2000, 25, 242–251. [Google Scholar]
- Akasaka, T.; Nishida, J.; Imaeda, T.; Shimamura, T.; Amadio, P.C.; An, K.N. Effect of hyaluronic acid on the excursion resistance of tendon graft, a biomechanical in vitro study in a modified human model. Clin. Biomech 2006, 21, 810–815. [Google Scholar]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar]
- Hannon, G.J. RNA interference. Nature 2002, 418, 244–251. [Google Scholar]
- Sledz, C.A.; Williams, B.R.G. RNA interference in biology and disease. Blood 2005, 106, 787– 794. [Google Scholar]
- Wang, Z.; Rao, D.D.; Senzer, N.; Nemunaitis, J. RNA interference and cancer therapy. Pharm. Res 2011, 28, 2983–2995. [Google Scholar]
- Dykxhoorn, D.M.; Lieberman, J. The silent revolution, RNA interference as basic biology, research tool, and therapeutic. Annu. Rev. Med 2005, 56, 401–423. [Google Scholar]
- Khan, U.; Occleston, N.L.; Khaw, P.T.; McGrouther, D.A. Differences in proliferative rate and collagen lattice contraction between endotenon and synovial fibroblasts. J. Hand Surg. Am 1998, 23, 266–273. [Google Scholar]
- Nyska, M.; Nyska, A.; Rivlin, E.; Porat, S.; Pines, M.; Shoshan, S.; Nagler, A. Topically applied halofuginone, an inhibitor of collagen type I transcription, reduces peritendinous fibrous adhesions following surgery. Connect. Tissue Res 1996, 34, 97–103. [Google Scholar]
- Li, F.; Fan, C.; Cheng, T.; Jiang, C.; Zeng, B. Efficient inhibition of fibroblast proliferation and collagen expression by ERK2 siRNAs. Biochem. Biophys. Res. Commun 2009, 382, 259–263. [Google Scholar]
- Li, F.; Ruan, H.; Fan, C.; Zeng, B.; Wang, C.; Wang, X. Efficient inhibition of the formation of joint adhesions by ERK2 small interfering RNAs. Biochem. Biophys. Res. Commun 2010, 391, 795–799. [Google Scholar]
- Kashiwagi, K.; Mochizuki, Y.; Yasunaga, Y.; Ishida, O.; Deie, M.; Ochi, M. Effects of transforming growth factor-beta 1on the early stages of healing of the Achilles tendon in a rat model. Scand J. Plast. Reconstr. Surg. Hand Surg 2004, 38, 193–197. [Google Scholar]
- Abrahamsson, S.O.; Lohmander, S. Differential effects of insulin-like growth factor-I on matrix and DNA synthesis in various regions and types of rabbit tendons. J. Orthop. Res 1996, 14, 370–376. [Google Scholar]
- Wang, E.D. Tendon repair. J. Hand Ther 1998, 11, 105–110. [Google Scholar]
- Hayashida, T.; Decaestecker, M.; Schnaper, H.W. Cross-talk between ERK MAP kinase and Smad-signaling pathways enhances TGF-beta dependent responses in human mesangial cells. FASEB J 2003, 17, 1576–1578. [Google Scholar]
- Vantaggiato, C.; Formentini, I.; Bondanza, A.; Bonini, C.; Naldini, L.; Brambilla, R. ERK1 and ERK2 mitogen-activated protein kinases affect Ras-dependent cell signaling differentially. J. Biol. 2006, 5. [Google Scholar] [CrossRef]
- Frémin, C.; Ezan, F.; Boisselier, P.; Bessard, A.; Pagès, G.; Pouysségur, J.; Baffet, G. ERK2 but not ERK1 plays a key role in hepatocyte replication, an RNAi-mediated ERK2 knockdown approach in wild-type and ERK1 null hepatocytes. Hepatology 2007, 45, 1035–1045. [Google Scholar]
- Lefloch, R.; Pouysségur, J.; Lenormand, P. Single and combined silencing of ERK1 and ERK2 reveals their positive contribution to growth signaling depending on their expression levels. Mol. Cell Biol 2008, 28, 511–527. [Google Scholar]
- Samuels, I.S.; Karlo, J.C.; Faruzzi, A.N.; Pickering, K.; Herrup, K.; Sweatt, J.D.; Saitta, S.C.; Landreth, G.E. Deletion of ERK2 mitogen-activated protein kinase identifies its key roles in cortical neurogenesis and cognitive function. J. Neurosci 2008, 28, 6983–6995. [Google Scholar]
- Sioud, M.; Sørensen, D.R. Cationic liposome-mediated delivery of siRNAs in adult mice. Biochem. Biophys. Res. Commun 2003, 312, 1220–1225. [Google Scholar]
- Khoury, M.; Louis-Plence, P.; Escriou, V. Efficient new cationic liposome formulation for systemic delivery of small interfering RNA silencing tumor necrosis factor-α in experimental arthritis. Arthritis Rheum 2006, 54, 1867–1877. [Google Scholar]
- Lois, C.; Hong, E.J.; Pease, S.; Brown, E.J.; Baltimore, D. Germline transmission and tissuespecific expression of transgenes delivered by lentiviral vectors. Science 2002, 295, 868–872. [Google Scholar]
- Pfeifer, A.; Ikawa, M.; Dayn, Y.; Verma, I.M. Transgenesis by lentiviral vectors, Lack of gene silencing in mammalian embryonic stem cells and preimp lantation embryos. Proc. Natl. Acad. Sci. USA 2002, 99, 2140–2145. [Google Scholar]
- Lai, Z.; Brady, R.O. Gene transfer into the central nervous system in vivo using a recombinant lentivirus vector. J. Neurosci. Res 2002, 67, 363–371. [Google Scholar]
- Yu, X.; Zhan, X.; D’Costa, J.; Tanavde, V.M.; Ye, Z.; Peng, T.; Malehorn, M.T.; Yang, X.; Civin, C.I.; Cheng, L. Lentiviral vectors with two independent internal promoters transfer high-level expression of multiple transgenes to human hematopoietic stem-progenitor cells. Mol. Ther 2003, 7, 827–838. [Google Scholar]
- Ishiyama, N.; Moro, T.; Ishihara, K.; Ohe, T.; Miura, T.; Konno, T.; Ohyama, T.; Kimura, M.; Kyomoto, M.; Nakamura, K.; Kawaguchi, H. The prevention of peritendinous adhesions by a phospholipid polymer hydrogel formed in situ by spontaneous intermolecular interactions. Biomaterials 2010, 31, 4009–4016. [Google Scholar]
- Güdemez, E.; Ekşioğlu, F.; Korkusuz, P.; Aşan, E.; Gürsel, I.; Hasirci, V. Chondroitin sulfate-coated polyhydroxyethyl methacrylate membrane prevents adhesion in full-thickness tendon tears of rabbits. J. Hand Surg. Am 2002, 27, 293–306. [Google Scholar]







© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ruan, H.; Liu, S.; Li, F.; Li, X.; Fan, C. Prevention of Tendon Adhesions by ERK2 Small Interfering RNAs. Int. J. Mol. Sci. 2013, 14, 4361-4371. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms14024361
Ruan H, Liu S, Li F, Li X, Fan C. Prevention of Tendon Adhesions by ERK2 Small Interfering RNAs. International Journal of Molecular Sciences. 2013; 14(2):4361-4371. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms14024361
Chicago/Turabian StyleRuan, Hongjiang, Shen Liu, Fengfeng Li, Xujun Li, and Cunyi Fan. 2013. "Prevention of Tendon Adhesions by ERK2 Small Interfering RNAs" International Journal of Molecular Sciences 14, no. 2: 4361-4371. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms14024361