Shape and Site Dependent in Vivo Degradation of Mg-Zn Pins in Rabbit Femoral Condyle
Abstract
:1. Introduction
2. Results
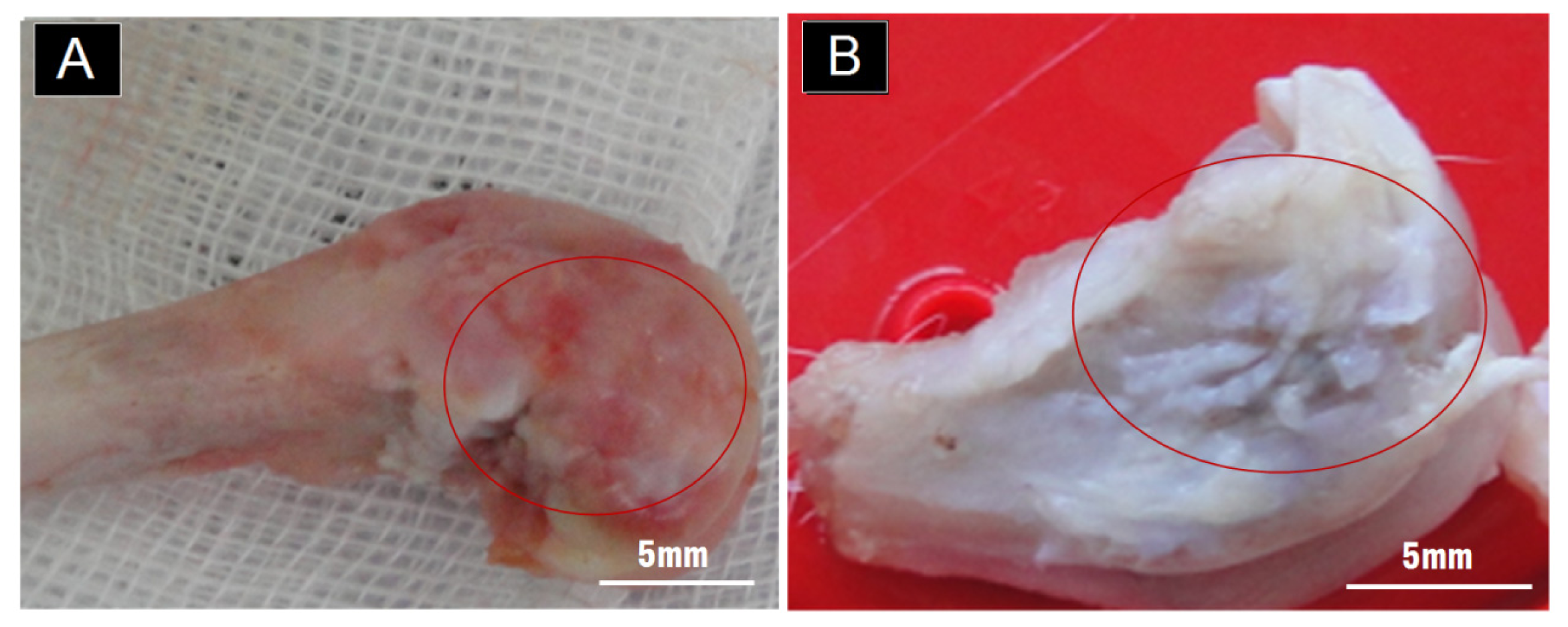
2.1. Gross Observation on the Outer Surface of the Condyle
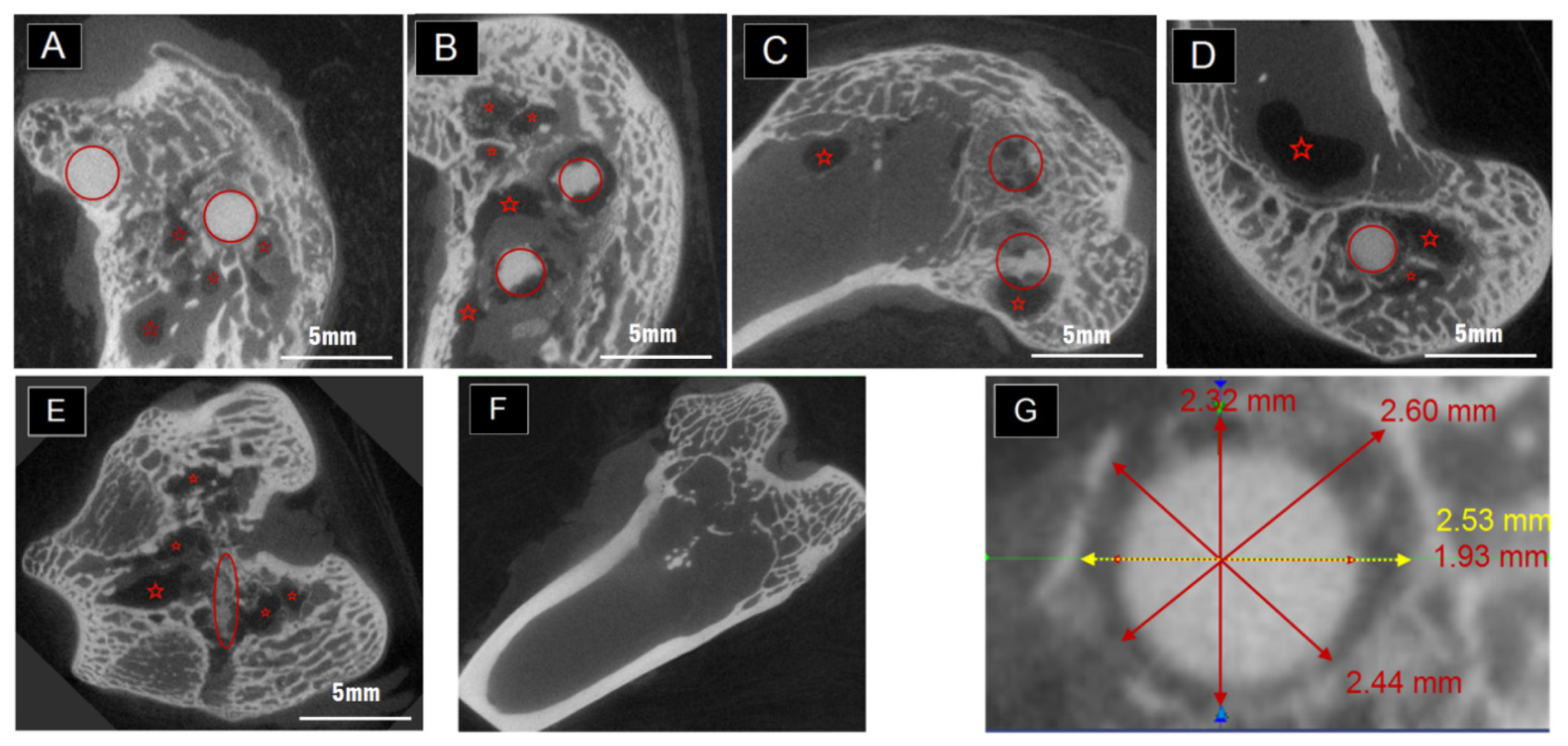
2.2. The Degradation Inside the Condyles Evaluated by Micro-CT
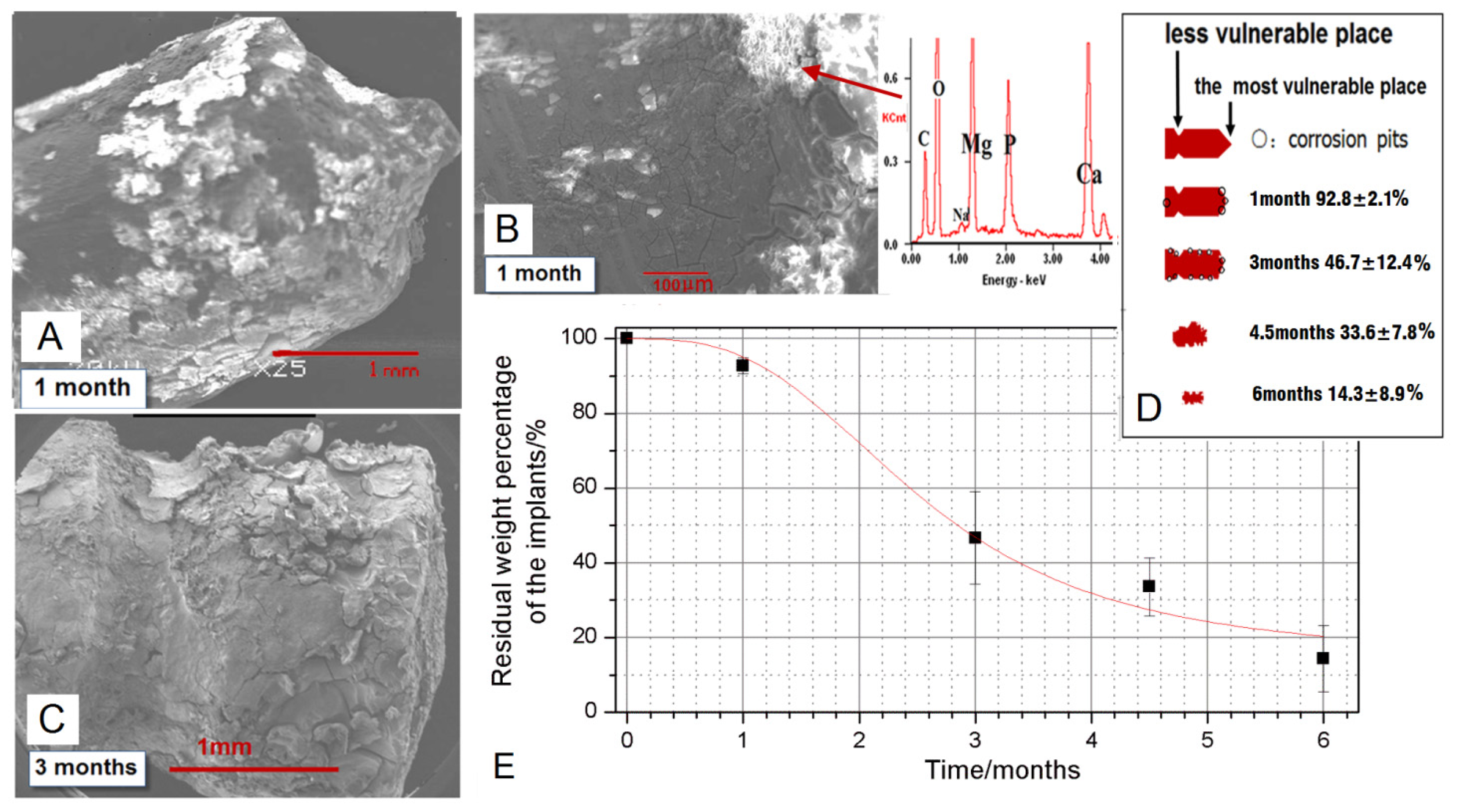
2.3. The Degradation Evaluated by SEM and EDX
3. Discussion
3.1. Degradation of the Mg-6Zn Alloy in Vivo
3.2. Shape Dependent Degradation of the Pins
3.3. Implantation Sites Dependent Degradation of the Pins
4. Materials and Methods
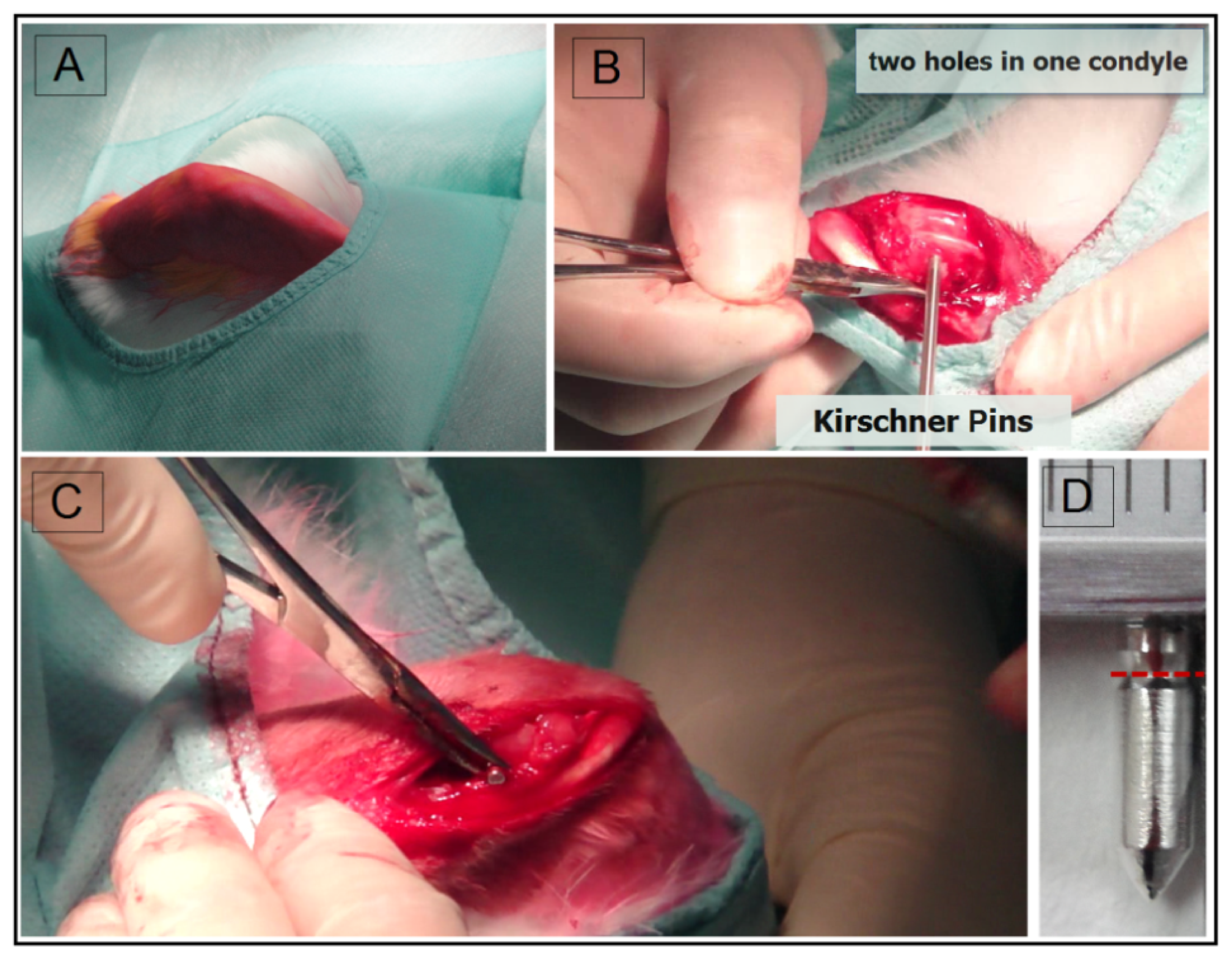
4.1. Surgery
4.2. Gross Evaluation
4.3. Ex Vivo Micro-Computed Tomography
4.4. SEM and EDX
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Janning, C.; Willbold, E.; Vogt, C.; Nellesen, J.; Meyer-Lindenberg, A.; Windhagen, H.; Thorey, F.; Witte, F. Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodelling. Acta Biomater 2010, 6, 1861–1868. [Google Scholar]
- Witte, F.; Ulrich, H.; Palm, C.; Willbold, E. Biodegradable magnesium scaffolds: Part II: Peri-implant bone remodeling. J. Biomed. Mater. Res. Part A 2007, 81A, 757–765. [Google Scholar]
- He, Y.; Tao, H.; Zhang, Y.; Jiang, Y.; Zhang, S.; Zhao, C.; Li, J.; Zhang, B.; Song, Y.; Zhang, X. Biocompatibility of bio-Mg-Zn alloy within bone with heart, liver, kidney and spleen. Chin. Sci. Bull 2009, 54, 484–491. [Google Scholar]
- Erdmann, N.; Angrisani, N.; Reifenrath, J.; Lucas, A.; Thorey, F.; Bormann, D.; Meyer-Lindenberg, A. Biomechanical testing and degradation analysis of MgCa0.8 alloy screws: A comparative in vivo study in rabbits. Acta Biomater 2011, 7, 1421–1428. [Google Scholar]
- Von der Höh, N.; Bormann, D.; Lucas, A.; Denkena, B.; Hackenbroich, C.; Meyer-lindenberg, A. Influence of different surface machining treatments of magnesium-based resorbable implants on the degradation behavior in rabbits. Adv. Eng. Mater 2009, 11, B47–B54. [Google Scholar]
- Vigorita, V.J.; Ghelman, B. Orthopaedic Pathology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1999. [Google Scholar]
- Willbold, E.; Kaya, A.A.; Kaya, R.A.; Beckmann, F.; Witte, F. Corrosion of magnesium alloy AZ31 screws is dependent on the implantation site. Mater. Sci. Eng. B 2011, 20, 1835–1840. [Google Scholar]
- Xu, L.; Yu, G.; Zhang, E.; Pan, F.; Yang, K. In vivo corrosion behavior of Mg-Mn-Zn alloy for bone implant application. J. Biomed. Mater. Res. A 2007, 83A, 703–711. [Google Scholar]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y.; et al. Research on an Mg-Zn alloy as a degradable biomaterial. Acta Biomater 2010, 6, 626–640. [Google Scholar]
- Wong, H.M.; Yeung, K.W.K.; Lam, K.O.; Tam, V.; Chu, P.K.; Luk, K.D.K. Abiodegradable polymer-based coating to control the performance ofmagnesium alloy orthopaedic implants. Biomaterials 2010, 31, 2084–2096. [Google Scholar]
- Erdmann, N.; Bondarenko, A.; Hewicker-Trautwein, M.; Angrisani, N.; Reifenrath, J.; Lucas, A. Evaluation of the soft tissue biocompatibility of MgCa0.8 andsurgical steel 316L in vivo: A comparative study in rabbits. Biomed. Eng. Online 2010, 9, 63. [Google Scholar]
- Krause, A.; von der Höh, N.; Bormann, D.; Krause, C.; Bach, F.; Windhagen, H. Degradation behaviour and mechanical properties of magnesium implants inrabbit tibiae. J. Mater. Sci 2010, 45, 624–632. [Google Scholar]
- Dziuba, D.; Meyer-Lindenberg, A.; Seitz, J.M.; Waizy, H.; Angrisani, N.; Reifenrath, J. Long-term in vivo degradation behaviour and biocompatibility of themagnesium alloy ZEK100 for use as a biodegradable bone implant. Acta Biomater 2013, 9, 8548–8560. [Google Scholar]
- ZainalAbidin, N.I.; Rolfe, B.; Owen, H.; Malisano, J.; Martin, D.; Hofstetter, J.; Uggowitzer, P.J.; Atrens, A. The in vivo and in vitro corrosion of high-purity magnesiumand magnesium alloys WZ21 and AZ91. Corros. Sci 2013, 75, 354–366. [Google Scholar]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg-Ca alloys for use asbiodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar]
- Von der Höh, N.; Bormann, D.; Lucas, A.; Thorey, F.; Meyer-Lindenberg, A. Comparison of the in vivo degradation progress of solid magnesiumalloy cylinders and screw-shaped magnesium alloy cylinders in a rabbitmodel. Mater. Sci. Forum 2010, 638–642, 742–747. [Google Scholar]
- Antonialli, S.; Italo, A.; Clamiro, B. Numerical evaluation of reduction of stress shielding in laser coated hip prostheses. Mater. Res 2011, 14, 331–334. [Google Scholar]
- Zhang, E.; Xu, L.; Yu, G.; Pan, F.; Yang, K. In vivo evaluation of biodegradable magnesium alloy bone implant in the first 6 months implantation. J. Biomed. Mater. Res. A 2009, 90A, 882–893. [Google Scholar]
- Xu, S.F.; Lin, K.L.; Wang, Z.; Jiang, C.J.; Wang, L.; Lu, J.X. Reconstruction of calvarial defect of rabbits using porous calcium silicate bioactive ceramics. Biomaterials 2008, 29, 2588–2596. [Google Scholar]
- Lu, J.X.; Gallur, A.; Flautre, B.; Anselme, K.; Descamps, M.; Thierry, B. Comparative study of tissue reactions to calcium phosphate ceramics among cancellous, cortical, and medullar bone sites in rabbits. J. Biomed. Mater. Res 1998, 42, 357–367. [Google Scholar]
- Xue, W.; Liu, X.; Zheng, X.; Ding, C. In vivo evaluation of plasma-sprayed wollastonite coating. Biomaterials 2005, 26, 3455–3460. [Google Scholar]
- Xie, Y.Z.; Chopin, D.; Morin, C.; Hardouin, P.; Zhu, Z.A.; Tang, J. Evaluation of the osteogenesis and biodegradation of porous biphasic ceramic in the human spine. Biomaterials 2006, 27, 2761–2767. [Google Scholar]
- Liu, S.; Jin, F.; Lin, K.; Chang, J.; Lu, J.; Sun, J.; Dai, K.; Fan, C. Effect of calcium silicate on in vitro physiochemical properties and in vivo osteogenisis, degradability and bioactivity of porous β-tricalcium phosphate bioceramics. Biomed. Mater 2013, 8, 025008:1–025008:9. [Google Scholar]
- Gan, Y.K.; Dai, K.R.; Zhang, P.; Tang, T.T.; Zhu, Z.A.; Lu, J.X. The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion. Biomaterials 2008, 29, 3973–3982. [Google Scholar]
- Cancedda, R.; Giannoni, P.; Mastrogiacomo, M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials 2007, 28, 4240–4250. [Google Scholar]
- Witte, F.; Ulrich, H.; Rudert, M.; Willbold, E. Biodegradable magnesium scaffolds: Part 1: Appropriate inflammatory response. J. Biomed. Mater. Res. A 2007, 81, 748–756. [Google Scholar]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar]
- Hampp, C.; Ullmann, B.; Reifenrath, J.; Angrisani, N.; Dziuba, D.; Bormann, D.; Seitz, J.M.; Meyer-Lindenberg, A. Research on the biocompatibility of the new magnesium alloy LANd442—An in vivo study in the rabbit tibiaover 26 weeks. Adv Eng Mater 2012, 14, B28–B37. [Google Scholar]
- Kuhlmann, J.; Bartsch, I.; Willbold, E.; Schuchardt, S.; Holz, O.; Hort, N.; Höhe, D.; Heineman, W.R.; Witte, F. Fast escape of hydrogen from gas cavities around corroding magnesiumimplants. Acta Biomater 2013, 9, 8714–8721. [Google Scholar]


© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Han, P.; Tan, M.; Zhang, S.; Ji, W.; Li, J.; Zhang, X.; Zhao, C.; Zheng, Y.; Chai, Y. Shape and Site Dependent in Vivo Degradation of Mg-Zn Pins in Rabbit Femoral Condyle. Int. J. Mol. Sci. 2014, 15, 2959-2970. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms15022959
Han P, Tan M, Zhang S, Ji W, Li J, Zhang X, Zhao C, Zheng Y, Chai Y. Shape and Site Dependent in Vivo Degradation of Mg-Zn Pins in Rabbit Femoral Condyle. International Journal of Molecular Sciences. 2014; 15(2):2959-2970. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms15022959
Chicago/Turabian StyleHan, Pei, Moyan Tan, Shaoxiang Zhang, Weiping Ji, Jianan Li, Xiaonong Zhang, Changli Zhao, Yufeng Zheng, and Yimin Chai. 2014. "Shape and Site Dependent in Vivo Degradation of Mg-Zn Pins in Rabbit Femoral Condyle" International Journal of Molecular Sciences 15, no. 2: 2959-2970. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms15022959




