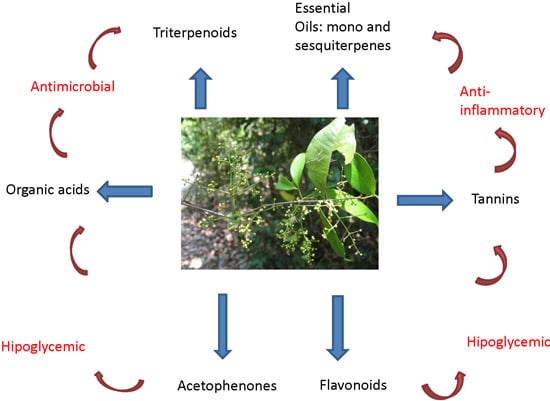
Constituents and Pharmacological Activities of Myrcia (Myrtaceae): A Review of an Aromatic and Medicinal Group of Plants
Abstract
:1. Introduction
2. Traditional Uses
3. Volatiles
| Species | Part of the Plant (Yield: v/w) | Compounds (Relative Abundance, %>5) Classes of Substances; Total | Ref. |
|---|---|---|---|
| M. acuminatissima O.Berg | Fresh leaves (0.12%) | β-pinene (5.0), linalool (22.3), terpinen-4-ol (5.2), β-caryophyllene (8.1), spathulenol (7.5), caryophyllene oxide (5.5) MH: 13.9%, OM: 35.1%, SH: 15.7%, OS: 28.0%, T: 97.0% | [20] |
| M. alagoensis O.Berg | Fresh leaves (0.3%) | β-caryophyllene (7.9), germacrene D (11.1), germacrene B (26.7), (2E,6E)-farnesoic acid (7.3) S: 79.9%, T: 80.5% | [21] |
| Dry leaves (0.4%) | β-caryophyllene (7.8), germacrene D (6.4), δ-cadinene (5.4), selina-3,7(11)-diene (5.4), germacrene B (23.1) S: 75.4%, T: 75.5% | [21] | |
| M. amazonica DC. | Fresh leaves (0.65%) | germacrene D (10.09), germacrene B (9.59), 1-epi-cubenol (20.22), α-muurolol (6.21) | [22] |
| Dry leaves (0.96%) | germacrene D (16.56), germacrene B (11.09), 1-epi-cubenol (14.72) | [22] | |
| M. arborescens O.Berg | Fresh leaves (0.2%) | α-muurolol (6.2), caryophyllene oxide (26.3), spathulenol (8.9), globulol (15.9), 5-epi-7-epi-α-eudesmol (5.9) S: 94.8%, T: 96.2% | [23] |
| M. bombycina (O.Berg) Nied. | Fresh leaves (0.95%) | α-pinene (23.9), β-pinene (12.4), limonene (7.0), γ-eudesmol (7.8) MH: 53.0%, OM: 5.4%, SH: 8.0%, OS: 24.0%, T: 93.5% | [20] |
| M. bracteata DC. | Leaves (0.71%) | α-bisabolol oxide (10.37), α-bisabolol (45.86) SH: 13.76%, OS: 63.49%, S: 77.25% | [24] |
| Leaves and fine stems (0.1%) a | (E)-nerolidol (80.8) | [25] | |
| Leaves and fine stems (0.3%) a | (E)-β-farnesene (33.9), β-curcumene (9.8), β-bisabolol (8.2) | [25] | |
| Leaves and fine stems (0.1%) a | germacrene B (8.8), spathulenol (31.0) | [25] | |
| M. cuprea (O.Berg) Kiaersk. | Leaves (<0.05%) | β-caryophyllene (9.57), α-humulene (7.03), γ-selinene (21.75), α-selinene (11.84), (Z)-α-bisabolene (9.51), (E,E)-α-farnesene (10.52) SH: 82.41%, S: 82.41%, OTH: 11.02% | [24] |
| Leaves and fine stems (0.3%) a | myrcene (48.1), β-caryophyllene (19.9), δ-cadinene (6.9) | [25] | |
| Leaves and fine stems (0.1%) a | α-pinene (15.9), myrcene (19.2), β-caryophyllene (39.1) | [25] | |
| Leaves and fine stems (>0.1%) a | β-caryophyllene (38.1), germacrene D (21.8), germacrene B (19.5) | [25] | |
| M. fallax (Rich.) DC. | Leaves (0.09%) | α-pinene (7.68), β-pinene (11.88), β-elemene (11.21), β-caryophyllene (5.55), selin-11-en-4α-ol (7.56) MH: 22.29%, M: 24.02%, SH: 41.83%, OS: 24.20%, S: 66.03% | [24] |
| Leaves (0.25%) | α-pinene (7.7), β-pinene (6.9), β-caryophyllene (6.0), carotol (9.9), guaiol (31.0) T: 83.4% | [26] | |
| Flowers (0.30%) | α-pinene (6.0), guaiol (27.5), aristolene (24.5) T: 83.2% | [26] | |
| Fresh leaves (0.25%) | α-bisabolol (83.8) SH: 7.2%, OS: 86.5%, T: 94.3% | [20] | |
| M. aff. fosteri Croat | Leaves | β-bisabolol oxide (19.2), α-bisabolol (19.2), bisabolol oxide B (7.0), undeca-4,6-diene (5.4) SH: 8.2%, OS: 65.9%, T: 76.7% | [27] |
| M. glabra (O.Berg) D.Legrand | Fresh leaves (0.11%) | α-copaene (6.1), β-caryophyllene (9.5), β-selinene (5.8), α-selinene (9.4), valerianol (13.2) SH: 54.4%, OS: 27.4%, OTH: 9.4%, T: 92.0% | [20] |
| M. hatschbachii D.Legrand | Fresh leaves (0.1%) | germacrene D (6.4%), γ-cadinene (8.1), α-cadinol (6.1), β-caryophyllene (23.3) S: 95.2%, T: 97.9% | [23] |
| M. lageana D.Legrand | Fresh leaves (0.3%) | (E)-nerolidyl acetate (25.3), germacrene D (23.4) S: 98.5%, T: 99.3% | [23] |
| M. laruotteana Camb. | Unripe fruits (0.3%) | spathulenol (5.4), globulol (6.3), α-bisabolol oxide B (11.5), α-bisabolol (23.6), globulol (6.3), (2E,6E)-methyl farnesoate (5.8) SH: 5.8%, OS: 75.8%, T: 82.8% | [28] |
| Leaves (0.05%) | spathulenol (7.3), globulol (6.2), guaiol (6.1), 1-epi-cubenol (5.0), α-cadinol (8.0), α-bisabolol (20.7), 14-hydroxy-α-muurolene (19.9) SH: 10.2%, OS: 79.6%, T: 90.4% | [29] | |
| Flowers (0.07%) | spathulenol (8.6), globulol (6.6), guaiol (7.7), 1-epi-cubenol (5.0), α-cadinol (6.5), α-bisabolol (28.1), 14-hydroxy-α-muurolene (13.7) SH: 9.1%, OS: 83.1%, T: 95.5% | [29] | |
| M. multiflora (Lam) DC. | Leaves (1.16%) | α-gurjunene (6.40), β-caryophyllene (10.72), γ-selinene (5.12), α-selinene (8.67), selin-11-en-4α-ol (10.67) MH: 6.14%, OS: 5.23%, M: 11.37%, SH: 53.91%, OS: 17.57%, S: 71.48% | [24] |
| Fresh leaves (0.20%) | β-caryophyllene (7.5), germacrene D (8.7), bicyclogermacrene (6.3), δ-cadinene (5.2), MW 222 (7.4), cubenol (5.9) SH: 44.9%, OS: 32.3%, n.i.: 5.9%, T: 87.5% | [20] | |
| M. myrtillifolia DC. | Leaves (0.14%)b | α-pinene (80.4), α-terpineol (7.0) MH: 85.4%, OM: 13.0%, T: 99.7% | [30] |
| Flowers (0.26%) b | α-pinene (76.2) MH: 77.1%, OM: 9.9%, T: 88.8% | [30] | |
| Fruits (0.37%) b | α-pinene (88.1) MH: 91.9%, T: 96.8% | [30] | |
| M. obtecta (O.Berg) Kiaersk. | Fresh leaves (0.1%) | α-pinene (7.2), ar-curcumene (19.0), β-bisabolene (8.5), α-copaene (8.0), α-humulene (6.2) M: 16.2%, S: 79.1%, T: 95.3% | [23] |
| M. obtecta (O.Berg) Kiaersk. | Leaves (0.01%) b | α-terpineol (11.2), α-guainene (5.8), trans-calamenene (29.3), 1-epi-cubenol (5.6) M: 16.7%, SH: 56.4%, OS: 20.9%, T: 95.6% | [31] |
| Flowers (n.i.) | methyl salicilate (88.2) T: 97.9% | [31] | |
| M. oligantha O.Berg | Fresh leaves (0.1%) | δ-cadinene (17.9), 1-epi-cubenol (7.2), cubenol (5.7), β-caryophyllene (6.5), caryophyllene oxide (5.4), bicyclogermacrene (8.3), spathulenol (10.2) S: 96.8%, T: 99.9% | [23] |
| M. ovata Cambess. | Leaves (0.9%) | neral (35.8), geranial (50.4) T: 92.1% | [17,32] |
| Leaves (1.27%; w/w) | OM: 91.78%, T: 93.55% | [33] | |
| M. pubiflora DC. | Fresh leaves (1.1%) | tricyclene (5.27), 1,8-cineole (5.35), caryophyllene oxide (22.16), mustakone (11.34) T: 72.7% | [34] |
| M. pubipetala Miq. | Fresh leaves (0.1%) | germacrene D (7.2), β-caryophyllene (13.3), bicyclogermacrene (25.2), spathulenol (31.7), n-heneicosane (14.9) OTH: 14.9%, S: 84.8%, T: 99.7% | [23] |
| M. richardiana (O.Berg) Kiaersk. | Fresh leaves (0.1%) | β-caryophyllene (20.6), caryophyllene oxide (19.3), α-humulene (5.1), bicyclogermacrene (5.7) S: 90.0%, T: 90.0% | [23] |
| M. rostrata DC. | Fresh leaves (0.2%) | δ-cadinene (5.7), τ-muurolol (5.1), caryophyllene oxide (13.1), bicyclogermacrene (6.8), spathulenol (17.3) S: 93.3%, T: 93.3% | [23] |
| M. rufipila McVaugh | Leaves (0.42%) a | β-caryophyllene (7.07), γ-elemene (10.49), germacrene D (9.09), bicyclogermacrene (7.49), δ-cadinene (7.36), germacrene B (6.70) SH: 72.35%, OS: 19.93%, S: 92.28% | [24] |
| Leaves (0.18%) a | β-caryophyllene (5.66), germacrene D (10.31), δ-cadinene (10.12), α-cadinol (6.20) SH: 65.87%, OS: 23.69%, S: 89.56% | [24] | |
| M. salzmanni O.Berg | Leaves (n.i.) b | β-caryophyllene (25.9), α-humulene(12.9), MW 222 (11.7), MW 220 (14.2), MW 222 (10.0) SH: 49.2%, OS: 10.1%, NI: 36.2%, T: 95.5% | [35] |
| Flowers (n.i.) | β-caryophyllene (13.8), α-humulene (10.9), MW 222 (10.0), MW 220 (12.6), cis-β-elemone (6.2), MW 222 (7.1) SH: 36.4%, OS: 20.1%, NI: 38.6%, T: 95.4% | [35] | |
| M. selloii (Spreng.) N.Silveira | Fresh leaves (0.5%) | germacrene D (6.7), δ-cadinene (14.5), τ-cadinol (9.3), α-cadinol (17.2), β-caryophyllene (9.0), bicyclogermacrene (10.2) S: 99.2%, T: 99.9% | [23] |
| M. splendens (Sw.) DC. | Fresh leaves (0.44%) | (Z)-α-bisabolene (79.65) SH: 94.54%, S: 98.34%, T: 98.34% | [36] |
| Fresh stems (0.15%) | β-caryophyllene (23.8), germacrene D (25.3), bicyclogermacrene (7.1), caryophyllene oxide (10.5) T: 97.2% | [37] | |
| Leaves (n.i.) | trans-2-hexenal (9.5), germacrene D (35.9), δ-cadinene (5.8), epi-α-cadinol (6.8), valerianol (16.3) SH: 55.7%, OS: 31.8%, T: 96.9% | [38] | |
| M. sylvatica (G.Mey) DC. | Leaves and fine stems (>0.1%) a | spathulenol (13.8), caryophyllene oxide (16.6), selin-11-en-4α-ol (24.7) | [25] |
| Leaves and fine stems (0.3%) a | cis-calamenene (30.1), α-calacorene (11.5), spathulenol (18.7) | [25] | |
| Leaves and fine stems (>0.1%) a | β-bisabolene (14.7), spathulenol (40.2) | [25] | |
| M. tomentosa (Aubl.) DC. | Aerial parts (0.54%) b | (E)-β-farnesene (6.94), γ-muurolene (18.04), bicyclogermacrene (11.51), (2E,6E)-methyl farnesoate (36.95) SH: 47.22%, OS: 52.02%, T: 99.24% | [39] |
| Fresh flowers (0.31%) | spathulenol (7.36), globulol (5.97), (2Z,6Z)-farnesal (6.86), (2Z,6Z)-farnesol (10.65), (2E,6E)-farnesal (5.36), (2E,6E)-methyl farnesoate (14.28), benzyl salicylate (5.99) OS: 46.69%, OTH: 24.61%, T: 72.71% | [39] | |
| Stem bark (0.31%) | (2E,6E)-methyl farnesoate (14.39), hexadecanoic acid (22.05) SH: 6.26%, OS: 9.06%, OTH: 60.4%, T: 76.27% | [39] | |
| Leaves (0.1–0.8) b | spathulenol (18.35), globulol (7.66), (2E,6E)-methyl farnesoate (46.38) | [40] | |
| Leaves (0.1–0.8) b | γ-muurolene (14.20), bicyclogermacrene (14.38), δ-amorphene (18.83) | [40] | |
| Leaves (0.1–0.8) b | γ-muurolene (6.62), bicyclogermacrene (8.04), globulol (57.48) | [40] | |
| Leaves (0.1–0.8) b | γ-muurolene (7.67), bicyclogermacrene (5.85), (2E,6E)-methyl farnesoate (60.69) | [40] | |
| Leaves (0.1–0.8) b | β-caryophyllene (12.66), γ-muurolene (40.16), bicyclogermacrene (13.74), δ-amorphene (6.31) | [40] |
4. Biological and Antioxidant Activities of the Essential Oils of Myrcia Species and Their Major Constituents
4.1. Anti-Inflammatory and Antinociceptive Effect
4.2. Antimicrobial Activity
4.3. Larvicidal Activity
4.4. Antiproliferative Activity
4.5. Antioxidant Capacity
5. Activity of the Major Compounds from Myrcia Essential Oils
6. Non-Volatiles
6.1. Flavonoids
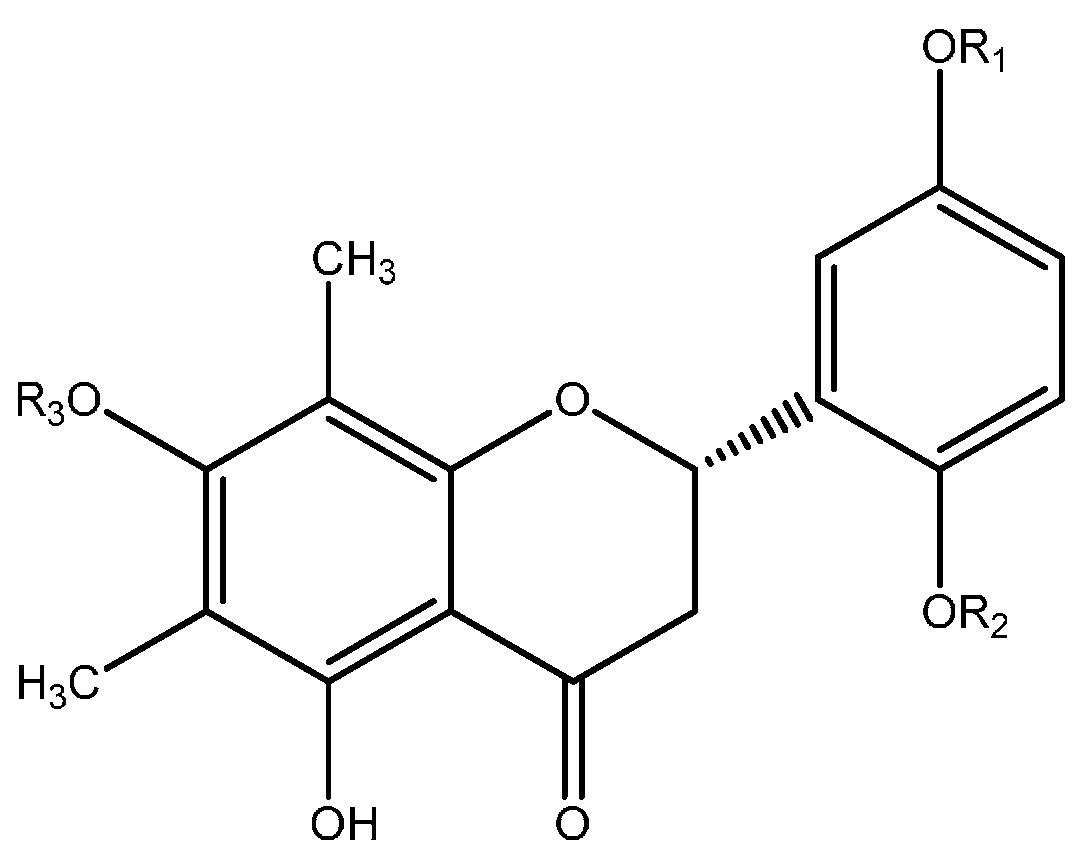
| Compound | R1 | R2 | R3 |
|---|---|---|---|
| 1 | H | H | O-β-d-glucopyranosyl |
| 2 | CH3 | H | O-β-d-glucopyranosyl |
| 3 | H | O-β-d-glucopyranosyl | H |
| 4 | H | H | (6ʹʹ-O-p-coumaroyl)-O-β-d-glucopyranosyl |
| 5 | H | H | (6ʹʹ-O-p-hydroxybenzoyl)-O-β-d-glucopyranosyl |

| Compound | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 6 | OH | H | OH | O-α-l-rhamnopyranosyl |
| 7 | OH | CH3 | OH | O-α-l-rhamnopyranosyl |
| 8 | OH | H | OH | O-α-l-rhamnopyranosyl |
| 9 | OH | H | OH | (2ʹʹ-O-galloyl)-O-α-l-rhamnopiranosyl |
| 10 | OH | H | H | O-α-l-arabinopyranosyl |
| 11 | OH | H | H | O-α-l-arabinofuranosyl |
| 12 | H | H | H | O-α-l-arabinofuranosyl |
| 13 | OH | H | OH | OH |
| 14 | H | H | H | O-Deoxyhexosyl |
| 15 | H | H | H | O-Hexosyl |
| 16 | OH | H | OH | O-β-d-galactopyranosyl |
| 17 | OH | H | OH | O-α-arabinofuranosyl |
| 18 | OH | H | OH | O-α-arabinopyranosyl |
| 19 | OH | H | OH | (O-galloyl)-O-hexosyl |
| 20 | H | H | OH | OH |
| 21 | H | H | OH | O-β-d-galactopyranosyl |
| 22 | H | H | OH | O-β-d-xylofuranosyl |
| 23 | H | H | OH | O-β-d-xylopyranosyl |
| 24 | H | H | OH | O-α-l-arabinofuranosyl |
| 25 | H | H | OH | (6ʹʹ-O-galloyl)-O-β-galactopyranosyl |
| 26 | H | H | OH | (O-galloyl)-pentosyl |
| 27 | OH | H | OH | (6ʹʹ-O-galloyl)-O-β-d-galactopyranosyl |
| 28 | OH | H | OH | O-β-d-xylopyranosyl |
| 29 | H | H | OH | O-α-l-arabinopyranosyl |
| 30 | H | H | OH | O-α-l-rhamnopyranosyl |
| 31 | H | H | H | O-β-d-galactopyranosyl |
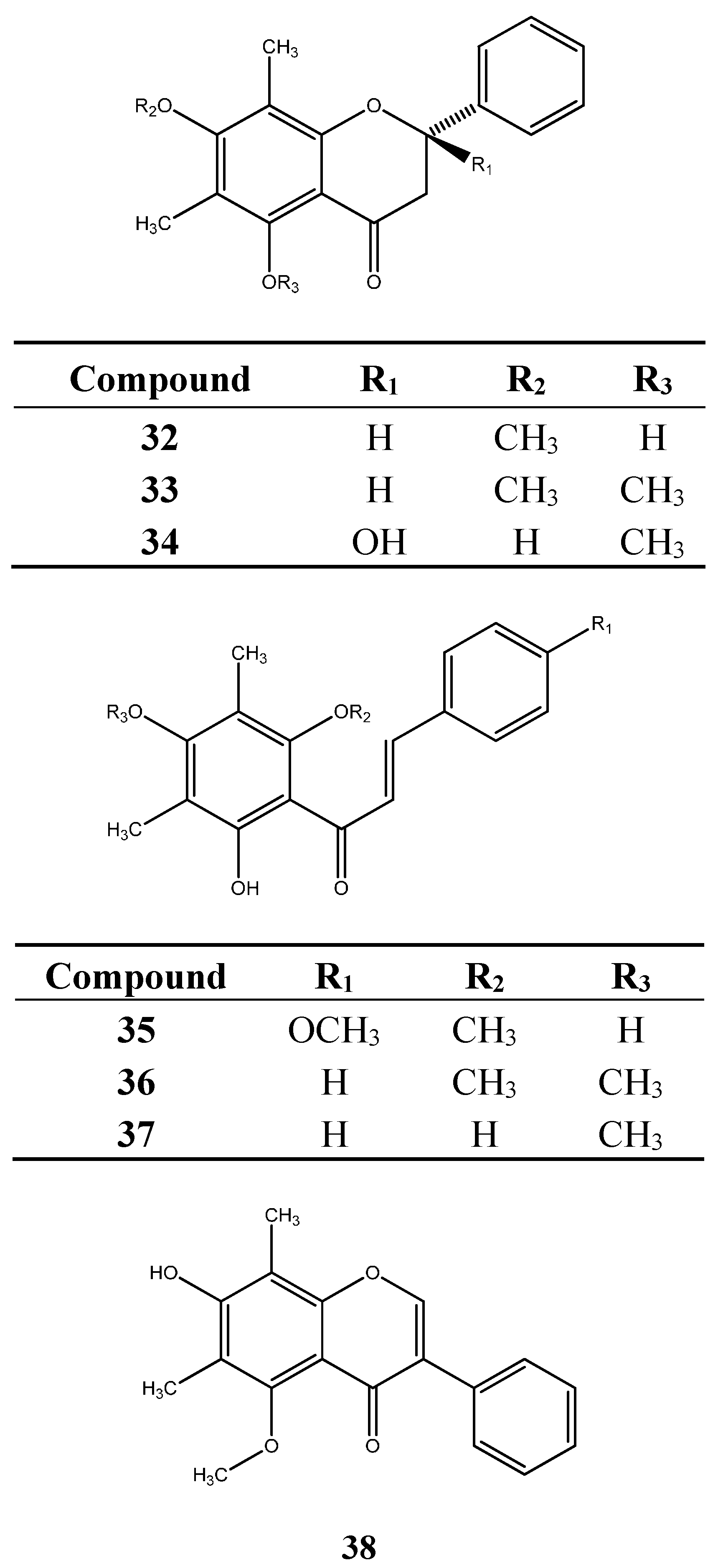
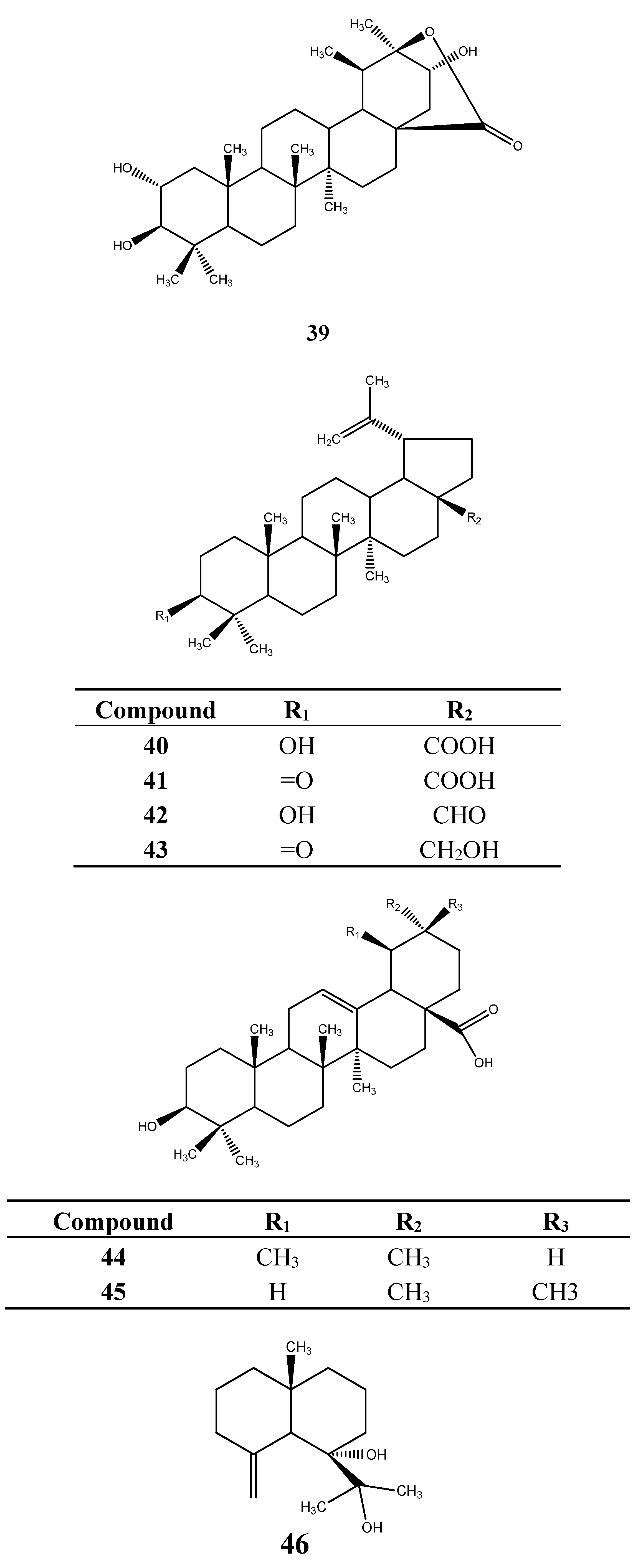
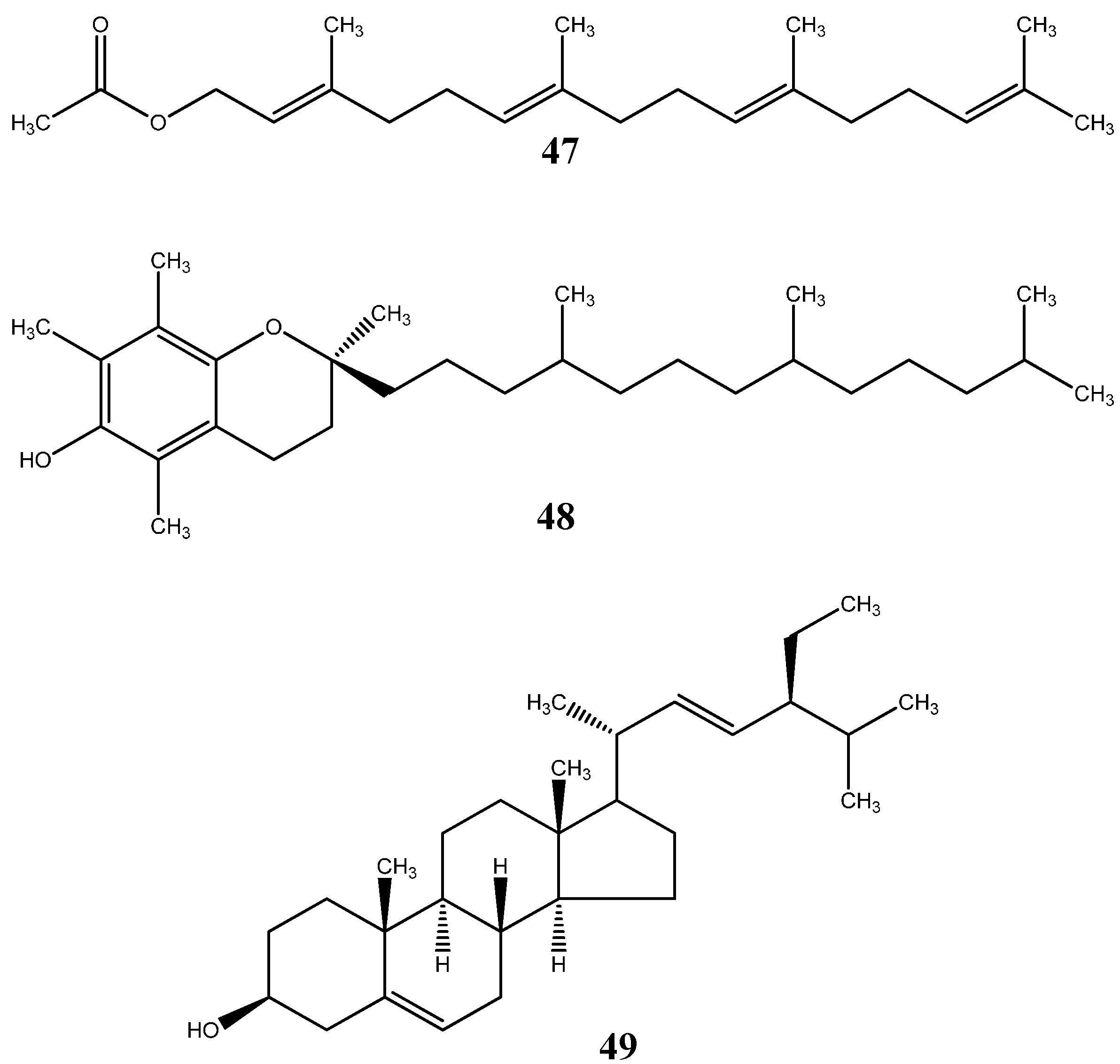
6.2. Terpenoids
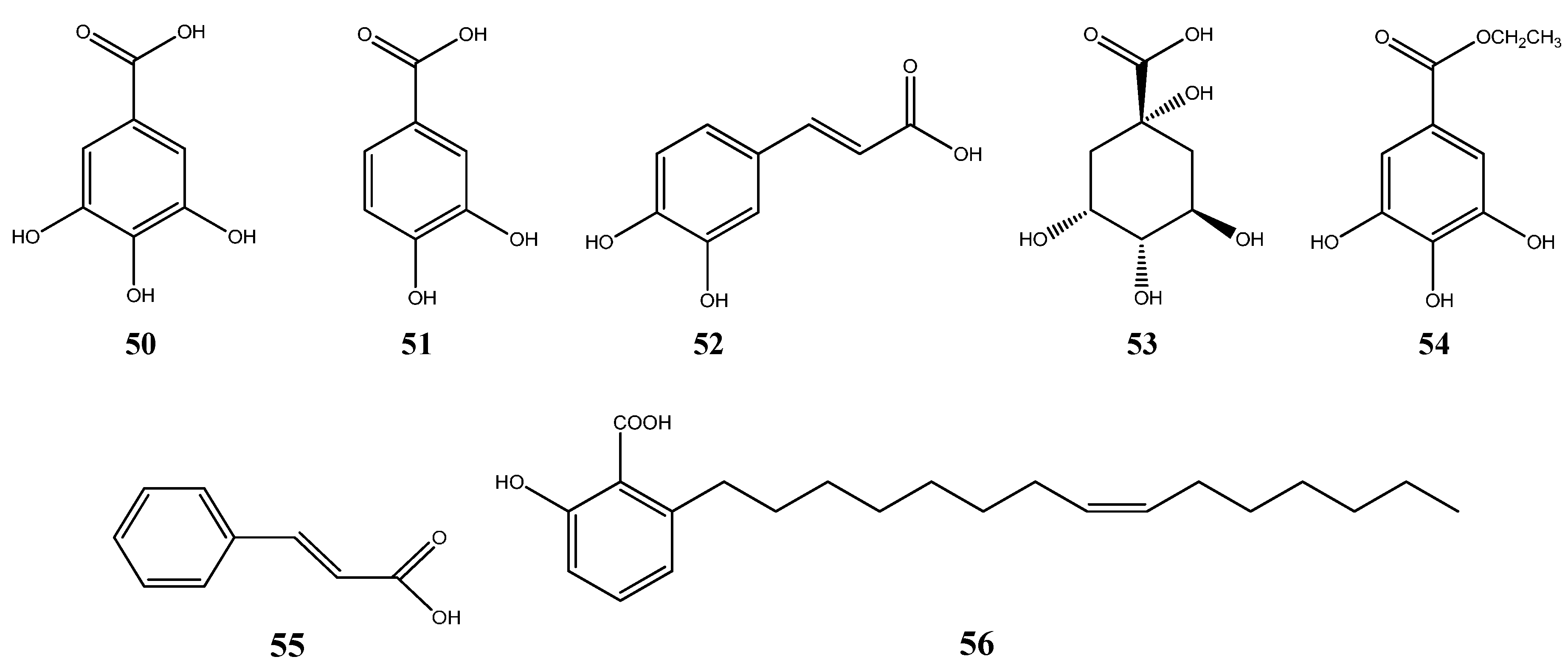
6.3. Organic Acids
6.4. Acetophenones and Related Compounds

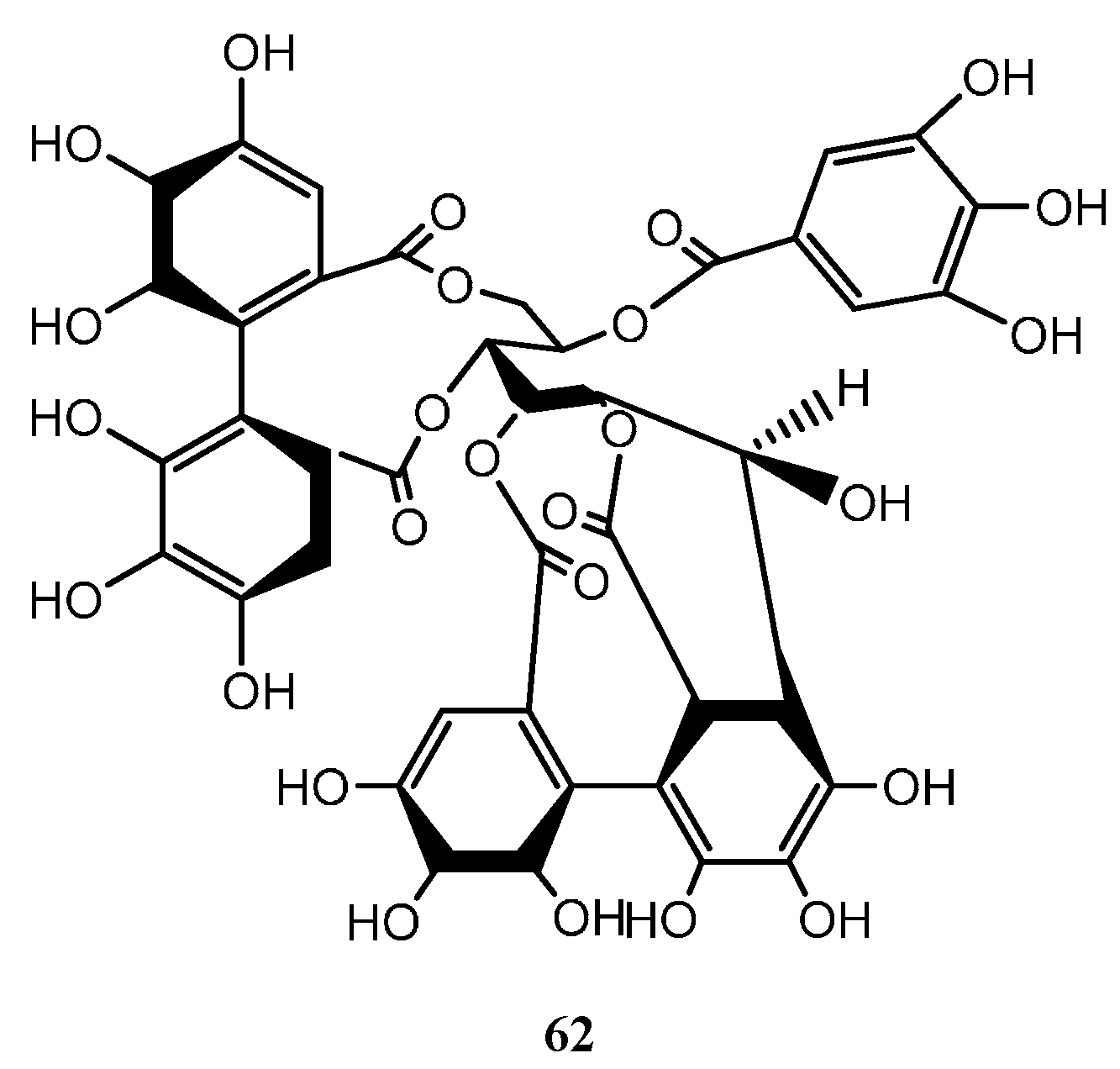
6.5. Tannins

| Compound | R1 | R2 | R3 |
|---|---|---|---|
| 63 | H | H | Acetyl |
| 64 | Acetyl | H | Acetyl |
| 65 | Acetyl | Acetyl | H |
| 66 | H | Acetyl | Acetyl |
| 67 | Acetyl | Acetyl | Acetyl |
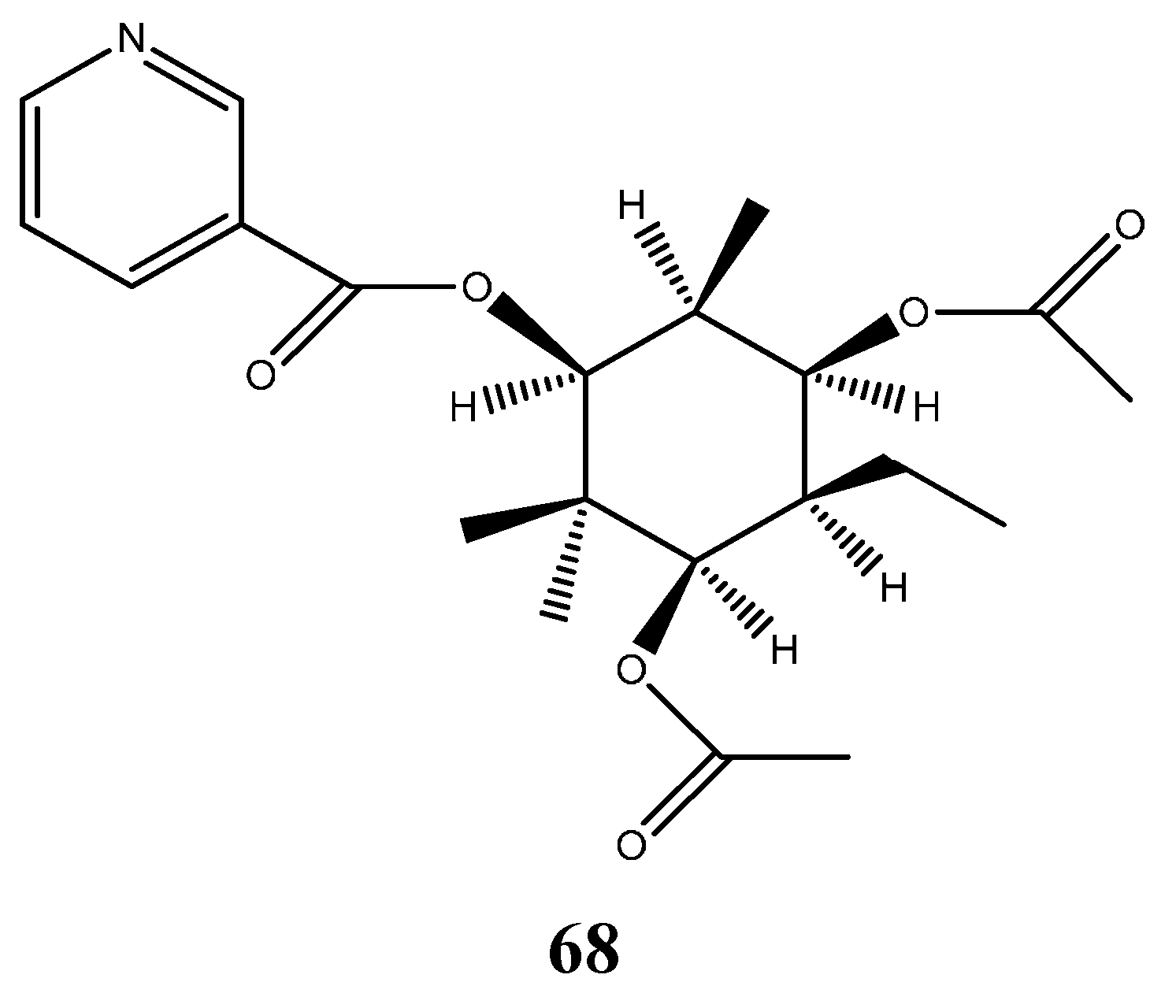
6.6. Alkaloid
7. Pharmacological Effects of Myrcia Extracts and Isolated Compounds
7.1. Hypoglycemic Potential
7.2. Antiobesity and Mixed Hypolipidemic Effects
7.3. Anti-Hemorrhagic Activity
7.4. Phytotoxic Effect and Allelophatic Potential
7.5. Hepatoprotective Effect
7.6. Antioxidant Effects
7.7. Others
8. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Govaerts, R.; Sobral, M.; Ashton, P.; Barrie, F.; Holst, B.K.; Landrum, L.L.; Matsumoto, K.; Mazine, F.F.; Nic Lughadha, E.; Proença, C.; et al. Word Checklist of Myrtaceae; Kew Publishing, Royal Botanic Gardens: Kew, UK, 2014. [Google Scholar]
- Sobral, M.; Proença, C.; Souza, M.; Mazine, F.; Lucas, E. Myrtaceae in Lista de Espécies da Flora do Brasil (List of species from Brazil’s flora). Jardim Botânico do Rio de Janeiro. Available online: http://reflora.jbrj.gov.br/jabot/floradobrasil/FB10660 (accessed on 7 July 2015).
- Landrum, L.R.; Kawasaki, M.L. The genera of Myrtaceae in Brazil: An illustrate synoptic treatment and identification keys. Brittonia 1997, 49, 508–536. [Google Scholar] [CrossRef]
- Souza, V.C.; Lorenzi, H. Botânica Sistemática: Guia Ilustrado para Identificação das Famílias de Fanerógamas Nativas e Exóticas no Brasil, Baseado no APG II (Systematic Botany: Illustrated Guide for the Identification of Native and Exotic Phanerogams in Brazil, Based on APG II), 2nd ed.; Instituto Plantarum: Nova Odessa, Brazil, 2008; pp. 297–302. [Google Scholar]
- Cruz, A.V.M.; Kaplan, M.A.C. Uso medicinal de espécies das famílias Myrtaceae e Melastomataceae no Brasil (Medicinal use of Myrtaceae and Melastomataceae species in Brazil). Floresta Ambient. 2004, 11, 47–52. [Google Scholar]
- Lucas, E.J.; Matsumoto, K.; Harris, S.A.; Nic Lughadha, E.M.; Benardini, B.; Chase, M.W. Phylogenetics, morphology, and evolution of the large genus Myrcia s.l. (Myrtaceae). Int. J. Plant Sci. 2011, 172, 915–934. [Google Scholar] [CrossRef]
- Staggemeier, V.G.; Diniz-Filho, J.A.F.; Forest, F.; Lucas, E. Phylogenetic analysis in Myrcia section Aulomyrcia and inferences on plant diversity in the Atlantic rainforest. Ann. Bot. 2015. [Google Scholar] [CrossRef] [PubMed]
- Staggemeier, V.G.; Diniz-Filho, J.A.F.; Zipparro, V.B.; Gressler, E.; Castro, E.R.; Mazine, F.; Costa, I.R.; Lucas, E.; Morellato, L.P. Clade-specific responses regulate phenological patterns in Neotropical Myrtaceae. Perspect. Plant Ecol. Evol. Syst. 2015. [Google Scholar] [CrossRef]
- Rosario, A.S.; Baumgratz, J.F.A.; Secco, R.S. Contribuição à taxonomia de Marlierea (Mirciinae; Myrtaceae) no Brasil (Contribution to the taxonomy of Marlierea (Mirciinae; Myrthaceae) in Brazil). Rodriguesia 2014, 1, 245–250. [Google Scholar] [CrossRef]
- Le Cointe, P. A Amazônia Brasileira III: Árvores e Plantas Úteis (Indígenas e Aclimatadas) (Brazilian Amazon III: Useful Trees and Plants (Natives and Acclimatized)); Livraria Clássica: Belém, Brazil, 1934; p. 366. [Google Scholar]
- Coimbra, R. Manual de Fitoterapia (Manual of Phytotherapy), 2nd ed.; Editora Cejup: Belém, Brazil, 1994; p. 219. [Google Scholar]
- Cruz, G.L. Dicionários de Plantas Úteis do Brasil (Dictionary of Useful Plants from Brazil), 5th ed.; Bertrand Brasil: Rio de Janeiro, Brazil, 1995; p. 498. [Google Scholar]
- Van den Berg, M.E. Plantas Medicinais na Amazônia: Contribuição ao Seu Conhecimento Sistemático (Medicinal Plants in the Amazon: Contribution to Its Systematic Knowledge), 3rd ed.; Museu Paraense Emílio Goeldi, Coleção Adolpho Ducke: Belém, Brazil, 2010; p. 220. [Google Scholar]
- Silva, F.K.S.; Rosário, A.S.; Secco, R.S.; Zoghbi, M.G.B. Levantamento das espécies conhecidas como pedra-ume-caá (Myrtaceae), com ênfase nas comercializadas na cidade de Belém, Pará, Brasil (Survey of the species known as pedra-ume-caá (Myrtaceae), with emphasis of those commercialized in the city of Belém, Pará, Brazil). Biota Amazôn. 2015, 5, 7–15. [Google Scholar]
- Jorge, L.I.F.; Aguiar, J.P.L.; Silva, M.L.P. Anatomia foliar de pedra-ume-caá (Myrcia sphaerocarpa, Myrcia guianensis, Eugenia punicifolia—Myrtaceae) (Leaf anatomy of pedra-ume-caá (Myrcia sphaerocarpa, Myrcia guianensis, Eugenia punicifolia—Myrtaceae)]. Acta Amazon. 2000, 30, 49–57. [Google Scholar] [CrossRef]
- Santos, E.B.; Dantas, G.S.; Santos, H.B.; Diniz, M.F.F.M.; Sampaio, F.C. Estudo etnobotânico de plantas medicinais para problemas bucais no município de João Pessoa, Brasil (Ethnobotanical study of medicinal plants for mouth diseases in the municipality of João Pessoa, Brazil). Braz. J. Pharmacogn. 2009, 19, 321–324. [Google Scholar] [CrossRef]
- Cândido, C.S.; Portella, C.S.A.; Laranjeira, B.J.; Silva, S.S.; Arriaga, A.M.C.; Santiago, G.M.P.; Gomes, G.A.; Almeida, P.C.; Carvalho, C.B.M. Effects of Myrcia ovata Cambess. essential oil on planktonic growth of gastrointestinal microorganisms and biofilm formation of Enterococcus faecalis. Braz. J. Microbiol. 2010, 41, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.A.F.; Moura, V.M.; Raposo, J.D.A.; Sousa, L.F.; Oliveira, R.B.; Santos, L.S.; Araújo, R.N.M.; Silva, A.M.M.; Aranha, E.P.; Suemitsu, C.; et al. The effect of the aqueous extract of Myrcia guianensis (Aubl) DC and its fractions against the hemorrhagic activity of Bothrops jararaca venom. J. Med. Plants Res. 2013, 7, 3139–3146. [Google Scholar]
- Stefanello, M.E.A.; Pascoal, A.C.R.F.; Salvador, M.J. Essential oils from Neotropical Myrtaceae: Chemical diversity and biological properties. Chem. Biodivers. 2011, 8, 73–94. [Google Scholar] [CrossRef] [PubMed]
- Henriques, A.T.; Sobral, M.; Bridi, R.; Vérin, P.; Menut, C.; Lamarty, G.; Bessière, J.M. Essential oils from five Southern Brazilian species of Myrcia (Myrtaceae). J. Essent. Oil Res. 1997, 9, 13–18. [Google Scholar] [CrossRef]
- Silva, A.N.; Uetanabaro, A.P.T.; Lucchese, A.M. Chemical composition and antibacterial activity of essential oils from Myrcia alagoensis (Myrtaceae). Nat. Prod. Commun. 2013, 8, 269–271. [Google Scholar]
- Calao, V.Y.P. Caracterização físico-química, composição e capacidade antioxidante do óleo essencial de Myrcia amazonica DC. (Myrtaceae) (Physical-chemical characterization, composition and antioxidant capacity of the essential oil of Myrcia amazonica DC. (Myrthaceae)). Master’s Thesis, Universidade Federal do Oeste do Pará, Santarém, Brazil, April 2014. [Google Scholar]
- Limberger, R.P.; Sobral, M.; Henriques, A.T.; Menut, C.; Bressière, J.M. Óleos voláteis de espécies de Myrcia nativas do Rio Grande do Sul (Volatile oils of Myrcia species native of Rio Grande do Sul). Quím. Nova 2004, 27, 916–919. [Google Scholar] [CrossRef]
- Pereira, R.A.; Zoghbi, M.G.B.; Bastos, M.N.C. Essential oils of twelve species of Myrtaceae growing wild in the sandbank of the Resex Maracanã, State of Pará, Brazil. J. Essent. Oil Bear. Plants 2010, 13, 440–450. [Google Scholar] [CrossRef]
- Zoghbi, M.G.B.; Andrade, E.H.A.; Silva, M.H.L.; Carreira, L.M.M.; Maia, J.G.S. Essential oils from three Myrcia species. Flavour Fragr. J. 2003, 8, 421–424. [Google Scholar] [CrossRef]
- Alarcón, L.D.; Peña, A.E.; Gonzales, C.N.; Quintero, A.; Meza, M.; Usubillaga, A.; Velasco, J. Composition and antibacterial activity of the essential oil of Myrcia falax (Rich.) DC. from Venezuela. Rev. Soc. Quím. Perú 2009, 75, 221–227. [Google Scholar]
- Tenorio, A.I.S.; Vargas, D.; Espinosa, A.; Diaz, A.; Gupta, M.P. Chemical composition of leaf essential oils of Calyptranthes microphylla B. Holts & M.L, Myrcia aff fosteri Croat and Eugenia octopleura Krug & Urb from Panama. J. Essent. Oil Res. 2011, 23, 29–33. [Google Scholar]
- Stefanello, M.E.A.; Riva, D.; Simionatto, E.L.; Carvalho, J.E.; Ruiz, A.L.T.G.; Salvador, M.J. Chemical composition and cytotoxic activity of essential oil from Myrcia laruotteana fruits. J. Essent. Oil Res. 2011, 23, 7–10. [Google Scholar] [CrossRef]
- Stefanello, M.E.A.; Cervi, A.C.; Wisniewski, A., Jr.; Simionatto, E.L. Essential oil composition of Myrcia laruotteana Camb. J. Essent. Oil Res. 2007, 19, 466–467. [Google Scholar] [CrossRef]
- Cerqueira, M.D.; Souza-Neta, L.C.; Passos, M.G.V.M.; Lima, E.O.; Roque, N.F.; Martins, D.; Guedes, M.L.S.; Cruz, F.G. Seasonal variation and antimicrobial activity of Myrcia myrtifolia essential oils. J. Braz. Chem. Soc. 2007, 18, 998–1003. [Google Scholar] [CrossRef]
- Stefanello, M.E.A.; Cervi, A.C.; Wisniewski, A., Jr.; Simionattto, E.L. Composição e variação sazonal do óleo essencial de Myrcia obtecta (O.Berg) Kiaersk. var. obtecta, Myrtaceae (Composition and seasonal variation of the essential oil of Myrcia obtecta (O.Berg) Kiaersk. var. obtecta, Myrtaceae). Braz. J. Pharmacogn. 2010, 20, 82–86. [Google Scholar]
- Lima, M.A.A.; Oliveira, F.F.M.; Gomes, G.A.; Lavor, P.L.; Santiago, G.M.P.; Nagao-Dias, A.T.; Arriaga, A.M.C.; Lemos, T.L.G.; Carvalho, M.G. Evaluation of larvicidal activity of the essential oils of plants from Brazil against Aedes aegypti (Diptera: Culicidae). Afr. J. Biotechnol. 2011, 10, 11716–11720. [Google Scholar]
- Santos, G.C.M.; Gomes, G.A.; Gonçalves, G.M.; Sousa, L.M.; Santiago, G.M.P.; Carvalho, M.G.; Marinho, B.G. Essential oil from Myrcia ovata: Chemical composition, antinociceptive and anti-inflammatory properties in mice. Planta Med. 2014, 80, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.S.; Guimarães, A.G.; Santana, M.T.; Siqueira, R.S.; Passos, L.O.; Machado, S.M.F.; Ribeiro, A.S.; Sobral, M.; Almeida, J.R.G.S.; Quintans-Júnior, L.J. Phytochemical screening, antinociceptive and anti-inflammatory effects of the essential oil of Myrcia pubiflora in mice. Braz. J. Pharmacogn. 2012, 22, 181–188. [Google Scholar] [CrossRef]
- Cerqueira, M.D.; Marques, E.J.; Martins, D.; Roque, N.F.; Cruz, F.G. Variação sazonal da composição do óleo essencial de Myrcia salzmannii Berg (Myrtaceae) (Seasonal variation of the chemical composition of the esssential oil of Myrcia salzmannii Berg (Myrtaceae)). Quím. Nova 2009, 32, 1544–1548. [Google Scholar] [CrossRef]
- Nakamura, M.J.; Monteiro, S.S.; Bizarri, C.H.B.; Siani, A.C.; Ramos, M.F.S. Essential oils of four Myrtaceae species from the Brazilian southeast. Biochem. Sys. Ecol. 2010, 38, 1170–1175. [Google Scholar] [CrossRef]
- Jiménez, D.; Araque, M.; Rojas, L.; Cordero, A.; Briceño, B. Componentes volátiles y actividad antibacteriana del vástago de Myrcia splendens (Sw.) DC. (Volatiles and antibacterial activity of the stems of Myrcia splendens (Sw.) DC.). Rev. Fac. Farm. 2012, 54, 7–11. [Google Scholar]
- Cole, R.A.; Haber, W.A.; Setzer, W.N. The leaf oil composition of Myrcia splendens from Monteverde, Costa Rica. J. Essent. Oil Bear. Plants 2008, 11, 41–44. [Google Scholar] [CrossRef]
- Sá, F.A.S.; Borges, L.L.; Paula, J.A.M.; Sampaio, B.L.; Ferri, P.H.; Paula, J.R. Essential oils in aerial parts of Myrcia tomentosa: Composition and variability. Braz. J. Pharmacogn. 2012, 22, 1233–1240. [Google Scholar] [CrossRef]
- Borges, L.L.; Alves, S.F.; Bara, M.T.F.; Conceição, E.C.; Herri, P.H.; Paula, J.R. Influence of environmental factors on the composition of essential oils from leaves of Myrcia tomentosa (Aubl.) DC. Bol. Latinoam. Caribe Plant. Med. Aromat. 2013, 12, 572–580. [Google Scholar]
- Siani, A.C.; Azevedo, M.B.M.; Ramos, M.F.S.; Trigo, J.R. Monoterpenes and sesquiterpenes of Neotropical Myrtaceae. In Current Trends in Phytochemistry; Epifano, F., Ed.; Research Signpost: Kerala, India, 2008; pp. 223–251. [Google Scholar]
- Quintans-Júnior, L.J.; Guimarães, A.G.; Santana, M.T.; Araújo, B.E.S.; Moreira, F.V.; Bonjardim, L.R.; Araújo, A.A.S.; Siqueira, J.S.; Antoniolli, A.R.; Botelho, M.A.; et al. Citral reduces nociceptive and inflammatory response in rodents. Rev. Bras. Plantas Med. 2011, 21, 497–502. [Google Scholar] [CrossRef]
- Chavan, M.J.; Wakte, P.S.; Shinde, D.B. Analgesic and anti-inflammatory activity of caryophyllene oxide from Annona squamosal L. bark. Phytomedicine 2010, 17, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Liapi, C.; Anifantis, G.; Chinou, I.; Kourounakis, A.P.; Thedosopoulus, S.; Galanopoulou, P. Antinociceptive properties of 1,8-cineole and β-pinene, from the essential oil of Eucalyptus camaldulensis leaves, in rodents. Planta Med. 2007, 73, 1274–1254. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Sanchez-Moreiras, A.M.; Abel, C.; Sohrabi, R.; Lee, S.; Gershenzon, J.; Tholl, D. The major volatile organic compound emitted from Arabidopsis thaliana, the sesquiterpene β-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 2012, 193, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.; Buchbauer, G. A review on recent research results (2008–2010) on essential oils as antimicrobials and antifungals. A review. Flavour Fragr. J. 2012, 27, 13–39. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Shimada, H.; Nishida, N.; Li, Y.; Togushida, I.; Yamahara, J.; Matsuda, H. Antidiabetic principles of natural medicines. II. Aldose reductase and α-glucosidase inhibitors from Brazilian natural medicine, the leaves of Myrcia multiflora DC. (Myrtaceae): Structures of Myrciacitrins I and II and Myrciaphenones A and B. Chem. Pharm. Bull. 1998, 46, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Nishida, N.; Yoshikawa, M. Antidiabetic principles of natural medicines. V. Aldose reductase inhibitors from Myrcia multiflora DC. (2): Structures of Myrciacitrins III, VI and V. Chem. Pharm. Bull. 2002, 50, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, L.L.; Vilega, W.; Dokkedal, A.L. Characterization of flavonoids and phenolic acids in Myrcia bella Cambess. Using FIA-ESI-IT-MSn and HPLC-PAD-ESI-IT-MS combined with NMR. Molecules 2013, 18, 8402–8416. [Google Scholar] [CrossRef] [PubMed]
- Moresco, H.H.; Pereira, M.; Bretanha, L.C.; Micke, G.A.; Pizzolatti, M.G.; Brighente, I.M.C. Myricitrin as the main constituent of two species of Myrcia. J. Appl. Pharm. Sci. 2014, 4, 1–7. [Google Scholar]
- Batista, A.N.L.; Colombo, R.; Pascoli, I.C.; Teles, H.L.; Silva, G.H.; Bomfim, G.C.C.; Burgos, R.C.R.; Cavalheiro, A.J.; Bolzani, V.S.; Silva, D.H.S.; et al. Development and validation of a HPLC method for standardization of herbal and commercial extracts of Myrcia uniflora. Braz. J. Pharmacogn. 2011, 21, 402–406. [Google Scholar] [CrossRef]
- Ferreira, A.C.F.; Neto, J.C.; Silva, A.C.M.; Kuster, R.M.; Carvalho, D.P. Inhibition of thyroid peroxidase by Myrcia uniflora flavonoids. Chem. Res. Toxicol. 2006, 19, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Imatomi, M.; Novaes, P.; Matos, A.P.; Gualtieri, S.C.J.; Molinillo, J.M.G.; Lacret, R.; Varela, R.M.; Macías, F.A. Phytotoxic effect of bioactive compounds isolated from Myrcia tomentosa (Myrtaceae) leaves. Biochem. Sys. Ecol. 2013, 46, 29–35. [Google Scholar] [CrossRef]
- Wubshet, S.G.; Moresco, H.H.; Tahtah, Y.; Brighente, I.M.C.; Staerk, D. High-resolution bioactivity profiling combined with HPLC-HRMS-SPE-NMR: α-Glucosidase inhibitors and acetylated ellagic acid rhamnosides from Myrcia palustris DC. (Myrtaceae). Phytochemistry 2015. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.D. Compostos fenólicos e terpenos de Myrcia hiemalis e Myrcia myrtifolia (Myrtaceae) (Phenolics and terpenes from Myrcia hiemalis and Myrcia myrtifolia (Myrtaceae)). Ph.D. Thesis, Universidade Federal da Bahia, Instituto de Química, Salvador, Brazil, 2012. [Google Scholar]
- Souza Filho, A.P.S.; Santos, R.A.; Santos, L.S.; Guilhon, G.M.P.; Santos, A.S.; Arruda, M.S.P.; Muller, A.H.; Arruda, A.C. Allelophatic potential of Myrcia guianensis. Planta Daninha 2006, 24, 649–656. [Google Scholar] [CrossRef]
- Ferreira, E.A.; Gris, E.F.; Rebello, J.M.; Correia, J.S.G.; Oliveira, L.F.S.; Wilhelm Filho, D.; Pedrosa, R.C. The 2ʹ,4ʹ,6ʹ-trihydroxyacetophenone isolated from Myrcia multiflora has antiobesity and mixed hypolipidemic effects with the reduction of lipid intestinal absorption. Planta Med. 2011, 77, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.D.; Souza-Neta, L.C.; Guedes, M.L.S.; Rivelino, R.; Cruz, F.G. Myrciaine, a new nicotinic ester from Myrcia blanchetiana (Myrtaceae). Tetrahedron Lett. 2013, 54, 1421–1423. [Google Scholar] [CrossRef]
- Vareda, P.M.P.; Saldanha, L.L.; Camaforte, N.A.P.; Violato, N.M.; Dokkedal, A.L.; Bosqueiro, J.R. Myrcia bella leaf extract presents hypoglycemic activity via PI3k/Akt insulin signaling pathway. Evid. Based Complement. Alternat. Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.A.; Gris, E.F.; Felipe, K.B.; Correia, J.S.G.; Cargnin-Ferreira, E.; Wilhelm Filho, D.; Pedrosa, R.C. Potent hepatoprotective effect in CCl4-induced hepatic injury in mice of phloroacetophenone from Myrcia multiflora. Lybian J. Med. 2010. [Google Scholar] [CrossRef]
- Salvador, M.J.; Lourenço, C.C.; Andreazza, N.L.; Pascoal, A.C.R.F.; Stefanello, M.E.A. Antioxidant capacity and phenolic content of four Myrtaceae plants of the South of Brazil. Nat. Prod. Commun. 2011, 6, 977–982. [Google Scholar] [PubMed]
- Alcântara, G.A.; Borges, L.L.; Paula, J.R. Seasonal variation in the content of phenolic compounds in barks of Myrcia rostrata DC. by influence of environmental factors. J. Pharm. Res. 2012, 5, 1306–1309. [Google Scholar]
- Borges, L.L.; Alves, S.F.; Sampaio, B.L.; Conceição, E.C.; Bara, M.T.F.B.; Paula, J.R. Environmental factors affecting the concentration of phenolic compounds in Myrcia tomentosa leaves. Braz. J. Pharmacogn. 2013, 23, 230–238. [Google Scholar] [CrossRef]
- Hecht, S.M. Biologically Active Extracts from Myrcia fallax (Myrtaceae) Peru and Method of Obtaining the Same. U.S. Patent 4,451,459, 23 July 1982. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascaes, M.M.; Guilhon, G.M.S.P.; Andrade, E.H.d.A.; Zoghbi, M.D.G.B.; Santos, L.D.S. Constituents and Pharmacological Activities of Myrcia (Myrtaceae): A Review of an Aromatic and Medicinal Group of Plants. Int. J. Mol. Sci. 2015, 16, 23881-23904. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161023881
Cascaes MM, Guilhon GMSP, Andrade EHdA, Zoghbi MDGB, Santos LDS. Constituents and Pharmacological Activities of Myrcia (Myrtaceae): A Review of an Aromatic and Medicinal Group of Plants. International Journal of Molecular Sciences. 2015; 16(10):23881-23904. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161023881
Chicago/Turabian StyleCascaes, Márcia Moraes, Giselle Maria Skelding Pinheiro Guilhon, Eloisa Helena de Aguiar Andrade, Maria Das Graças Bichara Zoghbi, and Lourivaldo Da Silva Santos. 2015. "Constituents and Pharmacological Activities of Myrcia (Myrtaceae): A Review of an Aromatic and Medicinal Group of Plants" International Journal of Molecular Sciences 16, no. 10: 23881-23904. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161023881