Photodynamic Therapy (PDT) with Chemotherapy for Advanced Lung Cancer with Airway Stenosis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Patient and Tumor Characteristics
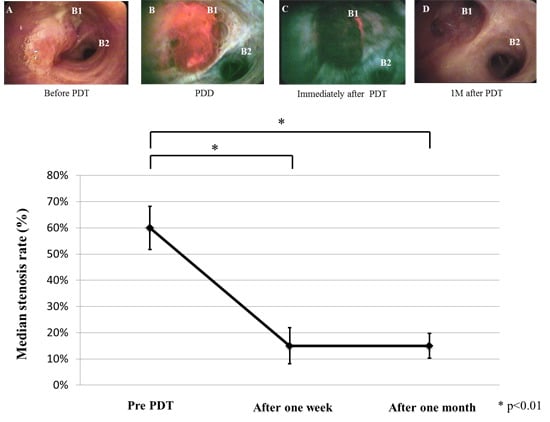
2.2. Treatment Results




| Case | Age | Patho | c-Stage | Chemotherapy | Location | Probe | before PDT S.R. | 1W after PDT S.R. | 1M after PDT S.R. | Survival (Day) | Outcome | CTCAE v4.0 (Grade) | KS (before) | KS (after) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 77 | Sq | IIA | CBDCA + TS-1 | LU (B1 + 2) | F and R | 45% | 15% | 15% | 224 | Death | 1 | 90 | 100 |
| 2 | 74 | LCNEC | IIIB | NDP + DOC | R Int | F and R | 100% | 60% | 15% | 160 | Death | 1 | 20 | 100 |
| 3 | 74 | Sq | IIB | CBDCA + TS-1 CBDCA + VNR | R Int | F and R | 60% | 15% | 30% | 638 | Death | 3 | 90 | 90 |
| 4 | 69 | Ad | IV | TS-1 | RM | F and R | 30% | 15% | 30% | 61 | Death | 1 | 20 | 90 |
| 5 | 75 | Sq | IV | CDDP + DOC | RM | F and R | 30% | 15% | 45% | 93 | Death | 3 | 80 | 90 |
| 6 | 74 | Giant | IV | CDDP + DOC | RL | F and R | 100% | 99% | 60% | 425 | Death | 1 | 70 | 100 |
| 7 | 72 | Sq | IV | CDDP + DOC | Carina | F | R 30% L 60% | R 15% L 15% | R 15% L 15% | 196 | Death | 3 | 90 | 100 |
| 8 | 58 | Sq | IIIB | CBDCA + TS-1 | RU (B2,3) | F and R | 45% | 30% | 15% | 614 | Alive | 1 | 90 | 100 |
| 9 | 77 | Sq | IIIA | NDP + DOC | RU | F | 99% | 15% | 15% | 38 | Death | 4 | 90 | 100 |
| 10 | 78 | ad | IIIA | CBDCA + PEM + Bev CBDCA + PTX + Bev | RU(B3) | F and R | 60% | 30% | 60% | 159 | Death | 1 | 100 | 100 |
| 11 | 80 | ad | IV | CBDCA + PEM + Bev | RU | F and R | 99% | 15% | 30% | 89 | Alive | 1 | 80 | 90 |
| 12 | 70 | sq | IIIB | CBDCA + nab-PTX CBDCA + TS-1 | R Int | F and R | 100% | 30% | 15% | 195 | Alive | 1 | 80 | 100 |
2.3. Tumor Responses on Computed Tomography

2.4. Discussion
3. Experimental Section
3.1. Patients
3.2. Photosensitizer and Laser Unit
3.3. Treatment Protocol
3.4. Evaluation of Effectiveness
3.5. Statistical Analyses
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Chin, C.S.; Litle, V.; Yun, J.; Weiser, T.; Swanson, S.J. Airway stents. Ann. Thorac. Surg. 2008, 85, S792–S796. [Google Scholar] [PubMed]
- Venuta, F.; Rendina, E.A.; de Giacomo, T.; Mercadante, E.; Francioni, F.; Pugliese, F.; Moretti, M.; Coloni, G.F. Nd:YAG laser resection of lung cancer invading the airway as a bridge to surgery and palliative treatment. Ann. Thorac. Surg. 2002, 74, 995–998. [Google Scholar] [CrossRef]
- Stephens, K.E.; Wood, D.E. Bronchoscopic management of central airway obstruction. J. Thorac. Cardiovasc. Surg. 2000, 119, 289–296. [Google Scholar] [CrossRef]
- Kennedy, T.C.; McWilliams, A.; Edell, E.; Sutedja, T.; Downie, G.; Yung, R.; Gazdar, A.; Mathur, P.N. Bronchial intraepithelial neoplasia/early central airways lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007, 132, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Usuda, J.; Ichinose, S.; Ishizumi, T.; Hayashi, H.; Ohtani, K.; Maehara, S.; Ono, S.; Honda, H.; Kajiwara, N.; Uchida, O.; et al. Outcome of photodynamic therapy using NPe6 for bronchogenic carcinomas in central airways >1.0 cm in diameter. Clin. Cancer Res. 2010, 16, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Usuda, J.; Okunaka, T.; Furukawa, K.; Honda, H.; Sakaniwa, N.; Suga, Y.; Hirata, T.; Ohtani, K.; Inoue, T.; et al. Basic and clinical research on photodynamic therapy at Tokyo Medical University Hospital. Lasers Surg. Med. 2006, 38, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Okunaka, T.; Yamamoto, H.; Tsuchida, T.; Usuda, J.; Kumasaka, H.; Ishida, J.; Konaka, C.; Kato, H. Effectiveness of photodynamic therapy and Nd-YAG laser treatment for obstructed tracheobronchial malignancies. Diagn. Ther. Endosc. 1999, 5, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Barr, H.; Tralau, C.J.; Boulos, P.B.; MacRobert, A.J.; Tilly, R.; Bown, S.G. The contrasting mechanisms of colonic collagen damage between photodynamic therapy and thermal injury. Photochem. Photobiol. 1987, 46, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Kinosita, K.; Saijo, T.; Hirata, T.; Saji, H.; Kato, H. Laser therapy and airway stenting for central-type lung cancer. Jpn. Med. Assoc. J. 2003, 46, 547–553. [Google Scholar]
- Nagata, Y.; Hiraoka, M.; Mizowaki, T.; Narita, Y.; Matsuo, Y.; Norihisa, Y.; Onishi, H.; Shirato, H. Survey of stereotactic body radiation therapy in Japan by the Japan 3-D Conformal External Beam Radiotherapy Group. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Jimenez, J.P.; Martinez-Ballarin, J.E.; Llunell, A.; Farrero, E.; Rodríguez, A.; Castro, M.J. Efficacy and safety of photodynamic therapy versus Nd-YAG laser resection in NSCLC with airway obstruction. Eur. Respir. J. 1999, 14, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.J.; Cheon, Y.K.; Lee, E.J.; Lee, T.Y.; Shim, C.S. Long-term outcome of photodynamic therapy with systemic chemotherapy compared to photodynamic therapy alone in patients with advanced hilar cholangiocarcinoma. Gut Liver 2014, 8, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, Y.; Nanashima, A.; Nonaka, T.; Uehara, M.; Isomoto, H.; Abo, T.; Nagayasu, T. Synergic effect of photodynamic therapy using talaporfin sodium with conventional anticancer chemotherapy for the treatment of bile duct carcinoma. J. Surg. Res. 2013, 181, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Usuda, J.; Kato, H.; Okunaka, T.; Furukawa, K.; Tsutsui, H.; Yamada, K.; Suga, Y.; Honda, H.; Nagatsuka, Y.; Ohira, T.; et al. Photodynamic therapy for lung cancers. J. Thorac. Oncol. 2006, 1, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, A.; Chantrain, C.F.; von Tiehl, K.; Buckley, S.; Rucker, N.; Shalinsky, D.R.; Shimada, H.; DeClerck, Y.A.; Gomer, C.J. The matrix metalloproteinase inhibitor prinomastat enhances photodynamic therapy responsiveness in a mouse tumor model. Cancer Res. 2004, 64, 2328–2332. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Furukawa, K.; Sato, M.; Okunaka, T.; Kusunoki, Y.; Kawahara, M.; Fukuoka, M.; Miyazawa, T.; Yana, T.; Matsui, K.; et al. Phase II clinical study of photodynamictherapy using mono-l-aspartyl chlorine e6 and diode laser for early superficial squamous cell carcinoma of the lung. Lung Cancer 2003, 42, 103–111. [Google Scholar] [CrossRef]
- Usuda, J.; Tsutsui, H.; Honda, H.; Ichinose, S.; Ishizumi, T.; Hirata, T.; Inoue, T.; Ohtani, K.; Maehara, S.; Imai, K.; et al. Photodynamic therapy for lung cancers based on novel photodynamic diagnosis using talaporfin sodium (NPe6) and autofluorescence bronchoscopy. Lung Cancer 2007, 58, 317–323. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, M.; Miyajima, K.; Kojika, M.; Kono, T.; Kato, H. Photodynamic Therapy (PDT) with Chemotherapy for Advanced Lung Cancer with Airway Stenosis. Int. J. Mol. Sci. 2015, 16, 25466-25475. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161025466
Kimura M, Miyajima K, Kojika M, Kono T, Kato H. Photodynamic Therapy (PDT) with Chemotherapy for Advanced Lung Cancer with Airway Stenosis. International Journal of Molecular Sciences. 2015; 16(10):25466-25475. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161025466
Chicago/Turabian StyleKimura, Masakazu, Kuniharu Miyajima, Masakazu Kojika, Takafumi Kono, and Harubumi Kato. 2015. "Photodynamic Therapy (PDT) with Chemotherapy for Advanced Lung Cancer with Airway Stenosis" International Journal of Molecular Sciences 16, no. 10: 25466-25475. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161025466