Enhanced Production of Bioactive Isoprenoid Compounds from Cell Suspension Cultures of Artemisia annua L. Using β-Cyclodextrins
Abstract
:1. Introduction
2. Results and Discussion
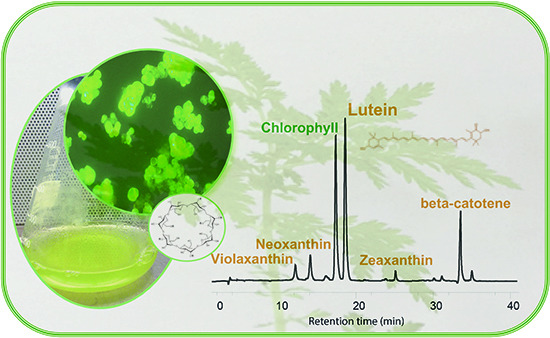
2.1. Effects of DIMEB on Intracellular and Extracellular Carotenoids and Quinones


2.2. Expression of Isoprenoid Biosynthetic Genes and Isoprenoid Production


3. Experimental Section
3.1. A. annua Suspension Cultured Cells
3.2. Elicitation of A. annua SCC
3.3. Carotenoid and Quinone Extraction from A. annua Cells and Spent Medium
3.4. HPLC Analysis of Carotenoid and Quinone

3.5. Relative Expression Analysis of Genes of Isoprenoid Biosynthesis
3.6. Real-Time PCR Experiment
| Gene | Sequence | Accession Number | |
|---|---|---|---|
| HMGR | Forward Reverse Probe | 5'-CATGCTTGAACCTACTTGGAGTCA-3' 3'-CAACACCGAACCAGCAACTATC-5' 5'-TGCGTGCATAGAATCACCAGGCTCA-3' | AF142473.1 |
| DXR | Forward Reverse Probe | 5'-CCCGTCTTGATCTTTGCAAGTT-3' 3'-GCAGAACAGCCAAATGCATT-5' 5'-AAGCACCGGACAACGTGAAATACCCG-3' | AF182287.2 |
| IDI * | Forward Reverse Probe | 5'-CAGACTTAGGTGAGGAGGGTCTCA-3' 3'-CCCACCACTTGAACAAGAAATTG-5' 5'-CTGTCGCCGTGGTTCAGGATTGTTG-3' | DQ666334.1 |
| FDS | Forward Reverse Probe | 5'-TCACCGCCGAATTGTTCA-3' 3'-TGCTTGTCAAGATCCTCTCCAA-5' 5'-ACTCATTTTACCTTCCAGTTGCCTGTGCAC-3' | U36376.1 AF112881.1 |
| GGDS | Forward Reverse Probe | 5'-AGGTTGTTTGCTGAGGAGTTGTT-3' 3'-CCACACCCCTCTCTGATTCAA-5' 5'-CCGAGGCGAAGCAGCAGTTGG-3' | EY076993.1 EY082045.1 |
| PS * | Forward Reverse Probe | 5'-GGGCGTATGTAAGCAAACCAA-3' 3'-CTTGATGATGCTGGTACAAGTGATT-5' 5'-AAGATAGTTGCTTTGCCTCTGGCATATGCA-3' | EY033375.1 |
| LCYB * | Forward Reverse Probe | 5'-TTTAGAAGGCACAAGACGGTTTT-3' 3'-AAAGTGAATAACTCAGGCAGAAACAA-5' 5'-ACCTCGTTACTGGCATGGGTTCTTGTCATC-3' | EY092112.1 |
| β-action | Forward Reverse Probe | 5'-CCATTGGTGCTGAGAGGTTCA-3' 3'-GCAGCTTCCATTCCGATCA-5' 5'-TGCCCTGAGGTCTTGTTCCAACCTTC-3' | EU531837.1 |
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cai, Z.; Kastell, A.; Knorr, D.; Smetanska, I. Exudation: An expanding technique for continuous production and release of secondary metabolites from plant cell suspension and hairy root cultures. Plant Cell Rep. 2012, 31, 461–467. [Google Scholar] [CrossRef]
- Waleczek, K.J.; Cabral Marques, H.M.; Hempel, B.; Schmidt, P.C. Phase solubility studies of pure (−)-α-bisabolol and camomile essential oil β-cyclodextrin. Eur. J. Pharm. Biopharm. 2003, 55, 247–251. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins. Drug solubilization and stabilization. J. Pharm. Sci. 1996, 85, 1017–1025. [Google Scholar] [CrossRef]
- Zamboni, A.; Gatto, P.; Cestaro, A.; Pilati, S.; Viola, R.; Mattivi, F.; Moser, C.; Velasco, R. Grapevine cell early activation of specific responses to DIMEB, a resveratrol elicitor. BMC Genomics 2009, 10. [Google Scholar] [CrossRef]
- Bru, R.; Selles, S.; Casado-Vela, J.; Belchí-Navarro, S.; Pedreño, M.A. Modified cyclodextrins are chemically defined glucan inducers of defense responses in grapevine cell cultures. J. Agric. Food Chem. 2006, 54, 65–71. [Google Scholar] [CrossRef]
- Durante, M.; Caretto, S.; Quarta, A.; de Paolis, A.; Nisi, R.; Mita, G. β-Cyclodextrins enhance artemisinin production in Artemisia annua suspension cell cultures. Appl. Microbiol. Biotechnol. 2011, 90, 1905–1913. [Google Scholar] [CrossRef]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Mattila, P.; Kumpulainen, J. Coenzymes Q9 and Q10: Contents in foods and dietary intake. J. Food Compos. Anal. 2001, 14, 409–417. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 2005, 26, 459–516. [Google Scholar] [CrossRef]
- Laule, O.; Furholz, A.; Chang, H.S.; Zhu, T.; Wang, X.; Heifetz, P.B.; Gruissem, W.; Lange, B.M. Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2003, 100, 6866–6871. [Google Scholar] [CrossRef]
- Caretto, S.; Quarta, A.; Durante, M.; Nisi, R.; de Paolis, A.; Blando, F.; Mita, G. Methyl jasmonate and miconazole differently affect arteminisin production and gene expression in Artemisia annua suspension cultures. Plant Biol. 2011, 13, 51–58. [Google Scholar] [CrossRef]
- Sabater-Jara, A.B.; Pedreño, M.A. Use of β-cyclodextrins to enhance phytosterol production in cell suspension cultures of carrot (Daucus carota L.). Plant Cell Tissue Organ Cult. 2013, 114, 249–258. [Google Scholar] [CrossRef]
- Sabater-Jara, A.B.; Onrubia, M.; Moyano, E.; Bonfill, M.; Palazón, J.; Pedreño, M.A.; Cusidó, R.M. Synergistic effect of cyclodextrins and methyl jasmonate on taxane production in Taxus x media cell cultures. Plant Biotechnol. J. 2014, 12, 1075–1084. [Google Scholar] [CrossRef]
- Wildholm, J.M. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 1972, 47, 189–194. [Google Scholar]
- Belchí-Navarro, S.; Almagro, L.; Lijavetzky, D.; Bru, R.; Pedreño, M.A. Enhanced extracellular production of trans-resveratrol in Vitis vinifera suspension cultured cells by using cyclodextrins and methyljasmonate. Plant Cell Rep. 2012, 31, 81–89. [Google Scholar] [CrossRef]
- Lopez-Nicolàs, J.M.; Rodriguez-Bonilla, P.; Garcia-Carmona, F. Cyclodextrins and antioxidants. Crit. Rev. Food Sci. Nutr. 2014, 54, 251–276. [Google Scholar] [CrossRef]
- Zidovetzki, R.; Levitan, I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochim. Biophys. Acta 2007, 1768, 1311–1324. [Google Scholar] [CrossRef]
- Lijavetzky, D.; Almagro, L.; Belchi-Navarro, S.; Martinez-Zapater, J.M.; Bru, R.; Pedreno, A. Synergistic effect of methyljasmonate and cyclodextrin on stilbene biosynthesis pathway gene expression and resveratrol production in Monastrell grapevine cell cultures. BMC Res. Notes 2008, 1. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. The 1-deoxy-d-xylulose-5-phosphate pathway of isoprenoid. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 47–65. [Google Scholar]
- Carretero-Paulet, L.; Cairó, A.; Botella-Pavía, P.; Besumbes, O.; Campos, N.; Boronat, A.; Rodríguez-Concepción, M. Enhanced flux through the methylerythritol 4-phosphate pathway in Arabidopsis plants overexpressing deoxyxylulose 5-phosphate reductoisomerase. Plant Mol. Biol. 2006, 62, 683–695. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Biosynthesis, accumulation and emission of carotenoids, alpha-tocopherol, plastoquinone, and isoprene in leaves under high photosynthetic irradiance. Photosynth. Res. 2007, 92, 163–79. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 51, 473–497. [Google Scholar] [CrossRef]
- Sadler, G.; Davis, J.; Dezman, D. Rapid extraction of lycopene and β-carotene from reconstituted tomato paste and pink grapefruit homogenates. J. Food Sci. 1990, 55, 1460–1461. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Collins, J.K.; Pair, S.D.; Roberts, W. Lycopene content differs among red-fleshed watermelon cultivars. J. Sci. Food Agric. 2001, 81, 983–987. [Google Scholar] [CrossRef]
- Fraser, P.D.; Pinto, M.S.E.; Holloway, D.E.; Bramley, P.M. Application of high-performance liquid chromatography with photodiode array detection to the metabolic profiling of plant isoprenoids. Plant J. 2000, 24, 551–558. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzello, F.; De Paolis, A.; Durante, M.; Blando, F.; Mita, G.; Caretto, S. Enhanced Production of Bioactive Isoprenoid Compounds from Cell Suspension Cultures of Artemisia annua L. Using β-Cyclodextrins. Int. J. Mol. Sci. 2014, 15, 19092-19105. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms151019092
Rizzello F, De Paolis A, Durante M, Blando F, Mita G, Caretto S. Enhanced Production of Bioactive Isoprenoid Compounds from Cell Suspension Cultures of Artemisia annua L. Using β-Cyclodextrins. International Journal of Molecular Sciences. 2014; 15(10):19092-19105. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms151019092
Chicago/Turabian StyleRizzello, Francesca, Angelo De Paolis, Miriana Durante, Federica Blando, Giovanni Mita, and Sofia Caretto. 2014. "Enhanced Production of Bioactive Isoprenoid Compounds from Cell Suspension Cultures of Artemisia annua L. Using β-Cyclodextrins" International Journal of Molecular Sciences 15, no. 10: 19092-19105. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms151019092