Carbon Fluxes between Primary Metabolism and Phenolic Pathway in Plant Tissues under Stress
Abstract
:1. Plant Phenolic Secondary Metabolites
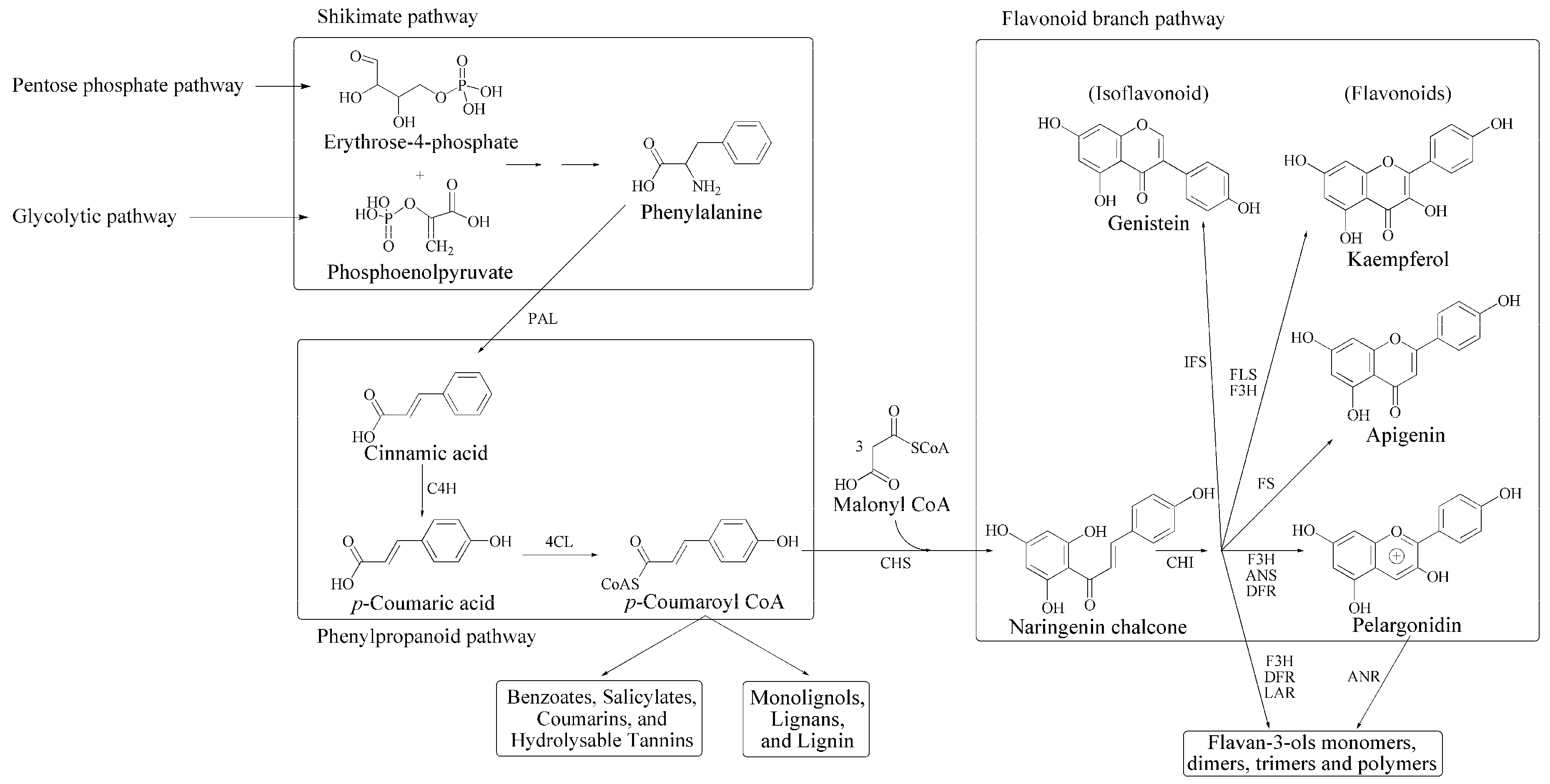
2. Plant Phenolics and Their Role in Defense against Environmental Stresses
3. Costs of Resistance
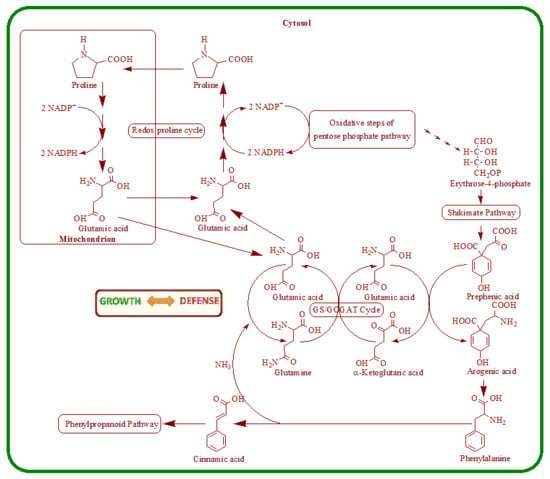
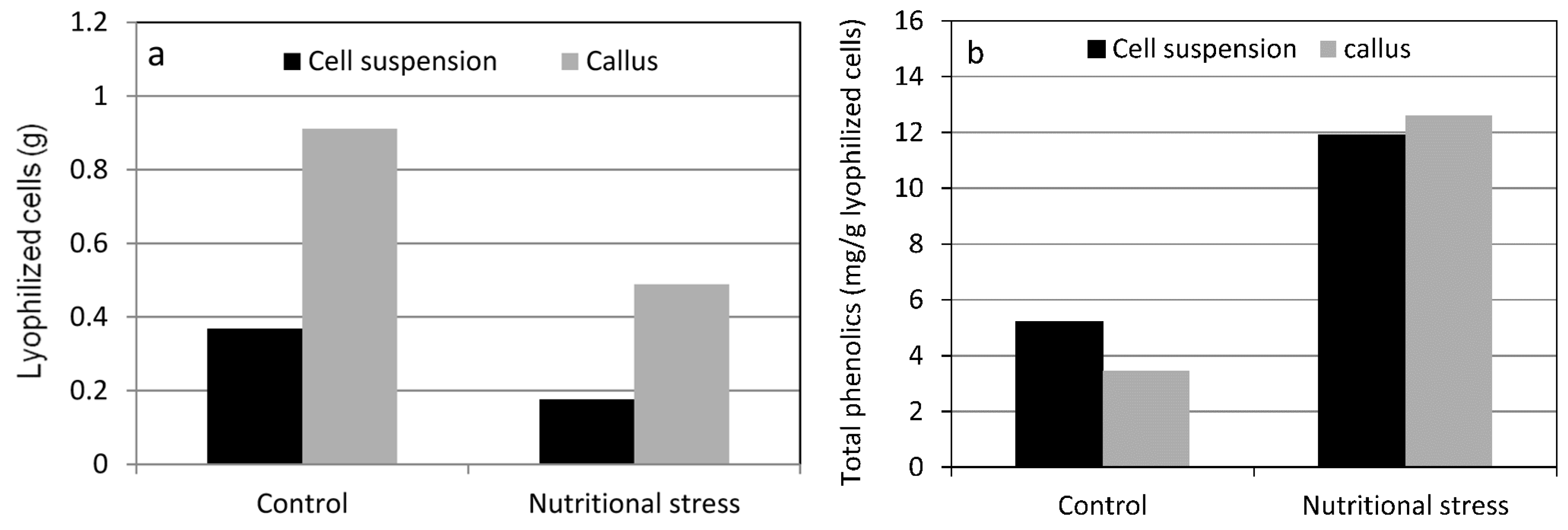
4. Nutritional Stress Induces Supply Pathways from Primary Metabolism to Phenolic Secondary Product Formation

5. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Chappell, J. Metabolic engineering of natural products in plants; tools of the trade and challenges for the future. Curr. Opin. Biotechnol. 2008, 19, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V. Phenolic Compounds: Introduction. In Handbook of Natural Products; Ramawat, K.G., Merillon, J.M., Eds.; Springer-Verlag: Berlin Heidelberg, Germany, 2013; pp. 1543–1580. [Google Scholar]
- Wink, M. Plant breeding: Importance of plant secondary metabolites for protection against pathogens and herbivores. Theor. Appl. Genet. 1988, 75, 225–233. [Google Scholar] [CrossRef]
- Pichersky, E.; Gang, D.R. Genetics and biochemistry of secondary metabolites in plants: An evolutionary perspective. Trends Plant Sci. 2000, 5, 439–445. [Google Scholar] [CrossRef]
- Osbourn, A.E.; Qi, X.; Townsend, B.; Qin, B. Dissecting plant secondary metabolism—Constitutive chemical defences in cereals. New Phytol. 2003, 159, 101–108. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Noel, J.P.; Austin, M.B.; Bomati, E.K. Structure-function relationships in plant phenylpropanoid biosynthesis. Curr. Opin. Plant Biol. 2005, 8, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Ornston, L.N.; Yeh, W.K. Origins of metabolic diversity: Evolutionary divergence by sequence repetition. Proc. Natl. Acad. Sci. USA 1979, 76, 3996–4000. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Biochemistry of Plant Secondary Metabolism; Sheffield Academic Press: Sheffield, UK; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Lehfeldt, C.; Shirley, A.M.; Meyer, K.; Ruegger, M.O.; Cusumano, J.C.; Viitanen, P.V.; Strack, D.; Chapple, C. Cloning of the SNG1 gene of Arabidopsis reveals a role for a serine carboxypeptidase-like protein as an acyltransferase in secondary metabolism. Plant Cell 2000, 12, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Tauber, E.; Last, K.S.; Olive, P.J.; Kyriacou, C.P. Clock gene evolution and functional divergence. J. Biol. Rhythms 2004, 19, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Broun, P. Transcriptional control of flavonoid biosynthesis: A complex network of conserved regulators involved in multiple aspects of differentiation in Arabidopsis. Curr. Opin. Plant Biol. 2005, 8, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, N.C.; Fett-Neto, A.G. Plant secondary metabolism and challenges in modifying its operation: An overview. In Plant Secondary Metabolism Engineering—Methods and Application, Methods in Molecular Biology; Fett-Neto, A.G., Ed.; Humana Press: New York, NY, USA, 2010; Volume 643, pp. 1–13. [Google Scholar]
- Lattanzio, V.; Cardinali, A.; Linsalata, V. Plant phenolics: A biochemical and physiological perspective. In Recent Advances in Polyphenols Research; Cheynier, V., Sarni-Manchado, P., Quideau, S., Eds.; Wiley-Blackwell Publishing: Oxford, UK, 2012; Volume 3, pp. 1–39. [Google Scholar]
- Robards, R.; Antolovich, M. Analytical chemistry of fruit bioflavonoids. A review. Analyst 1997, 122, 11R–34R. [Google Scholar] [CrossRef]
- Harborne, J.B. Plant phenolics. In Encyclopedia of Plant Physiology, New Series, Secondary Plant Products; Bell, E.A., Charlwood, B.V., Eds.; Springer-Verlag: Berlin, Germany, 1980; Volume 8, pp. 329–402. [Google Scholar]
- Lattanzio, V.; Kroon, P.A.; Quideau, S.; Treutter, D. Plant phenolics—Secondary metabolites with diverse functions. In Recent Advances in Polyphenol Research; Daayf, F., Lattanzio, V., Eds.; Wiley-Blackwell Publishing: Oxford, UK, 2008; Volume 1, pp. 1–35. [Google Scholar]
- Swain, T. Evolution of flavonoid compounds. In The Flavonoids; Harborne, J.B., Mabry, T.J., Mabry, H., Eds.; Chapman & Hall: London, UK, 1975; pp. 1096–1138. [Google Scholar]
- Boudet, A.M.; Graziana, A.; Ranjeva, R. Recent advances in the regulation of the prearomatic pathway. In The Biochemistry of Plant Phenolics; van Sumere, C.F., Lea, P.J., Eds.; Clarendon Press: London, UK, 1985; pp. 135–160. [Google Scholar]
- Hrazdina, G.; Jensen, R.A. Spatial organization of enzymes in plant metabolic pathways. Annu. Rev. Plant. Physiol. Plant Mol. Biol. 1992, 43, 241–267. [Google Scholar] [CrossRef]
- Hrazdina, G. Compartmentation in phenolic metabolism. Acta Hortic. 1994, 381, 86–96. [Google Scholar] [CrossRef]
- Schmid, J.; Amrhein, N. Molecular organization of the shikimate pathway in higher plants. Phytochemistry 1995, 39, 737–749. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid biosynthesis: A colorful model for genetics, biochemistry, cell biology and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.B.; Noel, J.P. The chalcone synthase superfamily of type III polyketide synthases. Nat. Prod. Rep. 2003, 20, 79–110. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Boudet, A.M. Evolution and current status of research in phenolic compounds. Phytochemistry 2007, 68, 2722–2735. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.E.; Cook, M.E.; Busse, J.S. The origin of plants: Body plan changes contributing to a major evolutionary radiation. Proc. Natl. Acad. Sci. USA 2000, 97, 4535–4540. [Google Scholar] [CrossRef] [PubMed]
- Lowry, B.; Lee, D.; Hébant, C. The origin of land plants: A new look at an old problem. Taxon 1980, 29, 183–197. [Google Scholar] [CrossRef]
- Gottlieb, O.R. Phytochemical evolution. Rev. Acad. Pol. Sci. Ex. Fis. Nat. 1986, 16, 39–45. [Google Scholar]
- Hertweck, C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. 2009, 48, 4688–4716. [Google Scholar] [CrossRef] [PubMed]
- McClure, J.W. Physology and function of flavonoids. In The Flavonoids; Harborne, J.B., Mabry, T.J., Mabry, H., Eds.; Chapman & Hall: London, UK, 1975; pp. 970–1055. [Google Scholar]
- Gottlieb, O.R. The role of oxygen in phytochemical evolution towards diversity. Phytochemistry 1989, 28, 2545–2558. [Google Scholar] [CrossRef]
- Gottlieb, O.R.; Kaplan, M.A.C. Phytochemical evolution: The redox theory. Nat. Prod. Lett. 1993, 2, 171–176. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced secondary metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Shulaev, V.; Cortes, D.; Miller, G.; Mittler, R. Metabolomics for plant stress response. Physiol. Plant. 2008, 132, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, I.; de Vos, R.C.H.; Bones, A.M.; Hall, R.D. Plant molecular stress responses face climate change. Trends Plant Sci. 2010, 15, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Saito, K. Integrated metabolomics for abiotic stress responses in plants. Curr. Opin. Plant Biol. 2015, 24, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Wittstock, U.; Gershenzon, J. Constitutive plant toxins and their role in defense against herbivores and pathogens. Curr. Opin. Plant Biol. 2002, 5, 300–307. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In Phytochemistry: Advances in Research; Imperato, F., Ed.; Research Signpost: Trivandrum, India, 2006; pp. 23–67. [Google Scholar]
- Somssich, I.E.; Bollmann, J.; Hahlbrock, K.; Kombrink, E.; Schulz, W. Differential early activation of defense-related genes in elicitor-treated parsley cells. Plant Mol. Biol. 1989, 12, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Somssich, I.E.; Wernert, P.; Kiedrowski, S.; Hahlbrock, K. Arabidopsis thaliana defense-related protein ELI3 is an aromatic alcohol:NADP+ oxidoreductase. Proc. Natl. Acad. Sci. USA 1996, 93, 14199–14203. [Google Scholar] [CrossRef] [PubMed]
- Somssich, I.E.; Hahlbrock, K. Pathogen defense in plants—A paradigm of biological complexity. Trends Plant Sci. 1998, 3, 86–90. [Google Scholar] [CrossRef]
- Ligterink, W.; Kroj, T.; zur Nieden, U.; Hirt, H.; Scheel, D. Receptor-mediated activation of a MAP kinase in pathogen defense of plants. Science 1997, 276, 2054–2057. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Arpaia, S.; Cardinali, A.; di Venere, D.; Linsalata, V. Role of endogenous flavonoids in resistance mechanism of Vigna to aphids. J. Agric. Food Chem. 2000, 48, 5316–5320. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.S.J. Importance of flavonoids in insect-plant interactions: Feeding and oviposition. Phytochemistry 2001, 56, 245–252. [Google Scholar] [CrossRef]
- Simmonds, M.S.J. Flavonoid-insect interactions: Recent advances in our knowledge. Phytochemistry 2003, 64, 21–30. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoids in seeds and grains: Physiological function, agronomic importance and the genetics of biosynthesis. Seed Sci. Res. 1998, 8, 415–422. [Google Scholar]
- Constabel, C.P.; Barbehenn, R. Defensive roles of polyphenol oxidase in plants. In Induced Plant Resistance to Herbivory; Schaller, A., Ed.; Springer Science + Business Media B.V.: New York, NY, USA, 2008; pp. 253–269. [Google Scholar]
- Barbehenn, R.; Dukatz, C.; Holt, C.; Reese, A.; Martiskainen, O.; Salminen, J.-P.; Yip, L.; Tran, L.; Constabel, C.P. Feeding on poplar leaves by caterpillars potentiates foliar peroxidase action in their guts and increases plant resistance. Oecologia 2010, 164, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Barbehenn, R.; Weir, Q.; Salminen, J.-P. Oxidation of ingested phenolics in the tree feeding caterpillar Orgyia leucostigma depends on foliar chemical composition. J. Chem. Ecol. 2008, 34, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Barbehenn, R.V.; Jaros, A.; Lee, G.; Mozola, C.; Weir, Q.; Salminen, J.-P. Tree resistance to Lymantria dispar caterpillars: Importance and limitations of foliar tannin composition. Oecologia 2009, 159, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Barbehenn, R.V.C.; Jaros, A.; Lee, G.; Mozola, C.; Weir, Q.; Salminen, J.-P. Hydrolyzable tannins as “quantitative defenses”: Limited impact against Lymantria dispar caterpillars on hybrid poplar. J. Insect Physiol. 2009, 55, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Purrington, C.B. Costs of resistance. Curr. Opin. Plant Biol. 2000, 3, 305–308. [Google Scholar] [CrossRef]
- Stamp, N. Out of the quagmire of plant defense hypotheses. Q. Rev. Biol. 2003, 78, 23–55. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.M.; Jenkins, G.I. Distinct UV-B and UV-A/Blue light signal transduction pathways induce chalcone synthase gene expression in Arabidopsis cells. Plant Cell 1996, 8, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Frohnmeyer, H.; Staiger, D. Ultraviolet-B radiation mediated responses in plants. Balancing damage and protection. Plant Physiol. 2003, 133, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Hectors, K.; Prinsen, E.; Coen, W.D.; Jansen, M.A.K.; Guisez, Y. Arabidopsis thaliana plants acclimated to low dose rates of ultraviolet B radiation show specific changes in morphology and gene expression in the absence of stress symptoms. New Phytol. 2007, 175, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, M.M.; Ballaré, C.L.; Bornman, J.F.; Flint, S.D.; Bjorn, L.O.; Teramura, A.H.; Kulandaivelu, G.; Tevini, M. Terrestrial ecosystems, increased solar ultraviolet radiation and interactions with other climatic change factors. Photochem. Photobiol. Sci. 2003, 2, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Grace, S.C.; Logan, B.A. Energy dissipation and radical scavenging by the plant phenylpronanoid pathway. Philos. Trans. R. Soc. Lond. B 2000, 355, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Mazza, C.A.; Boccalandro, H.E.; Giordano, C.V.; Battista, D.; Scopel, A.L.; Ballaré, C.L. Functional significance and induction by solar radiation of ultra-violet-absorbing sunscreens in field-grown soybean crops. Plant Physiol. 2000, 122, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R.; Chapin, F.S., III. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv. Ecol. Res. 2000, 30, 1–67. [Google Scholar]
- Scheible, W.-R.; Morcuende, R.; Czechowski, T.; Fritz, C.; Osuna, D.; Palacios-Rojas, N.; Schindelasch, D.; Thimm, O.; Udvardi, M.K.; Stitt, M. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol. 2004, 136, 2483–2499. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.P.; Chapin, F.S., III; Klein, D.R. Carbon nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 1983, 40, 357–368. [Google Scholar] [CrossRef]
- Niemann, G.J.; Pureveen, J.B.M.; Eijkel, G.B.H.; Poorter, H.; Boon, J.J. Differences in relative growth rate in 11 grasses correlate with differences in chemical composition as determined by pyrolysis mass spectrometry. Oecologia 1992, 89, 567–573. [Google Scholar] [CrossRef]
- Poorter, H.; Bergkotte, M. Chemical composition of 24 wild species differing in relative growth rate. Plant Cell Environ. 1992, 15, 221–229. [Google Scholar] [CrossRef]
- Lillo, C.; Lea, U.S.; Ruoff, P. Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ. 2008, 31, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Haukioja, E.; Ossipov, V.; Koricheva, J.; Honkanen, T.; Larsson, S.; Lempa, K. Biosynthetic origin of carbon-based secondary compounds: Cause of variable responses of woody plants to fertilization? Chemoecology 1998, 8, 133–139. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B.; Bačkor, M.; Repčák, M. Phenylalanine ammonia-lyase activity and phenolic compounds accumulation in nitrogen-deficient Matricaria chamomilla leaf rosettes. Plant Sci. 2007, 172, 393–399. [Google Scholar] [CrossRef]
- Glynn, C.; Herms, D.A.; Orians, C.M.; Hansen, R.C.; Larsson, S. Testing the growth-differentiation balance hypothesis: Dynamic responses of willows to nutrient availability. New Phytol. 2007, 176, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Mercure, S.A.; Daoust, B.; Samson, G. Causal relationship between growth inhibition, accumulation of phenolic metabolites, and changes of UV-induced fluorescences in nitrogen-deficient barley plants. Can. J. Bot. 2004, 6, 815–821. [Google Scholar] [CrossRef]
- Jin, C.W.; You, G.Y.; He, Y.F.; Tang, C.X.; Wu, P.; Zheng, S.J. Iron deficiency induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. Plant Physiol. 2007, 144, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.W.; You, G.Y.; Zheng, S.J. The iron deficiency-induced phenolics secretion plays multiple important roles in plant iron acquisition underground. Plant Signal. Behav. 2008, 3, 60–61. [Google Scholar] [CrossRef] [PubMed]
- Vigani, G.; Zocchi, G.; Bashir, K.; Phillipar, K.; Briat, J.F. Cellular iron homeostasis and metabolism in plants. Front. Plant Sci. 2013, 4, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Yonekura-Sakaibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Tohge, T.; Nishiyama, Y.; Hirai, M.Y.; Yano, M.; Nakajima, J.-I.; Awazuhara, M.; Inoue, E.; Takahashi, H.; Goodenowe, D.B.; Kitayama, M.; et al. Functional genomics by integrated analysis of metabolome and transcriptome of Arabidopsis plants over-expressing an MYB transcription factor. Plant J. 2005, 42, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Morcuende, R.; Bari, R.; Gibon, Y.; Zheng, W.; Pant, B.D.; Bläsing, O.; Usadel, B.; Czechowski, T.; Udvardi, M.K.; Stitt, M.; et al. Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant Cell Environ. 2007, 30, 85–112. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.-D.; Pant, P.; Erban, A.; Huhman, D.; Kopka, J.; Scheible, W.-R. Identification of primary and secondary metabolites with phosphorus status-dependent abundance in Arabidopsis, and of the transcription factor PHR1 as a major regulator of metabolic changes during phosphorus limitation. Plant Cell Environ. 2015, 38, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Rubio, V.; Linhares, F.; Solano, R.; Martin, A.C.; Iglesias, J.; Leyva, A.; Paz-Ares, J. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev. 2001, 15, 2122–2133. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, G.; Karabourniotis, G. Boron deficiency and concentrations and composition of phenolic compounds in Olea europaea leaves: A combined growth chamber and field study. Tree Physiol. 2005, 25, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Hamilton, J.G.; Zangerl, A.R.; DeLucia, E.H.; Berenbaum, M.R. The carbon-nutrient balance hypothesis: Its rise and fall. Ecol. Lett. 2001, 4, 86–95. [Google Scholar] [CrossRef]
- Oh, M.-M.; Trick, H.N.; Rajashekar, C.B. Secondary metabolism and antioxidants are involved in environmental adaptation and stress tolerance in lettuce. J. Plant Physiol. 2009, 166, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Lütz, C. Cell physiology of plants growing in cold environments. Protoplasma 2010, 244, 53–73. [Google Scholar] [CrossRef] [PubMed]
- Airaki, M.; Leterrier, M.; Mateos, R.M.; Valderrama, R.; Chaki, M.; Barroso, J.B.; del Rio, L.A.; Palma, J.M.; Corpas, F.J. Metabolism of reactive oxygen species and reactive nitrogen species in pepper (Capsicum annuum L.) plants under low temperature stress. Plant Cell Environ. 2012, 35, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; van Sumere, C.F. Changes in phenolic compounds during the development and cold storage of artichoke (Cynara scolymus L.) heads. Food Chem. 1987, 24, 37–50. [Google Scholar] [CrossRef]
- Lattanzio, V.; di Venere, D.; Linsalata, V.; Bertolini, P.; Ippolito, A.; Salerno, M. Low temperature metabolism of apple phenolics and quiescence of Phlyctaena vagabunda. J. Agric. Food Chem. 2001, 49, 5817–5821. [Google Scholar] [CrossRef] [PubMed]
- Stefanowska, M.; Kuras, M.; Kacperska, A. Low temperature induced modifications in cell ultrastructure and localization of phenolics in winter oilseed rape (Brassica napus L. var oleifera L.) leaves. Ann. Bot. 2002, 90, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Leyva, A.; Jarillo, J.A.; Salinas, J.; Martinez-Zapater, J.M. Low temperature induces the accumulation of phenylalanine ammonia-lyase and chalcone synthase mRNAs of Arabidopsis thaliana in a light-dependent manner. Plant Physiol. 1995, 108, 39–46. [Google Scholar] [PubMed]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Solecka, D.; Boudet, A.M.; Kacperska, A. Phenylpropanoid and anthocyanin changes in low-temperature treated oilseed rape leaves. Plant Physiol. Biochem. 1999, 37, 491–496. [Google Scholar] [CrossRef]
- Christie, P.J.; Alfenito, M.R.; Walbot, V. Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: Enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta 1994, 194, 541–549. [Google Scholar] [CrossRef]
- Schützendübel, A.; Polle, A. Plant responses to abiotic stresses: Heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 2002, 53, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Bazzaz, F.A.; Chiariello, N.R.; Coley, P.D.; Pitelka, L.F. Allocating resources to reproduction and defense. BioScience 1987, 37, 58–67. [Google Scholar] [CrossRef]
- Coley, P.D.; Bryant, J.P.; Chapin, F.S., III. Resource availability and plant antiherbivore defense. Science 1985, 230, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Bazzaz, F.A.; Grace, J. Plant Resource Allocation; Academic Press: London, UK, 1997. [Google Scholar]
- Bazzaz, F.A.; Ackerly, D.D.; Reekie, E.G. Reproductive allocation in plants. In Seeds: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CABI Publishing: Wallingford, UK, 2000; pp. 1–30. [Google Scholar]
- Matyssek, R.; Schnyder, H.; Oßwald, W.; Ernst, D.; Munch, J.C.; Pretzsch, H. Growth and Defence in Plants, Ecological Studies Volume 220; Springer-Verlag: Berlin Heidelberg, Germany, 2012. [Google Scholar]
- Heil, M.; Hilpert, A.; Kaiser, W.; Linsenmair, E. Reduced growth and seed set following chemical induction of pathogen defence: Does systemic acquired resistance (SAR) incur allocation costs? J. Ecol. 2000, 88, 645–654. [Google Scholar] [CrossRef]
- Heil, M.; Baldwin, I.T. Fitness costs of induced resistance: Emerging experimental support for a slippery concept. Trends Plant Sci. 2002, 7, 61–67. [Google Scholar] [CrossRef]
- Strauss, S.Y.; Rudgers, J.A.; Lau, J.A.; Irwin, R.E. Direct and ecological costs of resistance to herbivory. Trends Ecol. Evol. 2002, 17, 278–284. [Google Scholar] [CrossRef]
- Zangerl, A.R.; Arntz, A.M.; Berenbaum, M.R. Physiological price of an induced chemical defense: Photosynthesis, respiration, biosynthesis, and growth. Oecologia 1997, 109, 433–441. [Google Scholar] [CrossRef]
- Pavia, H.; Toth, G.; Aberg, P. Trade-offs between phlorotannin production and annual growth in natural populations of the brown seaweed Ascophyllum nodosum. J. Ecol. 1999, 87, 761–771. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Bloom, A.J.; Field, C.B.; Waring, R.H. Plant responses to multiple environmental factors. BioScience 1987, 37, 49–57. [Google Scholar] [CrossRef]
- Brown, J.K.M. A cost of disease resistance: Paradigm or peculiarity? Trends Genet. 2003, 19, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Burdon, J.J.; Thrall, P.H. The fitness costs to plants of resistance to pathogens. Genome Biol. 2003, 4, 227.1–227.3. [Google Scholar] [CrossRef] [PubMed]
- Siemens, D.H.; Lischke, H.; Maggiulli, N.; Schürch, S.; Roy, B.A. Cost of resistance and tolerance under competition: The defense-stress benefit hypothesis. Evol. Ecol. 2003, 17, 247–263. [Google Scholar] [CrossRef]
- Dietrich, R.; Ploss, K.; Heil, M. Growth responses and fitness costs after induction of pathogen resistance depend on environmental conditions. Plant Cell Environ. 2005, 28, 211–222. [Google Scholar] [CrossRef]
- Hernández, I.; van Breusegem, F. Opinion on the possible role of flavonoids as energy escape valves: Novel tools for nature’s Swiss army knife. Plant Sci. 2010, 179, 297–301. [Google Scholar] [CrossRef]
- Selmar, D.; Kleinwächter, M. Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind. Crop. Prod. 2013, 42, 558–566. [Google Scholar] [CrossRef]
- Kleinwächter, M.; Selmar, D. New insights explain that drought stress enhances the quality of spice and medicinal plants: Potential applications. Agron. Sustain. Dev. 2015, 35, 121–131. [Google Scholar] [CrossRef]
- Lattanzio, V.; Cardinali, A.; Ruta, C.; Morone Fortunato, I.; Lattanzio, V.M.T.; Linsalata, V.; Cicco, N. Relationship of secondary metabolism to growth in oregano (Origanum vulgare L.) shoot cultures under nutritional stress. Environ. Exp. Bot. 2009, 65, 54–62. [Google Scholar] [CrossRef]
- Logemann, E.; Tavernaro, A.; Schulz, W.; Somssich, I.E.; Hahlbrock, K. UV light selectively co-induces supply pathways from primary metabolism and flavonoid secondary product formation in parsley. Proc. Natl. Acad. Sci. USA 2000, 97, 1903–1907. [Google Scholar] [CrossRef] [PubMed]
- Henkes, S.; Sonnewald, U.; Badur, R.; Flachmann, R.; Stitt, M. A small decrease of plastid transketolase activity in antisense tobacco transformants has dramatic effects on photosynthesis and phenylpropanoid metabolism. Plant Cell 2001, 13, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Nakane, E.; Kawakita, K.; Doke, N.; Yoshioka, H. Elicitation of primary and secondary metabolism during defense in the potato. J. Gen. Plant. Pathol. 2003, 69, 378–384. [Google Scholar] [CrossRef]
- Lloyd, J.C.; Zakhleniuk, O.V. Responses of primary and secondary metabolism to sugar accumulation revealed by microarray expression analysis of the Arabidopsis mutant, pho3. J. Exp. Bot. 2004, 55, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Leser, C.; Treutter, D. Effects of nitrogen supply on growth, contents of phenolic compounds and pathogen (scab) resistance of apple trees. Physiol. Plant. 2005, 123, 49–56. [Google Scholar] [CrossRef]
- Fritz, C.; Palacios-Rojas, N.; Feil, R.; Stitt, M. Regulation of secondary metabolism by the carbon-nitrogen status in tobacco: Nitrate inhibits large sectors of phenylpropanoid metabolism. Plant J. 2006, 46, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; Keller, M.M.; Ballaré, C.L. To grow or defend? Low red: Far-red ratios reduce jasmonate sensitivity in Arabidopsis seedlings by promoting DELLA degradation and increasing JAZ10 stability. New Phytol. 2014, 204, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A. Benefits and costs of induced plant defense for Lepidium virginicum (Brassicaceae). Ecology 2000, 81, 1804–1813. [Google Scholar] [CrossRef]
- Cipollini, D. Stretching the limits of plasticity: Can a plant defend against both competitors and herbivores? Ecology 2004, 85, 28–37. [Google Scholar] [CrossRef]
- Izaguirre, M.M.; Mazza, C.A.; Biondini, M.; Baldwin, I.T.; Ballaré, C.L. Remote sensing of future competitors: Impacts on plant defenses. Proc. Natl. Acad. Sci. USA 2006, 103, 7170–7174. [Google Scholar] [CrossRef] [PubMed]
- Ballaré, C.L. Illuminated behaviour: Phytochrome as a key regulator of light foraging and plant anti-herbivore defence. Plant Cell Environ. 2009, 32, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Schumaker, K.S.; Zhu, J.K. Cell signaling during cold, drought, and salt stress. Plant Cell 2002, 14, S165–S183. [Google Scholar] [PubMed]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, K.; Küster, H.; Rutten, T.; Fait, A.; Fernie, A.R.; Miersch, O.; Wasternack, C.; Emery, R.J.N.; Desel, C.; Hosein, F.; et al. ADP-glucose pyrophosphorylase-deficient pea embryos reveal specific transcriptional and metabolic changes of carbon-nitrogen metabolism and stress responses. Plant Physiol. 2009, 149, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defence mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Bachereau, F.; Marigo, G.; Asta, J. Effect of solar radiation (UV and visible) at high altitude on CAM-cycling and phenolic compound biosynthesis in Sedum album. Physiol. Plant. 1998, 104, 203–210. [Google Scholar] [CrossRef]
- Cooper-Driver, G.A.; Bhattacharya, M. Role of phenolics in plant evolution. Phytochemistry 1998, 49, 1165–1174. [Google Scholar] [CrossRef]
- Kidd, P.S.; Llugany, M.; Poschenrieder, C.; Gunsé, B.; Barcelò, J. The role of root exudates in aluminium resistance and silicon-induced amelioration of aluminium toxicity in three variety of maize (Zea mays L.). J. Exp. Bot. 2001, 52, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.J.; Chapman, W.; Jenkins, G.I.; Graham, I.; Martin, T.; Crozier, A. The effect of nitrogen and phosphorus deficiency on flavonol accumulation in plant tissues. Plant Cell Environ. 2001, 24, 1189–1197. [Google Scholar] [CrossRef]
- Casati, P.; Walbot, V. Gene expression profiling in response to ultraviolet radiation in Zea mays genotypes with varying flavonoid content. Plant Physiol. 2003, 132, 1739–1754. [Google Scholar] [CrossRef] [PubMed]
- Treutter, D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 2005, 7, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Bornman, J.F.; Ballaré, C.L.; Flint, S.D.; Kulandaivelu, G. Terrestrial ecosystems, increased solar ultraviolet radiation, and interactions with other climate change factors. Photochem. Photobiol. Sci. 2007, 6, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Olsen, K.M.; Lea, U.S.; Slimestad, R.; Verheul, M.; Lillo, C. Differential expression of the four Arabidopsis PAL genes –PAL1 and PAL2 have functional specialization in abiotic environmental triggered flavonoid synthesis. J. Plant Physiol. 2008, 165, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Adams-Phillip, L.; Briggs, A.G.; Bent, A.F. Disruption of poly(ADP-ribosyl)ation mechanisms alters responses of Arabidopsis to biotic stress. Plant Physiol. 2010, 152, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.S.; Bonner, C.A.; Theodorou, M.E.; Paxton, W.C.; Hrazdina, G.; Jensen, R.A. Response of aromatic pathway enzymes of plant suspension cells to phosphate limitation. Bioorg. Med. Chem. Lett. 1993, 3, 1415–1420. [Google Scholar] [CrossRef]
- Van der Plas, L.H.W.; Eijkelboom, C.; Hagendoorn, M.J.M. Relation between primary and secondary metabolism in plant cell suspensions. Plant Cell Tiss. Org. 1995, 43, 111–116. [Google Scholar] [CrossRef]
- Brown, J.K.M. Yield penalties of disease resistance in crops. Curr. Opin. Plant Biol. 2002, 5, 339–344. [Google Scholar] [CrossRef]
- Messina, F.J.; Durham, S.L.; Richards, J.H.; McArthur, E.D. Trade-off between plant growth and defense? A comparison of sagebrush populations. Oecologia 2002, 131, 43–51. [Google Scholar] [CrossRef]
- Cipollini, D.; Purrington, C.B.; Bergelson, J. Costs of induced responses in plants. Basic Appl. Ecol. 2003, 4, 79–85. [Google Scholar] [CrossRef]
- Walters, D.; Heil, M. Costs and trade-offs associated with induced resistance Physiol. Mol. Plant Pathol. 2007, 71, 3–17. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [PubMed]
- Vos, I.A.; Pieterse, C.M.J.; van Wees, S.C.M. Costs and benefits of hormone-regulated plant defences. Plant Pathol. 2013, 62, 43–55. [Google Scholar] [CrossRef]
- Shetty, K. Role of proline-linked pentose phosphate pathway in biosynthesis of plant phenolics for functional food and environmental applications: A review. Proc. Biochem. 2004, 9, 789–803. [Google Scholar] [CrossRef]
- Kushad, M.M.; Yelenosky, G. Evaluation of polyamine and proline levels during low temperature acclimation of citrus. Plant Physiol. 1987, 84, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Hare, P.D.; Cress, W.A. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1997, 21, 79–102. [Google Scholar] [CrossRef]
- Mehta, S.K.; Gaur, J.P. Heavy-metal-induced proline accumulation and its role in ameliorating metal toxicity in Chlorella vulgaris. New Phytol. 1999, 143, 253–259. [Google Scholar] [CrossRef]
- Fedin, I.S.; Grigorova, I.D.; Georgieva, K.M. Response of barley seedlings to UV-B radiation as affected by NaCl. J. Plant Physiol. 2003, 160, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Kiyosue, T.; Yoshiba, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is upregulated by proline but downregulated by dehydration in Arabidopsis. Plant Cell 1996, 8, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Hare, P.D.; Cress, W.A.; van Staden, J. Dissecting the roles of osmolyte accumulation in plants. Plant Cell Environ. 1998, 21, 535–553. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Sangam, S.; Amrutha, R.N.; Sri Laxmi, P.; Naidu, K.R.; Rao, K.R.S.S.; Sreenath, R.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Facchini, P.J. Plant secondary metabolism: Out of the evolutionary abyss. Trends Plant Sci. 1999, 4, 382–384. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.S.; Lister, C. Flavonoid functions in plants. In Flavonoids—Chemistry, Biochemistry and Applications; Andersen, Ø.M., Markham, K.R., Eds.; CRC Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 397–411. [Google Scholar]
- Lattanzio, V.; Cardinali, A.; di Venere, D.; Linsalata, V.; Palmieri, S. Browning phenomena in stored artichoke (Cynara scolymus L.) heads: Enzymic or chemical reactions? Food Chem. 1994, 50, 1–7. [Google Scholar] [CrossRef]
- Fahrendorf, T.; Ni, W.; Shorrosh, B.S.; Dixon, R.A. Stress responses in alfalfa (Medicago sativa L.) XIX. Transcriptional activation of oxidative pentose phosphate pathway genes at the onset of the isoflavonoid phytoalexin response. Plant Mol. Biol. 1995, 28, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Hare, P.D.; Cress, W.A.; van Staden, J. Proline synthesis and degradation: A model system for elucidating stress-related signal transduction. J. Exp. Bot. 1999, 50, 413–434. [Google Scholar] [CrossRef]
- Stewart, C.R.; Bogges, S.F.; Aspinall, D.; Paleg, L.G. Inhibition of proline oxidation by water stress. Plant Physiol. 1977, 59, 930–932. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, C.H.; Yeh, G.C.; Phang, J.M. Transfer of 1-pyrroline-5-carboxylate as oxiding potential from hepatocytes to erythrocytes. Biochem. J. 1982, 202, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Maggio, A.; Bressan, R.A.; Hasegawa, P.M.; Locy, R.D. Moderately increased constitutive proline does not alter osmotic stress tolerance. Physiol. Plant. 1997, 101, 240–246. [Google Scholar] [CrossRef]
- Fine, P.V.A.; Miller, Z.J.; Mesones, I.; Irazuzta, S.; Appel, H.M.; Stevens, M.H.H.; Sääksjärvi, I.; Schultz, J.C.; Coley, P.D. The growth-defense trade-off and habitat specialization by plants in Amazonian forests. Ecology 2006, 87, S150–S162. [Google Scholar] [CrossRef]
- Ncube, B.; Finnie, J.F.; van Staden, J. Dissecting the stress metabolic alterations in vitro Cyrtanthus regenerants. Plant Physiol. Biochem. 2013, 65, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Naliwajski, M.R.; Skłodowska, M. Proline and its metabolism enzymes in cucumber cell cultures during acclimation to salinity. Protoplasma 2014, 251, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Matyssek, R.; Agerer, R.; Ernst, D.; Munch, J.C.; Osswald, W.; Pretzch, H.; Priesack, E.; Schnyder, H.; Treutter, D. The plant’s capacity in regulating resource demand. Plant Biol. 2005, 7, 560–580. [Google Scholar] [CrossRef] [PubMed]
- Schloter, M.; Matyssek, R. Tuning growth versus defence–belowground interactions and plant resource allocation. Plant Soil 2009, 323, 1–5. [Google Scholar] [CrossRef]
- Margna, U. Control at the level of substrate supply. An alternative in the regulation of phenylpropanoid accumulation in plant cells. Phytochemistry 1977, 16, 419–426. [Google Scholar] [CrossRef]
- Margna, U.; Vainjärv, T.; Laanest, L. Different L-phenylalanine pools available for the biosynthesis of phenolics in buckwheat seedling tissues. Phytochemistry 1989, 28, 469–475. [Google Scholar] [CrossRef]
- Jones, C.G.; Hartley, S.E. A protein competition model of phenolic allocation. Oikos 1999, 86, 27–44. [Google Scholar] [CrossRef]
- Donaldson, J.R.; Kruger, E.L.; Lindroth, R.L. Competition- and resource-mediated tradeoffs between growth and defensive chemistry in trembling aspen (Populus tremuloides). New Phytol. 2006, 169, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Gao, X.; Liao, L.; Harberd, N.P.; Fu, X. Phosphate starvation root architecture and anthocyanin accumulation responses are modulated by the gibberellin-DELLA signaling pathway in Arabidopsis. Plant Physiol. 2007, 145, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Le Bot, J.; Benard, C.; Robin, C.; Bourgaud, F.; Adamowicz, S. The “trade-off” between synthesis of primary and secondary compounds in young tomato leaves is altered by nitrate nutrition: Experimental evidence and model consistency. J. Exp. Bot. 2009, 60, 4301–4314. [Google Scholar] [CrossRef] [PubMed]
- Endara, M.-J.; Coley, P.D. The resource availability hypothesis revisited: A meta-analysis. Funct. Ecol. 2011, 25, 389–398. [Google Scholar] [CrossRef]
- Arnholdt-Schmitt, B. Stress-induced cell reprogramming. A role for global genome regulation? Plant Physiol. 2004, 136, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993, 125, 27–58. [Google Scholar] [CrossRef]
- Nanda, A.K.; Andrio, E.; Marino, D.; Pauly, N.; Dunand, C. Reactive oxygen species during plant-microorganism early interactions. J. Integr. Plant Biol. 2010, 52, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Razal, R.A.; Ellis, S.; Singh, S.; Lewis, N.G.; Towers, G.H.N. Nitrogen recycling in phenylpropanoid metabolism. Phytochemistry 1996, 41, 31–35. [Google Scholar] [CrossRef]
- Van Heerden, P.S.; Towers, G.H.N.; Lewis, N.G. Nitrogen metabolism in lignifying Pinus taeda cell cultures. J. Biol. Chem. 1996, 271, 12350–12355. [Google Scholar] [CrossRef] [PubMed]
- Mckey, D.; Waterman, P.G.; Mbi, C.N.; Gartlan, J.S.; Struhsaker, T.T. Phenolic content of vegetation in two African rain forests: Ecological implications. Science 1978, 202, 61–64. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caretto, S.; Linsalata, V.; Colella, G.; Mita, G.; Lattanzio, V. Carbon Fluxes between Primary Metabolism and Phenolic Pathway in Plant Tissues under Stress. Int. J. Mol. Sci. 2015, 16, 26378-26394. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161125967
Caretto S, Linsalata V, Colella G, Mita G, Lattanzio V. Carbon Fluxes between Primary Metabolism and Phenolic Pathway in Plant Tissues under Stress. International Journal of Molecular Sciences. 2015; 16(11):26378-26394. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161125967
Chicago/Turabian StyleCaretto, Sofia, Vito Linsalata, Giovanni Colella, Giovanni Mita, and Vincenzo Lattanzio. 2015. "Carbon Fluxes between Primary Metabolism and Phenolic Pathway in Plant Tissues under Stress" International Journal of Molecular Sciences 16, no. 11: 26378-26394. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161125967