Mechanisms and Implications of Dual-Acting Methotrexate in Folate-Targeted Nanotherapeutic Delivery
Abstract
:1. Introduction

2. Results and Discussion
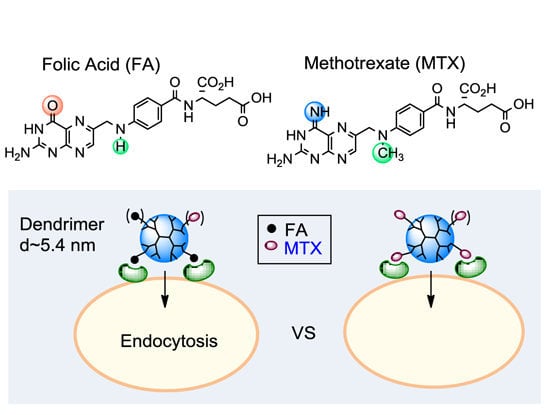
2.1. FA vs. MTX
2.1.1. Binding Affinity to Folate Receptor (FAR)

| Mw (g/mol) | a,b clogD | b,c tPSA (Å2) | d Permeability (×10−6 cm/s) | KD (Receptor) | Ki (Cellular Target) | |
|---|---|---|---|---|---|---|
| FA | 441.4 | −5.82 | 208 | 1.7 [68] | 0.4 nM (FAR) [61] | 0.48 μM (DHFR) [69] |
| MTX | 454.4 | −4.98 | 211 | 1.2 [68] | 20–100 nM (FAR) [61,65,66]; 4.3 μM (RFC) [64] | 1.2 nM (DHFR) [70]; 13 μM (TYMS) [71]; 96 μM (dCK) [72] |
2.1.2. Enzyme Pharmacology
2.2. Binding to a FAR(+) Model Surface as Studied by SPR Spectroscopy

2.2.1. Monovalent Ligands
| FA [50,51] | MTX [50,51] | G5(MTX)n (n = 5) [51] | G5(MTX)n (n = 10) [51] | |
|---|---|---|---|---|
| b kd (s−1) | 1.2 × 10−2 | 1.7 × 10−2 | 2.0 × 10−4 | 7.8 × 10−5 |
| c KD (M) | 2.0–11 × 10−6 | 2.4–4.0 × 10−5 | 2.1 × 10−9 | 5.5 × 10−10 |
| d β | - | 1 | 19,045 (3810) | 72,727 (7273) |
2.2.2. Multivalent MTX Ligands
2.3. Dendrimer Binding to FAR(+) Cells in Vitro Studied by Fluorescence Confocal Microscopy and Flow Cytometry

2.4. Inhibition of Dihydrofolate Reductase in a Cell-Free Condition

2.5. In Vitro Cytotoxicity Studied by XTT Assay

2.6. Role of MTX Release in Vitro

3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baker, J.R., Jr. Dendrimer-based nanoparticles for cancer therapy. Hematol. Am. Soc. Hematol. Educ. Program. 2009, 2009, 708–719. [Google Scholar]
- Majoros, I.J.; Williams, C.R.; Becker, A.; Baker, J.R., Jr. Methotrexate delivery via folate targeted dendrimer-based nanotherapeutic platform. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 502–510. [Google Scholar]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar]
- Agasti, S.S.; Rana, S.; Park, M.-H.; Kim, C.K.; You, C.-C.; Rotello, V.M. Nanoparticles for detection and diagnosis. Adv. Drug Deliv. Rev. 2010, 62, 316–328. [Google Scholar]
- Li, C.; Wallace, S. Polymer-drug conjugates: Recent development in clinical oncology. Adv. Drug Deliv. Rev. 2008, 60, 886–898. [Google Scholar]
- Patri, A.K.; Kukowska-Latallo, J.F.; Baker, J.J.R. Targeted drug delivery with dendrimers: Comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv. Drug Deliv. Rev. 2005, 57, 2203–2214. [Google Scholar]
- Cheng, Z.; Al Zaki, A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, A. Multifunctional nanoparticles: Cost vs. benefit of adding targeting and imaging capabilities. Science 2012, 338, 903–910. [Google Scholar]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers: Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar]
- Tomalia, D.A.; Naylor, A.M.; William, A.; Goddard, I. Starburst dendrimers: Molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew. Chem. Int. Ed. 1990, 29, 138–175. [Google Scholar]
- Zhou, Z.; Shen, Y.; Tang, J.; Jin, E.; Ma, X.; Sun, Q.; Zhang, B.; van Kirk, E.A.; Murdoch, W.J. Linear polyethyleneimine-based charge-reversal nanoparticles for nuclear-targeted drug delivery. J. Mater. Chem. 2011, 21, 19114–19123. [Google Scholar]
- Chertok, B.; David, A.E.; Yang, V.C. Polyethyleneimine-modified iron oxide nanoparticles for brain tumor drug delivery using magnetic targeting and intra-carotid administration. Biomaterials 2010, 31, 6317–6324. [Google Scholar]
- Sideratou, Z.; Kontoyianni, C.; Drossopoulou, G.I.; Paleos, C.M. Synthesis of a folate functionalized PEGylated poly(propylene imine) dendrimer as prospective targeted drug delivery system. Bioorg. Med. Chem. Lett. 2010, 20, 6513–6517. [Google Scholar]
- Paleos, C.M.; Tsiourvas, D.; Sideratou, Z.; Tziveleka, L. Acid- and salt-triggered multifunctional poly(propylene imine) dendrimer as a prospective drug delivery system. Biomacromolecules 2004, 5, 524–529. [Google Scholar]
- Kaminskas, L.M.; Kelly, B.D.; McLeod, V.M.; Sberna, G.; Owen, D.J.; Boyd, B.J.; Porter, C.J.H. Characterisation and tumour targeting of PEGylated polylysine dendrimers bearing doxorubicin via a pH labile linker. J. Control. Release 2011, 152, 241–248. [Google Scholar]
- Chen, H.-T.; Neerman, M.F.; Parrish, A.R.; Simanek, E.E. Cytotoxicity, hemolysis, and acute in vivo toxicity of dendrimers based on melamine, candidate vehicles for drug delivery. J. Am. Chem. Soc. 2004, 126, 10044–10048. [Google Scholar]
- Zhang, W.; Nowlan, D.T.; Thomson, L.M.; Lackowski, W.M.; Simanek, E.E. Orthogonal, convergent syntheses of dendrimers based on melamine with one or two unique surface sites for manipulation. J. Am. Chem. Soc. 2001, 123, 8914–8922. [Google Scholar]
- Chandran, S.S.; Nan, A.; Rosen, D.M.; Ghandehari, H.; Denmeade, S.R. A prostate-specific antigen–activated N-(2-hydroxypropyl) methacrylamide copolymer prodrug as dual-targeted therapy for prostate cancer. Mol. Cancer Ther. 2007, 6, 2928–2937. [Google Scholar]
- Hrkach, J.; von Hoff, D.; Ali, M.M.; Andrianova, E.; Auer, J.; Campbell, T.; de Witt, D.; Figa, M.; Figueiredo, M.; Horhota, A.; et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med. 2012, 4, 128ra39. [Google Scholar]
- Liu, Z.; Chen, K.; Davis, C.; Sherlock, S.; Cao, Q.; Chen, X.; Dai, H. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008, 68, 6652–6660. [Google Scholar]
- Dhar, S.; Liu, Z.; Thomale, J.R.; Dai, H.; Lippard, S.J. Targeted single-wall carbon nanotube-mediated Pt(IV) prodrug delivery using folate as a homing device. J. Am. Chem. Soc. 2008, 130, 11467–11476. [Google Scholar]
- Daniel, M.-C.; Astruc, D. Gold nanoparticles; assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar]
- Ghosh, S.K.; Pal, A.; Kundu, S.; Nath, S.; Pal, T. Fluorescence quenching of 1-methylaminopyrene near gold nanoparticles: Size regime dependence of the small metallic particles. Chem. Phys. Lett. 2004, 395, 366–372. [Google Scholar]
- Landmark, K.J.; DiMaggio, S.; Ward, J.; Kelly, C.; Vogt, S.; Hong, S.; Kotlyar, A.; Myc, A.; Thomas, T.P.; Penner-Hahn, J.E.; et al. Synthesis, characterization, and in vitro testing of superparamagnetic iron oxide nanoparticles targeted using folic acid-conjugated dendrimers. ACS Nano 2008, 2, 773–783. [Google Scholar]
- Choi, Y.-E.; Kwak, J.-W.; Park, J. W. Nanotechnology for early cancer detection. Sensors 2010, 10, 428–455. [Google Scholar]
- Mansoori, G.A.; Brandenburg, K.S.; Shakeri-Zadeh, A. A Comparative study of two folate-conjugated gold nanoparticles for cancer nanotechnology applications. Cancers 2010, 2, 1911–1928. [Google Scholar]
- Patra, C.R.; Bhattacharya, R.; Mukhopadhyay, D.; Mukherjee, P. Fabrication of gold nanoparticles for targeted therapy in pancreatic cancer. Adv. Drug Deliv. Rev. 2010, 62, 346–361. [Google Scholar]
- Shakeri-Zadeh, A.; Ghasemifard, M.; Ali Mansoori, G. Structural and optical characterization of folate-conjugated gold-nanoparticles. Phys. E 2010, 42, 1272–1280. [Google Scholar]
- Mammen, M.; Choi, S.K.; Whitesides, G.M. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Ed. 1998, 37, 2754–2794. [Google Scholar]
- Kiessling, L.L.; Gestwicki, J.E.; Strong, L.E. Synthetic multivalent ligands as probes of signal transduction. Angew. Chem. Int. Ed. 2006, 45, 2348–2368. [Google Scholar]
- Fasting, C.; Schalley, C.A.; Weber, M.; Seitz, O.; Hecht, S.; Koksch, B.; Dernedde, J.; Graf, C.; Knapp, E.-W.; Haag, R. Multivalency as a chemical organization and action principle. Angew. Chem. Int. Ed. 2012, 51, 10472–10498. [Google Scholar]
- Kukowska-Latallo, J.F.; Candido, K.A.; Cao, Z.; Nigavekar, S.S.; Majoros, I.J.; Thomas, T.P.; Balogh, L.P.; Khan, M.K.; Baker, J.R., Jr. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005, 65, 5317–5324. [Google Scholar]
- Majoros, I.J.; Thomas, T.P.; Mehta, C.B.; Baker, J.R., Jr. Poly(amidoamine) dendrimer-based multifunctional engineered nanodevice for cancer therapy. J. Med. Chem. 2005, 48, 5892–5899. [Google Scholar]
- Quintana, A.; Raczka, E.; Piehler, L.; Lee, I.; Myc, A.; Majoros, I.; Patri, A. K.; Thomas, T.; Mulé, J.; Baker, J.R., Jr. Design and function of a dendrimer-based therapeutic nanodevice targeted to tumor cells through the folate receptor. Pharm. Res. 2002, 19, 1310–1316. [Google Scholar]
- Wong, P.T.; Tang, K.; Coulter, A.; Tang, S.; Baker, J.R.; Choi, S.K. Multivalent dendrimer vectors with DNA intercalation motifs for gene delivery. Biomacromolecules 2014, 15, 4134–4145. [Google Scholar]
- Witte, A.B.; Leistra, A.N.; Wong, P.T.; Bharathi, S.; Refior, K.; Smith, P.; Kaso, O.; Sinniah, K.; Choi, S.K. Atomic force microscopy probing of receptor-nanoparticle interactions for riboflavin receptor targeted gold-dendrimer nanocomposites. J. Phys. Chem. B 2014, 118, 2872–2882. [Google Scholar]
- Witte, A.B.; Timmer, C.M.; Gam, J.J.; Choi, S.K.; Banaszak Holl, M.M.; Orr, B.G.; Baker, J.R.; Sinniah, K. Biophysical characterization of a riboflavin-conjugated dendrimer platform for targeted drug delivery. Biomacromolecules 2012, 13, 507–516. [Google Scholar]
- Shukla, R.; Thomas, T.P.; Peters, J.; Kotlyar, A.; Myc, A.; Baker, J.R., Jr. Tumor angiogenic vasculature targeting with PAMAM dendrimer-RGD conjugates. Chem. Commun. 2005, 5739–5741. [Google Scholar]
- Huang, B.; Otis, J.; Joice, M.; Kotlyar, A.; Thomas, T.P. PSMA-targeted stably-linked “dendrimer-glutamate urea-methotrexate” as a prostate cancer therapeutic. Biomacromolecules 2014, 15, 915–923. [Google Scholar]
- Thomas, T.P.; Shukla, R.; Kotlyar, A.; Liang, B.; Ye, J.Y.; Norris, T. B.; Baker, J.R., Jr. Dendrimer-epidermal growth factor conjugate displays superagonist activity. Biomacromolecules 2008, 9, 603–609. [Google Scholar]
- Zhou, Y.; Chakraborty, S.; Liu, S. Radiolabeled cyclic RGD peptides as radiotracers for imaging tumors and thrombosis by SPECT. Theranostics 2011, 1, 58–82. [Google Scholar]
- Serpe, L.; Gallicchio, M.; Canaparo, R.; Dosio, F. Targeted treatment of folate receptor-positive platinum-resistant ovarian cancer and companion diagnostics, with specific focus on vintafolide and etarfolatide. Pharm. Pers. Med. 2014, 7, 31–42. [Google Scholar]
- Pribble, P.; Edelman, M.J. EC145: A novel targeted agent for adenocarcinoma of the lung. Expert Opin. Investig. Drugs 2012, 21, 755–761. [Google Scholar]
- Dosio, F.; Milla, P.; Cattel, L. EC-145, a folate-targeted Vinca alkaloid conjugate for the potential treatment of folate receptor-expressing cancers. Curr. Opin. Investig. Drugs 2010, 11, 1424–1433. [Google Scholar]
- Mullen, D.G.; Fang, M.; Desai, A.; Baker, J.R., Jr.; Orr, B.G.; Banaszak Holl, M.M. A quantitative assessment of nanoparticle-ligand distributions: Implications for targeted drug and imaging delivery in dendrimer conjugates. ACS Nano 2010, 4, 657–670. [Google Scholar]
- Van Dongen, M.A.; Rattan, R.; Silpe, J.; Dougherty, C.; Michmerhuizen, N.; van Winkle, M.; Huang, B.; Choi, S. K.; Sinniah, K.; Orr, B.G.; et al. Poly(amidoamine) dendrimer-methotrexate conjugates: The mechanism of interaction with folate binding protein. Mol. Pharm. 2014, 11, 4049–4058. [Google Scholar]
- Van Dongen, M.A.; Silpe, J.E.; Dougherty, C.A.; Kanduluru, A.K.; Choi, S.K.; Orr, B.G.; Low, P.S.; Banaszak Holl, M.M. Avidity mechanism of dendrimer-folic acid conjugates. Mol. Pharm. 2014, 11, 1696–1706. [Google Scholar]
- Gu, F.; Zhang, L.; Teply, B.A.; Mann, N.; Wang, A.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc. Natl. Acad. Sci. USA 2008, 105, 2586–2591. [Google Scholar]
- Qian, H.; Jin, R. Controlling nanoparticles with atomic precision: The case of Au144(SCH2CH2Ph)60. Nano Lett. 2009, 9, 4083–4087. [Google Scholar]
- Zong, H.; Thomas, T.P.; Lee, K.-H.; Desai, A.M.; Li, M.-H.; Kotlyar, A.; Zhang, Y.; Leroueil, P.R.; Gam, J.J.; Banaszak Holl, M.M.; et al. Bifunctional pamam dendrimer conjugates of folic acid and methotrexate with defined ratio. Biomacromolecules 2012, 13, 982–991. [Google Scholar]
- Li, M.-H.; Choi, S.K.; Thomas, T.P.; Desai, A.; Lee, K.-H.; Kotlyar, A.; Banaszak Holl, M.M.; Baker, J.R., Jr. Dendrimer-based multivalent methotrexates as dual acting nanoconjugates for cancer cell targeting. Eur. J. Med. Chem. 2012, 47, 560–572. [Google Scholar]
- Silpe, J.E.; Sumit, M.; Thomas, T.P.; Huang, B.; Kotlyar, A.; van Dongen, M.A.; Banaszak Holl, M.M.; Orr, B.G.; Choi, S.K. Avidity modulation of folate-targeted multivalent dendrimers for evaluating biophysical models of cancer targeting nanoparticles. ACS Chem. Biol. 2013, 8, 2063–2071. [Google Scholar]
- Thomas, T.P.; Huang, B.; Choi, S.K.; Silpe, J.E.; Kotlyar, A.; Desai, A.M.; Gam, J.; Joice, M., Jr. Polyvalent PAMAM-methotrexate dendrimer as a folate receptor-targeted therapeutic. Mol. Pharm. 2012, 9, 2669–2676. [Google Scholar]
- Dervieux, T.; Furst, D.; Lein, D.O.; Capps, R.; Smith, K.; Walsh, M.; Kremer, J. Polyglutamation of methotrexate with common polymorphisms in reduced folate carrier, aminoimidazole carboxamide ribonucleotide transformylase, and thymidylate synthase are associated with methotrexate effects in rheumatoid arthritis. Arthritis Rheumatol. 2004, 50, 2766–2774. [Google Scholar]
- Elnakat, H.; Ratnam, M. Distribution, functionality and gene regulation of folate receptor isoforms: Implications in targeted therapy. Adv. Drug Deliv. Rev. 2004, 56, 1067–1084. [Google Scholar]
- Ross, J.F.; Chaudhuri, P.K.; Ratnam, M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer 1994, 73, 2432–2443. [Google Scholar]
- Thomas, T.P.; Goonewardena, S.N.; Majoros, I.J.; Kotlyar, A.; Cao, Z.; Leroueil, P.R.; Baker, J.R. Folate-targeted nanoparticles show efficacy in the treatment of inflammatory arthritis. Arthritis Rheumatol. 2011, 63, 2671–2680. [Google Scholar]
- Van Der Heijden, J.W.; Oerlemans, R.; Dijkmans, B.A.C.; Qi, H.; Laken, C.J.V.D.; Lems, W.F.; Jackman, A.L.; Kraan, M.C.; Tak, P.P.; Ratnam, M.; et al. Folate receptor β as a potential delivery route for novel folate antagonists to macrophages in the synovial tissue of rheumatoid arthritis patients. Arthritis Rheumatol. 2009, 60, 12–21. [Google Scholar]
- Nakashima-Matsushita, N.; Homma, T.; Yu, S.; Matsuda, T.; Sunahara, N.; Nakamura, T.; Tsukano, M.; Ratnam, M.; Matsuyama, T. Selective expression of folate receptor β and its possible role in methotrexate transport in synovial macrophages from patients with rheumatoid arthritis. Arthritis Rheumatol. 1999, 42, 1609–1616. [Google Scholar]
- Wu, M.; Gunning, W.; Ratnam, M. Expression of folate receptor type α in relation to cell type, malignancy, and differentiation in ovary, uterus, and cervix. Cancer Epidemiol. Biomark. Prev. 1999, 8, 775–782. [Google Scholar]
- Campbell, I.G.; Jones, T.A.; Foulkes, W.D.; Trowsdale, J. Folate-binding protein is a marker for ovarian cancer. Cancer Res. 1991, 51, 5329–5338. [Google Scholar]
- Kamen, B.A.; Capdevila, A. Receptor-mediated folate accumulation is regulated by the cellular folate content. Proc. Natl. Acad. Sci. USA 1986, 83, 5983–5987. [Google Scholar]
- Low, P.S.; Henne, W.A.; Doorneweerd, D.D. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc. Chem. Res. 2008, 41, 120–129. [Google Scholar]
- Lu, Y.; Low, P.S. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv. Drug Deliv. Rev. 2002, 54, 675–693. [Google Scholar]
- Sierra, E.E.; Brigle, K.E.; Spinella, M.J.; Goldman, I.D. Comparison of transport properties of the reduced folate carrier and folate receptor in murine L1210 leukemia cells. Biochem. Pharmacol. 1995, 50, 1287–1294. [Google Scholar]
- Nandini-Kishore, S.G.; Frazier, W.A. [3H]Methotrexate as a ligand for the folate receptor of Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA 1981, 78, 7299–7303. [Google Scholar]
- Rijnboutt, S.; Jansen, G.; Posthuma, G.; Hynes, J.B.; Schornagel, J.H.; Strous, G.J. Endocytosis of GPI-linked membrane folate receptor-a. J. Cell Biol. 1996, 132, 35–47. [Google Scholar]
- Wibowo, A.S.; Singh, M.; Reeder, K.M.; Carter, J.J.; Kovach, A.R.; Meng, W.; Ratnam, M.; Zhang, F.; Dann, C.E. Structures of human folate receptors reveal biological trafficking states and diversity in folate and antifolate recognition. Proc. Natl. Acad. Sci. USA 2013, 110, 15180–15188. [Google Scholar]
- Verwei, M.; van den Berg, H.; Havenaar, R.; Groten, J.P. Effect of folate-binding protein on intestinal transport of folic acid and 5-methyltetrahydrofolate across Caco-2 cells. Eur. J. Nutr. 2005, 44, 242–249. [Google Scholar]
- McAlinden, T.P.; Hynes, J.B.; Patil, S.A.; Westerhof, G.R.; Jansen, G.; Schornagel, J.H.; Kerwar, S.S.; Freisheim, J.H. Synthesis and biological evaluation of a fluorescent analog of folic acid. Biochemistry 1991, 30, 5674–5681. [Google Scholar]
- Ercikan-Abali, E.A.; Waltham, M.C.; Dicker, A.P.; Schweitzer, B.I.; Gritsman, H.; Banerjee, D.; Bertino, J.R. Variants of human dihydrofolate reductase with substitutions at leucine-22: Effect on catalytic and inhibitor binding properties. Mol. Pharmacol. 1996, 49, 430–437. [Google Scholar]
- Allegra, C.J.; Chabner, B.A.; Drake, J.C.; Lutz, R.; Rodbard, D.; Jolivet, J. Enhanced inhibition of thymidylate synthase by methotrexate polyglutamates. J. Biol. Chem. 1985, 260, 9720–9726. [Google Scholar]
- Uga, H.; Kuramori, C.; Ohta, A.; Tsuboi, Y.; Tanaka, H.; Hatakeyama, M.; Yamaguchi, Y.; Takahashi, T.; Kizaki, M.; Handa, H. A new mechanism of methotrexate action revealed by target screening with affinity beads. Mol. Pharmacol. 2006, 70, 1832–1839. [Google Scholar]
- Chen, C.; Ke, J.; Zhou, X.E.; Yi, W.; Brunzelle, J.S.; Li, J.; Yong, E.-L.; Xu, H.E.; Melcher, K. Structural basis for molecular recognition of folic acid by folate receptors. Nature 2013, 500, 486–489. [Google Scholar]
- Kirkwood, J.M.; Canellos, G.P.; Ervin, T.J.; Pitman, S.W.; Weichselbaum, R.; Miller, D. Increased therapeutic index using moderate dose methotrexate and leucovorin twice weekly vs. weekly high dose methotrexate-leucovorin in patients with advanced squamous carcinoma of the head and neck: A safe new effective regimen. Cancer 1981, 47, 2414–2421. [Google Scholar]
- Kaul, A.; OʼReilly, D.T.; Slack, R.K.; Collins, D.; Walmsley, J.; Duke, O.; Kiely, P.D.W. Tolerability of methotrexate and leflunomide combination therapy for inflammatory arthritis in routine clinical practice: Results of a four-centre study. Rheumatology 2008, 47, 1430–1431. [Google Scholar]
- Parker, N.; Turk, M.J.; Westrick, E.; Lewis, J.D.; Low, P.S.; Leamon, C.P. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 2005, 338, 284–293. [Google Scholar]
- Weitman, S.D.; Lark, R.H.; Coney, L.R.; Fort, D.W.; Frasca, V.; Zurawski, V.R.; Kamen, B.A. Distribution of the folate receptor gp38 in normal and malignant cell lines and tissues. Cancer Res. 1992, 52, 3396–3401. [Google Scholar]
- Thomas, T.P.; Joice, M.; Sumit, M.; Silpe, J.E.; Kotlyar, A.; Bharathi, S.; Kukowska-Latallo, J.; James, R.; Baker, J.; Choi, S.K. Design and in vitro validation of multivalent dendrimer methotrexates as a folate-targeting anticancer therapeutic. Curr. Pharm. Design. 2013, 19, 6594–6605. [Google Scholar]
- Thomas, T.P.; Majoros, I.J.; Kotlyar, A.; Kukowska-Latallo, J.F.; Bielinska, A.; Myc, A.; Baker, J. R., Jr. Targeting and inhibition of cell growth by an engineered dendritic nanodevice. J. Med. Chem. 2005, 48, 3729–3735. [Google Scholar]
- Dunbar, J.; Yennawar, H.P.; Banerjee, S.; Luo, J.; Farber, G.K. The effect of denaturants on protein structure. Protein Sci. 1997, 6, 1727–1733. [Google Scholar]
- Cody, V.; Luft, J.R.; Pangborn, W. Understanding the role of Leu22 variants in methotrexate resistance: Comparison of wild-type and Leu22Arg variant mouse and human dihydrofolate reductase ternary crystal complexes with methotrexate and NADPH. Acta Crystallogr. D Biol. Crystallogr. 2005, 61, 147–155. [Google Scholar]
- Vicent, M.J.; Ringsdorf, H.; Duncan, R. Polymer therapeutics: Clinical applications and challenges for development. Adv. Drug Deliv. Rev. 2009, 61, 1117–1120. [Google Scholar]
- Thomas, T.P.; Kukowska-Latallo, J.R. Biological application of PAMAM dendrimer nanodevices in vitro and in vivo. In Dendrimer-Based Nanomedicine; Majoros, I., Baker, J.R., Jr., Eds.; Pan Stanford: Hackensack, NJ, USA, 2008; pp. 175–207. [Google Scholar]
- Choi, S.K.; Thomas, T.P.; Li, M.-H.; Desai, A.; Kotlyar, A.; Baker, J.R. Photochemical release of methotrexate from folate receptor-targeting PAMAM dendrimer nanoconjugate. Photochem. Photobiol. Sci. 2012, 11, 653–660. [Google Scholar]
- Choi, S.K.; Verma, M.; Silpe, J.; Moody, R.E.; Tang, K.; Hanson, J.J.; Baker, J.R., Jr. A photochemical approach for controlled drug release in targeted drug delivery. Bioorg. Med. Chem. 2012, 20, 1281–1290. [Google Scholar]
- Choi, S.K.; Leroueil, P.; Li, M.-H.; Desai, A.; Zong, H.; van Der Spek, A.F.L.; Baker, J.R., Jr. Specificity and negative cooperativity in dendrimer–oxime drug complexation. Macromolecules 2011, 44, 4026–4029. [Google Scholar]
- Choi, S.K.; Thomas, T.P.; Leroueil, P.R.; Kotlyar, A.; van Der Spek, A.F.L.; Baker, J.R. Specific and cooperative interactions between oximes and PAMAM dendrimers as demonstrated by 1H NMR study. J. Phys. Chem. B 2012, 116, 10387–10397. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, P.T.; Choi, S.K. Mechanisms and Implications of Dual-Acting Methotrexate in Folate-Targeted Nanotherapeutic Delivery. Int. J. Mol. Sci. 2015, 16, 1772-1790. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms16011772
Wong PT, Choi SK. Mechanisms and Implications of Dual-Acting Methotrexate in Folate-Targeted Nanotherapeutic Delivery. International Journal of Molecular Sciences. 2015; 16(1):1772-1790. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms16011772
Chicago/Turabian StyleWong, Pamela T., and Seok Ki Choi. 2015. "Mechanisms and Implications of Dual-Acting Methotrexate in Folate-Targeted Nanotherapeutic Delivery" International Journal of Molecular Sciences 16, no. 1: 1772-1790. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms16011772