Characterization of Bactrocera dorsalis Serine Proteases and Evidence for Their Indirect Role in Insecticide Tolerance
Abstract
:1. Introduction
2. Results
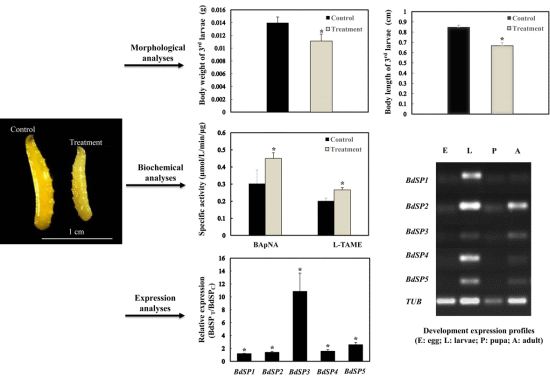
2.1. β-Cypermethrin Intake Slows Larval Development
2.2. Effect of β-Cypermethrin on Protease Activities
2.3. Amino Acid Similarities
2.4. Phylogenetic Analyses
2.5. Developmental Expression Profiles of BdSPs
2.6. Temporal Expression of BdSPs in Response to β-Cypermethrin
3. Discussion
4. Experimental Section
4.1. Insects
4.2. Exposure to β-Cypermethrin
4.3. Preparation of Enzyme Extracts
4.4. Protein Concentration and Protease Activity
4.5. RNA Isolation and First-Strand cDNA Synthesis
4.6. Sequence Analysis and Phylogenetic Tree Construction
4.7. Analysis of SP Gene Expression
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bateman, M.A. The ecology of fruit flies. Annu. Rev. Entomol 1972, 17, 493–518. [Google Scholar]
- Fletcher, B. The biology of dacine fruit flies. Annu. Rev. Entomol 1987, 32, 115–144. [Google Scholar]
- Clarke, A.R.; Armstrong, K.F.; Carmichael, A.E.; Milne, J.R.; Raghu, S.; Roderick, G.K.; Yeates, D.K. Invasive phytophagous pests arising through a recent tropical evolutionary radiation: The Bactrocera dorsalis complex of fruit flies. Annu. Rev. Entomol 2005, 50, 293–319. [Google Scholar]
- Wolfson, J.L.; Murdock, L.L. Diversity in digestive proteinase activity among insects. J. Chem. Ecol 1990, 16, 1089–1102. [Google Scholar]
- Murdock, L.; Shade, R.; Pomeroy, M. Effects of E-64, a cysteine proteinase inhibitor, on cowpea weevil growth, development, and fecundity. Environ. Entomol 1988, 17, 467–469. [Google Scholar]
- Chapman, R.F. The Insects: Structure and Function, 4th ed.; Cambridge University Press: New York, NY, USA, 1998; pp. 54–59. [Google Scholar]
- Srinivasan, A.; Giri, A.P.; Gupta, V.S. Structural and functional diversities in lepidopteran serine proteases. Cell Mol. Biol. Lett 2006, 11, 132–154. [Google Scholar]
- Li, H.M.; Buczkowski, G.; Mittapalli, O.; Xie, J.; Wu, J.; Westerman, R.; Schemerhorn, B.; Murdock, L.; Pittendrigh, B. Transcriptomic profiles of Drosophila melanogaster third instar larval midgut and responses to oxidative stress. Inset. Mol. Biol 2008, 17, 325–339. [Google Scholar]
- Blow, D.M. The tortuous story of Asp-His-Ser: Structural analysis of alpha-chymotrypsin. Trends. Biochem. Sci 1997, 22, 405. [Google Scholar]
- Hedstrom, L. Serine protease mechanism and specificity. Chem. Rev 2002, 102, 4501–4524. [Google Scholar]
- Kale, M.; Rathore, N.; John, S.; Bhatnagar, D. Lipid peroxidative damage on pyrethroid exposure and alterations in antioxidant status in rat erythrocytes: A possible involvement of reactive oxygen species. Toxicol. Lett 1999, 105, 197–205. [Google Scholar]
- Wolansky, M.; Harrill, J. Neurobehavioral toxicology of pyrethroid insecticides in adult animals: A critical review. Neurotoxicol. Teratol 2008, 30, 55–78. [Google Scholar]
- Vais, H.; Williamson, M.S.; Devonshire, A.L.; Usherwood, P.N.R. The molecular interactions of pyrethroid insecticides with insect and mammalian sodium channels. PestManag. Sci 2001, 57, 877–888. [Google Scholar]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol 2007, 52, 231–253. [Google Scholar]
- Huang, Y.; Shen, G.M.; Jiang, H.B.; Jiang, X.Z.; Dou, W.; Wang, J.J. Multiple P450 genes: Identification, tissue-specific expression and their responses to insecticide treatments in the oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidea). Pestic. Biochem. Physiol 2013, 106, 1–7. [Google Scholar]
- Hu, F.; Dou, W.; Wang, J.J.; Jia, F.X.; Wang, J.J. Multiple glutathione S-transferase genes: identification and expression in oriental fruit fly Bactrocera dorsalis. PestManag. Sci 2014, 70, 295–303. [Google Scholar]
- Hu, F.; Dou, W.; Wang, J.J.; Jia, F.X.; Wang, J.J. Purification and biochemical characterization of glutathione S-transferases from four field populations of Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Arch. Insect Biochem. Physiol 2011, 78, 201–215. [Google Scholar]
- Hsu, J.C.; Wu, W.J.; Haymer, D.S.; Liao, H.Y.; Feng, H.T. Alterations of the acetylcholinesterase enzyme in the oriental fruit fly Bactrocera dorsalis are correlated with resistance to the organophosphate insecticide fenitrothion. Insect Biochem. Mol. Biol 2008, 38, 146–154. [Google Scholar]
- Silva, L.B.; Reis, A.P.; Pereira, E.J.G.; Oliveira, M.G.A.; Guedes, R.N.C. Altered cysteine proteinase activity in insecticide-resistant strains of the maize weevil: purification and characterization. Comp. Bioche. Physiol. B 2010, 157, 80–87. [Google Scholar]
- Silva, L.B.; Reis, A.P.; Pereira, E.J.G.; Oliveira, M.G.A.; Guedes, R.N.C. Partial purification and characterization of trypsin-like proteinases from insecticide-resistant and -susceptible strains of the maize weevil Sitophilus zeamais. Comp. Bioche. Physiol. B 2010, 155, 12–19. [Google Scholar]
- Philippou, D.; Field, L.; Moores, G. Metabolic enzyme (s) confer imidacloprid resistance in a clone of Myzus persicae (Sulzer)(Hemiptera: Aphididae) from Greece. Pest Manag. Sci 2010, 66, 390–395. [Google Scholar]
- Araújo, R.; Guedes, R.N.C.; Oliveira, M.G.A.; Ferreira, G. Enhanced proteolytic and cellulolytic activity in insecticide-resistant strains of the maize weevil Sitophilus zeamais. J. Stored Prod. Res 2008, 44, 354–359. [Google Scholar]
- Natsuhara, K.; Shimada, K.; Tanaka, T.; Miyata, T. Phenobarbital induction of permethrin detoxification and phenobarbital metabolism in susceptible and resistant strains of the beet armyworm Spodoptera exigua (Hübner). Pestic. Biochem. Physiol 2004, 79, 33–41. [Google Scholar]
- Nath, B.S. Changes in carbohydrate metabolism in hemolymph and fat body of the silkworm, Bombyx mori L., exposed to organophosphorus insecticides. Pestic. Biochem. Physiol 2000, 68, 127–137. [Google Scholar]
- Perona, J.J.; Craik, C.S. Structural basis of substrate specificity in the serine proteases. Protein Sci 1995, 4, 337–360. [Google Scholar]
- Ge, Z.Y.; Wan, P.J.; Han, Z.J.; Golding, B. Cloning and characterization of trypsin-and chymotrypsin-like genes in the striped rice stem borer Chilo suppressalis. Genome 2012, 55, 281–288. [Google Scholar]
- Ross, J.; Jiang, H.; Kanost, M.R.; Wang, Y. Serine proteases and their homologs in the Drosophila melanogaster genome: An initial analysis of sequence conservation and phylogenetic relationships. Gene 2003, 304, 117–131. [Google Scholar]
- Zhan, Q.; Zheng, S.; Feng, Q.; Liu, L. A midgut-specific chymotrypsin cDNA (Slctlp1) from Spodoptera litura: Cloning, characterization, localization and expression analysis. Arch. Insect Biochem. Physiol 2011, 76, 130–143. [Google Scholar]
- Zhang, C.; Zhou, D.; Zheng, S.; Liu, L.; Tao, S.; Yang, L.; Hu, S.; Feng, Q. A chymotrypsin-like serine protease cDNA involved in food protein digestion in the common cutworm, Spodoptera litura: Cloning, characterization, developmental and induced expression patterns, and localization. J. Insect Physiol 2010, 56, 788–799. [Google Scholar]
- Bhosale, S.; Kallapur, V. Changes in the metabolic fuel reserves of the 5th instar, Bombyx mori, following endosulfan treatment. Entomon 1985, 10, 281. [Google Scholar]
- Karban, R.; Agrawal, A.A. Herbivore offense. Annu. Rev. Ecol. Syst 2002, 33, 641–664. [Google Scholar]
- Appel, H.M.; Martin, M.M. Significance of metabolic load in the evolution of host specificity of Manduca sexta. Ecology 1992, 73, 216–228. [Google Scholar]
- Nath, B.S.; Suresh, A.; Varma, B.M.; Kumar, R. Changes in protein metabolism in hemolymph and fat Body of the silkworm, Bombyx mori (Lepidoptera: Bombycidae) in response to organophosphorus insecticides toxicity. Ecotoxicol. Environ. Saf 1997, 36, 169–173. [Google Scholar]
- Shen, G.M.; Dou, W.; Huang, Y.; Jiang, X.Z.; Smagghe, G.; Wang, J.J. In silico cloning and annotation of genes involved in the digestion, detoxification and RNA interference mechanism in the midgut of Bactrocera dorsalis [Hendel (Diptera: Tephritidae)]. Insect Mol. Biol 2013, 22, 354–365. [Google Scholar]
- Davis, C.A.; Riddell, D.C.; Higgins, M.J.; Holden, J.J.; White, B.N. A gene family in Drosophila melanogaster coding for trypsin-like enzymes. Nucleic. Acids. Res 1985, 13, 6605–6619. [Google Scholar]
- Shen, G.M.; Huang, Y.; Jiang, X.Z.; Dou, W.; Wang, J.J. Effect of β-Cypermethrin exposure on the stability of nine housekeeping genes in Bactrocera dorsalis (Diptera: Tephritidae). Fla. Entomol 2013, 96, 442–450. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 1976, 72, 248–254. [Google Scholar]
- Hosseininaveh, V.; Bandani, A.; Azmayeshfard, P.; Hosseinkhani, S.; Kazzazi, M. Digestive proteolytic and amylolytic activities in Trogoderma granarium Everts (Dermestidae: Coleoptera). J. Stored Prod. Res 2007, 43, 515–522. [Google Scholar]
- Chen, C.C.; Gao, L.T.; Shi, B.J.; Wen, W.D.; Hu, Z.N.; Wu, W.J. Effects of periplocosides P and E from Periploca sepium on the proteinase activities in the midgut of larvae of Mythimna separata and Agrotis ypsilon (Lepidoptera Noctuidae). Acta. Entomol. Sin 2012, 55, 412–419. [Google Scholar]
- Hummel, B.C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can. J. Biochem. Physiol 1959, 37, 1393–1399. [Google Scholar]
- Jeanmougin, F.; Thompson, J.D.; Gouy, M.; Higgins, D.G.; Gibson, T.J. Multiple sequence alignment with Clustal X. Trends. Biochem. Sci 1998, 23, 403. [Google Scholar]
- Shen, G.M.; Jiang, H.B.; Wang, X.N.; Wang, J.J. Evaluation of endogenous references for gene expression profiling in different tissues of the oriental fruit fly Bactrocera dorsalis (Diptera: Tephritidae). BMC Mol. Biol 2010, 11, 76. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic. Acids Res 2001, 29, e45. [Google Scholar]




| Groups | Specific activity (nmol/mL/min/mg) | |
|---|---|---|
| BApNA | L-TAME | |
| Control | 302.67 ± 79.93 | 201.33 ± 16.92 |
| Treatment | 450.00 ± 32.97 * | 266.00 ± 13.89 * |
| Species | Serine protease | GenBank Accession number | Conserved regions a | Length | Enzyme specificity b | ||
|---|---|---|---|---|---|---|---|
| TAAHC | DIAL | GDSGGP | |||||
| Bactrocera. dorsalis | BdSP1 | GAAP01000017 | SATHC | DIGL | 265 | T(DGG) | |
| Bactrocera. dorsalis | BdSP2 | GAAP01000019 | DIAI | 259 | T(DGG) | ||
| Bactrocera. dorsalis | BdSP3 | GAAP01000020 | DLAL | 247 | E(GVD) | ||
| Bactrocera. dorsalis | BdSP4 | GAAP01000021 | 258 | T(DGG) | |||
| Bactrocera. dorsalis | BdSP5 | GAAP01000022 | 257 | E(GVD) | |||
| Glossina morsitans morsitans | GmmTR1 | ACB98719.1 | DVAV | 256 | T(DGG) | ||
| Glossina morsitans morsitans | GmmTR2 | ADD19605.1 | DYSL | 281 | T(DGG) | ||
| Aedes aegypti | AaSP1 | XP_004520098.1 | DLAL | 248 | E(GVD) | ||
| Aedes aegypti | AaSP2 | XP_001659492.1 | 247 | E(GVD) | |||
| Aedes aegypti | AaSP3 | XP_001659851.1 | DIAV | 274 | E(GVD) | ||
| Ceratitis capitata | CcSP | XP_004520096.1 | DVAL | 254 | E(GVD) | ||
| Ceratitis capitata | CcTR | XP_004517776.1 | DIAV | 257 | T(DGG) | ||
| Drosophila melanogaster | DmTR1 | AAA17449.1 | DIVI | 253 | T(DGG) | ||
| Drosophila melanogaster | DmTR2 | NP_525112.1 | DIAI | 256 | T(DGG) | ||
| Drosophila erecta | DeTR1 | XP_001976081.1 | DIAV | 256 | T(DGG) | ||
| Drosophila erecta | DeTR2 | XP_001976082.1 | DVGI | 262 | T(DGG) | ||
| Drosophila yakuba | DyTR | XP_002091228.1 | DIAI | 256 | T(DGG) | ||
| Phlebotomus papatasi | PpTR | AAM96943.1 | DFSL | 268 | T(DGW) | ||
| Manduca sexta | MsTR | P35046.1 | DIAI | 256 | T(DGG) | ||
| Hypoderma diana | HdSP | ACF98290.1 | DVAI | 256 | T(DGG) | ||
| Stomoxys calcitrans | SsSP | AAC39131.1 | DVAV | 254 | T(DGG) | ||
| Genes | Relative expression | |||
|---|---|---|---|---|
| Egg | Larva | Pupa | Adult | |
| BdSP1 | 1.0 | 7.48 ± 0.93 | 0.05 ± 0.02 | 1.61 ± 0.51 |
| BdSP2 | 1.0 | 189.62 ± 23.11 | 0.47 ± 0.16 | 118 ± 28.69 |
| BdSP3 | 1.0 | 4.04 ± 0.86 | 0.45 ± 0.22 | 2.21 ± 0.03 |
| BdSP4 | 1.0 | 6.71 ± 1.72 | 0.67 ± 0.25 | 1.10 ± 0.19 |
| BdSP5 | 1.0 | 7.86 ± 1.48 | 0.04 ± 0.02 | 3.56 ± 0.10 |
| Genes | GenBank accession number | Primer name and sequence (5′-3′) | PCR efficiency (%) | R2 |
|---|---|---|---|---|
| BdSP1 | GAAP01000017 | S: ACACACTCGGGTTTTAGCGT A: GAGGCGCAATCTTCACGTTG | 106.25 | 0.981 |
| BdSP2 | GAAP01000019 | S: AGAGGACGTATTGTTGGCGG A: CGGCACGGATTCTCAAAACC | 96.17 | 0.993 |
| BdSP3 | GAAP01000020 | S: ATCCCACGGGTCGTGTAGTA A: GTTGTGCCTGTTTCGACCAC | 108.43 | 0.986 |
| BdSP4 | GAAP01000021 | S: CAACGTGAAGATTGCGCCTC A: CCTTTTTGGCACAGCCTTCC | 91.95 | 0.989 |
| BdSP5 | GAAP01000022 | S: TTCCCCCATCAGGTCTCAC A: CCGAGTGTGCGTTGGATAC | 94.61 | 0.988 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hou, M.-Z.; Shen, G.-M.; Wei, D.; Li, Y.-L.; Dou, W.; Wang, J.-J. Characterization of Bactrocera dorsalis Serine Proteases and Evidence for Their Indirect Role in Insecticide Tolerance. Int. J. Mol. Sci. 2014, 15, 3272-3286. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms15023272
Hou M-Z, Shen G-M, Wei D, Li Y-L, Dou W, Wang J-J. Characterization of Bactrocera dorsalis Serine Proteases and Evidence for Their Indirect Role in Insecticide Tolerance. International Journal of Molecular Sciences. 2014; 15(2):3272-3286. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms15023272
Chicago/Turabian StyleHou, Ming-Zhe, Guang-Mao Shen, Dong Wei, Ya-Li Li, Wei Dou, and Jin-Jun Wang. 2014. "Characterization of Bactrocera dorsalis Serine Proteases and Evidence for Their Indirect Role in Insecticide Tolerance" International Journal of Molecular Sciences 15, no. 2: 3272-3286. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms15023272