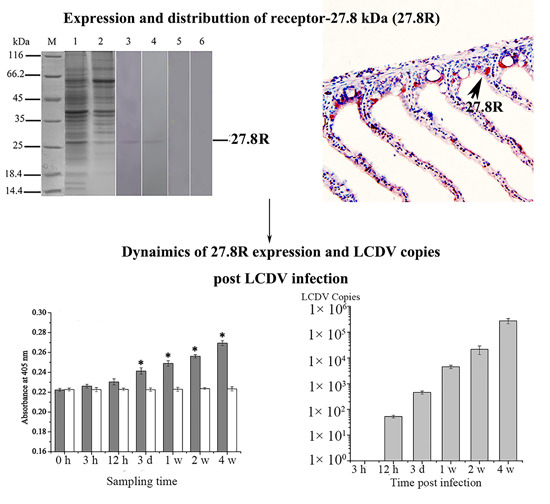
Tissue Localization of Lymphocystis Disease Virus (LCDV) Receptor-27.8 kDa and Its Expression Kinetics Induced by the Viral Infection in Turbot (Scophthalmus maximus)
Abstract
:1. Introduction
2. Results
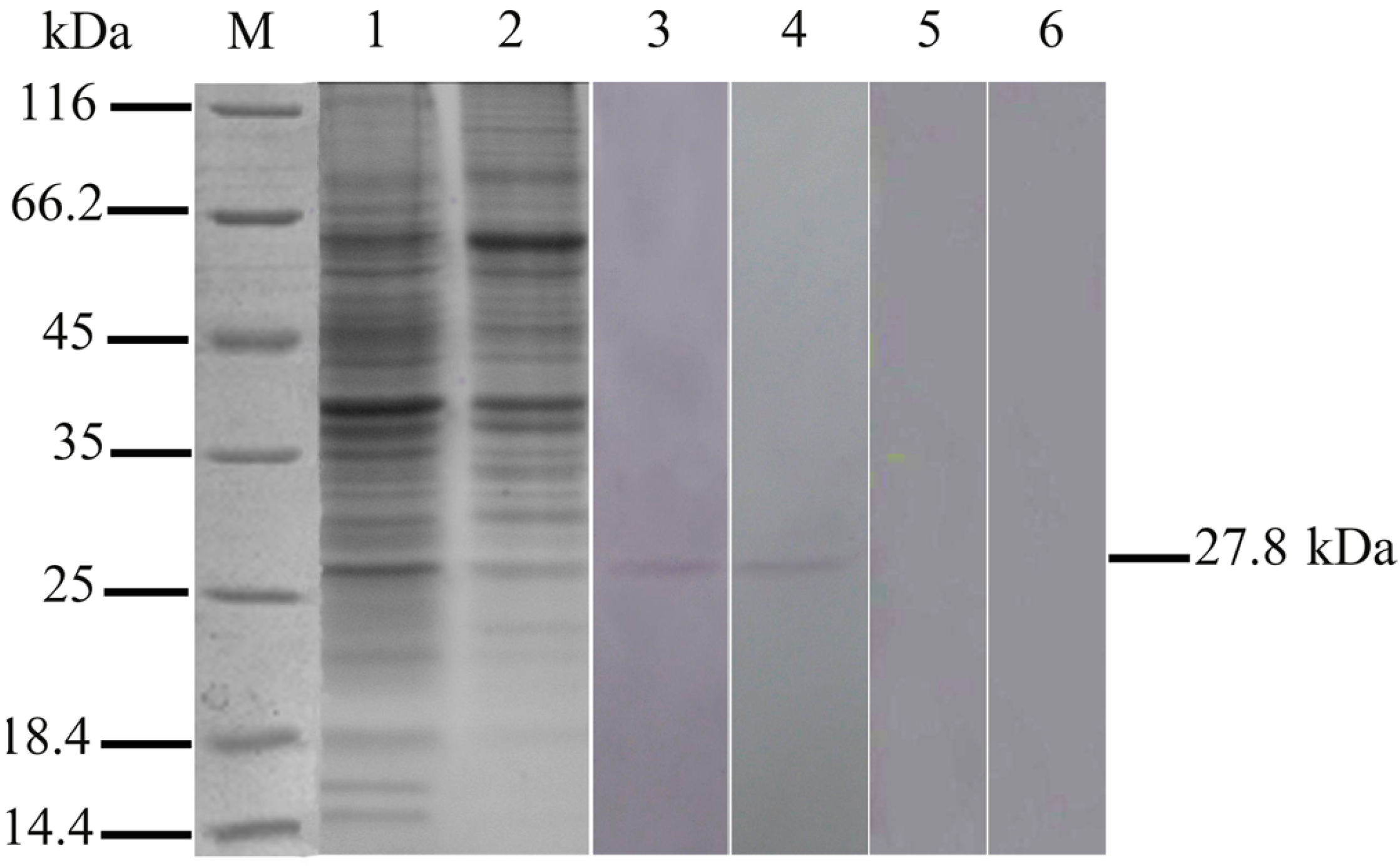
2.1. Expression of 27.8R in Turbot by Western Blotting
2.2. 27.8R Distribution in Turbot by IIFA

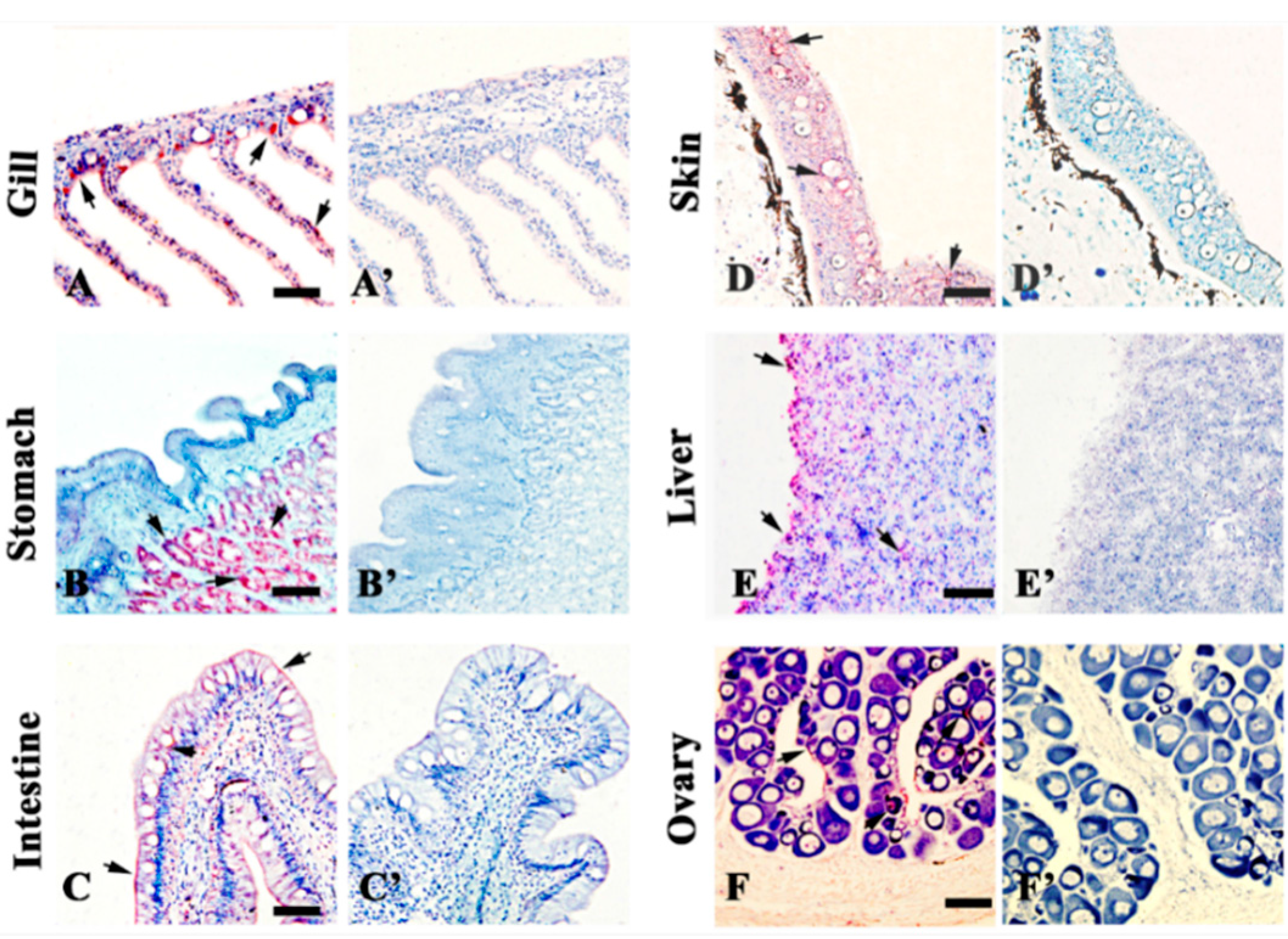
2.3. Localization of 27.8R by Immunohistochemistry
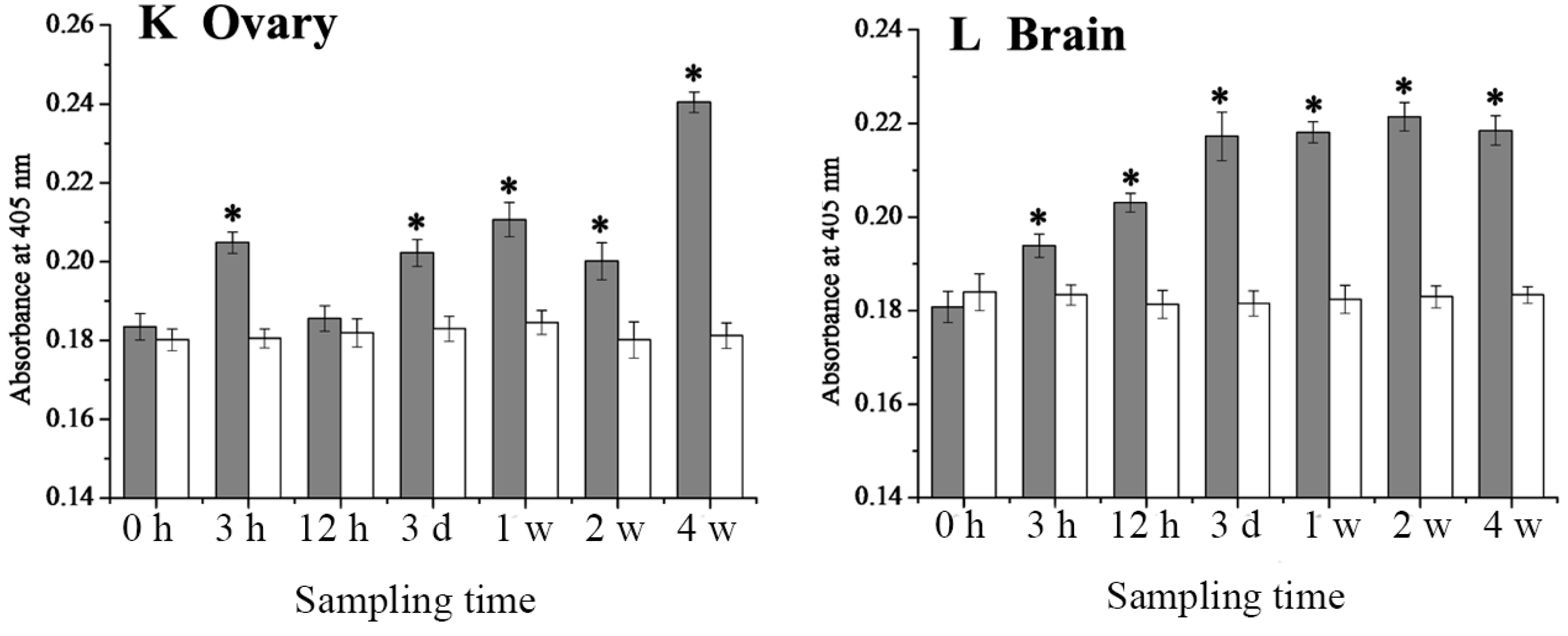
2.4. Expression Changes of 27.8R after LCDV Infection



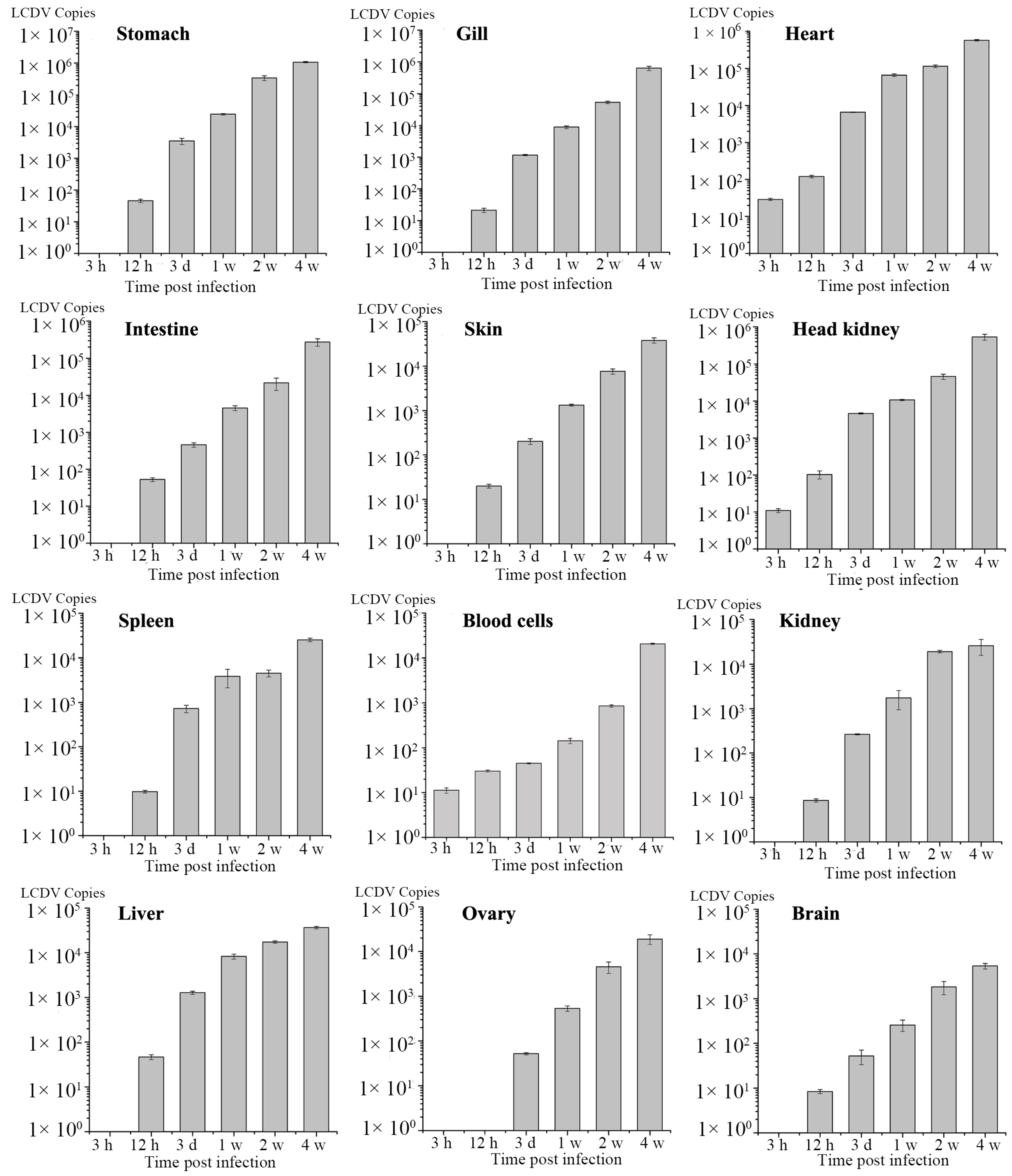
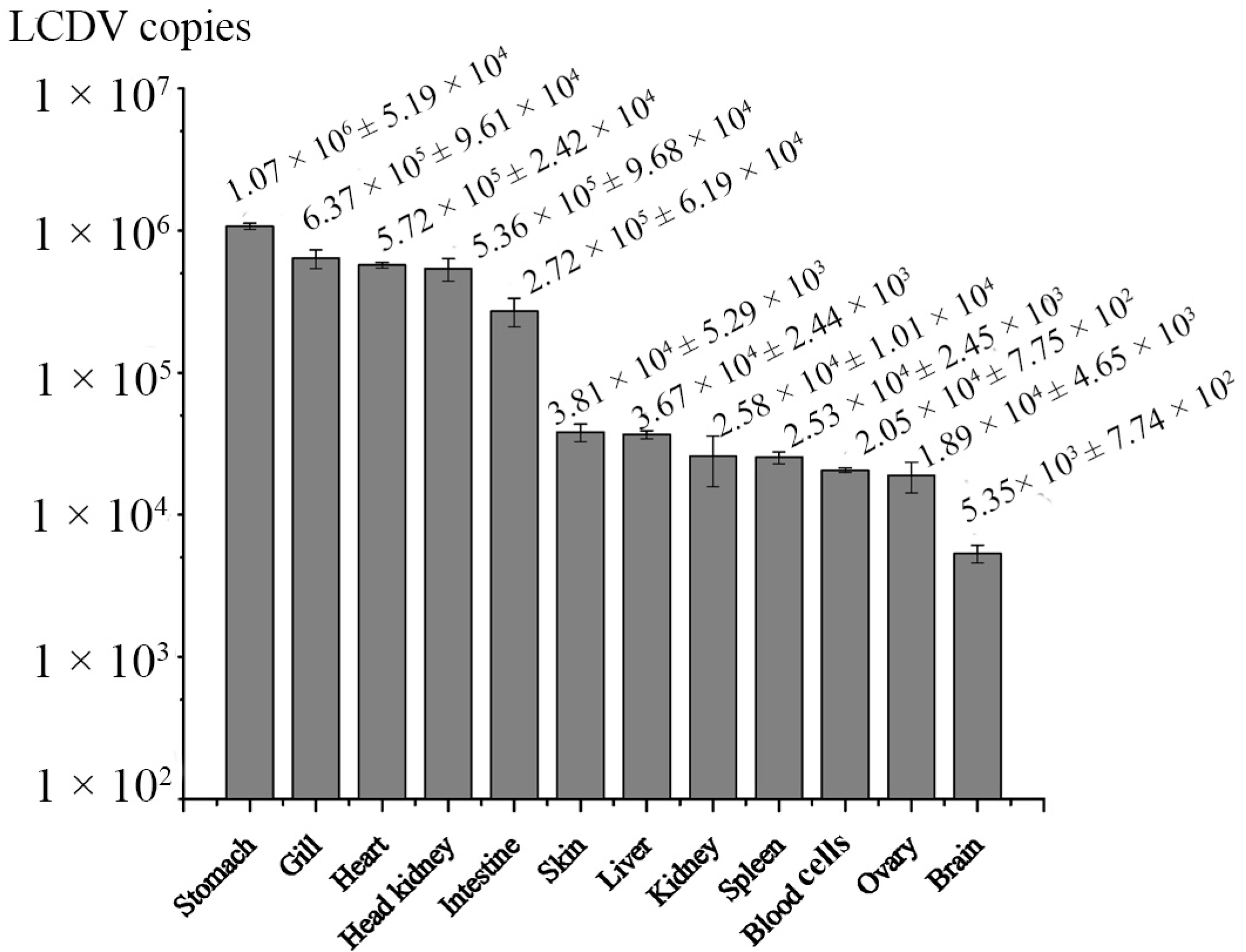
2.5. Dynamics of LCDV Copies in Fish Tissues
2.6. 27.8R Distribution and LCDV Antigens in Peripheral Blood Cells

3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Fish, Virus and Monoclonal Antibodies
4.3. SDS-PAGE and Western Blotting Analysis
4.4. Tissue Cryosections and IIFA
4.5. Tissue Paraffin Sections and Immunohistochemistry
4.6. Virus Infection and Sampling
4.7. ELISA
4.8. Real-Time Quantitative PCR
4.9. Blood Cell Smear Preparation and IIFA
4.10. Statistics
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shi, C.Y.; Wang, Y.G.; Huang, J.; Wang, Q.Y. The progress on viral diseases research of turbot Scophthalmus maximus. High Technol. Lett. 2003, 9, 99–105. [Google Scholar]
- Shi, C.Y.; Wang, Y.G.; Huang, J.; Wang, Q.Y. The first report of an iridovirus-like agent infection in farmed turbot Scophthalmus maximus in China. Aquaculture 2004, 236, 11–25. [Google Scholar] [CrossRef]
- Nishizawa, T.; Savaş, H.; Işıdan, H.; Üstündağ, C.; Iwamoto, H.; Yoshimizu, M. Genotyping and pathogenicity of viral hemorrhagic septicemia virus from free-living turbot (Psetta maxima) in a Turkish coastal area of the Black Sea. Appl. Environ. Microb. 2006, 72, 2373–2378. [Google Scholar] [CrossRef] [PubMed]
- Chinchar, V.G. Ranaviruses (family Iridovirudae): Emerging cold-blooded killers. Arch. Virol. 2002, 147, 447–470. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, R.; Hasegawa, O.; Lapatra, S.; Yoshimizu, M. Isolation and characterization of the Japanese flounder (Paralichthys olivaceus) lymphocystis disease virus. J. Aquat. Anim. Health 2002, 14, 114–123. [Google Scholar] [CrossRef]
- Yang, C.G.; Wang, X.L.; Wang, L.; Zhang, B.; Chen, S.L. A new Akirin1 gene in turbot (Scophthalmus maximus): Molecular cloning, characterization and expression analysis in response to bacterial and viral immunological challenge. Fish Shellfish Immunol. 2011, 30, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Zhang, Y.X.; Yang, C.G.; Zhang, B.; Chen, S.L. Cloning, characterization and expression analysis of a cold shock domain family member YB-1 in turbot Scophthalmus maximus. Fish Shellfish Immunol. 2012, 33, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, X.L.; Yang, C.G.; Zhao, X.J.; Zhang, Y.X.; Wang, T.Z.; Chen, S.L. Molecular cloning and multifunctional characterization of GRIM-19 (gene associated with retinoid-interferon-induced mortality 19) homologue from turbot (Scophthalmus maximus). Dev. Comp. Immunol. 2014, 43, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Imajoh, M.; Yagyu, K.I.; Oshima, S.I. Early interactions of marine birnavirus infection in several fish cell lines. J. Gen. Virol. 2003, 84, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Flint, M.; Tscherne, D.M. Cellular receptors and HCV entry. In Hepatitis C; Humana Press: New York, NY, USA, 2009; pp. 265–277. [Google Scholar]
- Tayyari, F.; Marchant, D.; Moraes, T.J.; Duan, W.; Mastrangelo, P.; Hegele, R.G. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat. Med. 2011, 17, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sheng, X.Z.; Xing, J.; Tang, X.Q.; Zhan, W.B. Identification of a 27.8 kDa protein from flounder gill cells involved in lymphocystis disease virus binding and infection. Dis. Aquat. Org. 2011, 94, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.Z.; Wang, M.; Xing, J.; Zhan, W.B. Monoclonal antibodies against 27.8 kDa protein receptor efficiently block lymphocystis disease virus infection in flounder Paralichthys olivaceus gill cells. Dis. Aquat. Org. 2012, 100, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Cano, I.; Valverde, E.J.; Garcia-Rosado, E.; Alonso, M.C.; Lopez-Jimena, B.; Ortiz-Delgado, J.B.; Borrego, J.J.; Sarasquete, C.; Castro, D. Transmission of lymphocystis disease virus to cultured gilthead seabream, Sparus aurata L., larvae. J. Fish Dis. 2013, 36, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Bek, E.J.; McMinn, P.C. The pathogenesis and prevention of encephalitis due to human enterovirus 71. Curr. Infect. Dis. Rep. 2012, 14, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Li, Y.Q.; Sheng, X.Z.; Xing, J.; Tang, X.Q. Detection of lymphocystis disease virus in Japanese flounder Paralichthys olivaceus and other marine teleosts from northern China. Chin. J. Oceanol. Limnol. 2010, 28, 1213–1220. [Google Scholar] [CrossRef]
- Colorni, A.; Diamant, A. Splenic and cardiac lymphocystis in the red drum, Sciaenops ocellatus (L.). J. Fish Dis. 1995, 18, 467–471. [Google Scholar] [CrossRef]
- Qu, L.Y.; Sun, X.Q.; Zhang, J.X. Electron ultramicroscope observation of lymphocystis disease of the left-eyed flounder Paralichthys olivaceus. J. Ocean Univ. China 2000, 30, 105–110. [Google Scholar]
- Gibson-Kueh, S.; Netto, P.; Ngoh-Lim, G.H.; Chang, S.F.; Ho, L.L.; Qin, Q.W.; Chua, F.H.C.; Ng, M.L.; Ferguson, H.W. The pathology of systemic iridoviral disease in fish. J. Comp. Pathol. 2003, 129, 111–119. [Google Scholar] [CrossRef]
- Sun, X.Q.; Huang, J.; Liu, Y.Q.; Qu, L.Y.; Hong, X.G.; Zhang, J.X. The Studies on diagnositic method of dot-blot and in situ hybridization for lymphocystis disease of cultured Paralichthys olivaceus. High Technol. Lett. 2003, 1, 89–94. [Google Scholar]
- Kvitt, H.; Heinisch, G.; Diamant, A. Detection and phylogeny of Lymphocystivirus in sea bream Sparus aurata based on the DNA polymerase gene and major capsid protein sequences. Aquaculture 2008, 275, 58–63. [Google Scholar] [CrossRef]
- Papi, A.; Johnston, S.L. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 (ICAM-1) via increased NF-κB-mediated transcription. J. Biol. Chem. 1999, 274, 9707–9720. [Google Scholar] [CrossRef] [PubMed]
- Wenzlow, N.; Plattet, P.; Wittek, R.; Zurbriggen, A.; Grone, A. Immunohistochemical demonstration of the putative canine distemper virus receptor CD150 in dogs with and without distemper. Vet. Pathol. 2007, 44, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.J.; Tang, X.Q.; Zhan, W.B.; Xing, J.; Sheng, X.Z. Expression kinetics of β-integrin in Chinese shrimp (Fenneropenaeus chinensis) hemocytes following infection with white spot syndrome virus. Fish Shellfish Immunol. 2013, 35, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Fan, P.H.; Jin, J.; Su, W.H.; An, D.; Xu, L.; Sun, S.Y.; Zhang, Y.; Meng, X.Y.; Gao, F.; et al. Establishment of cell lines with increased susceptibility to EV71/CA16 by stable over expression of SCARB2. Virol. J. 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.H.; Tang, X.Q.; Sheng, X.Z.; Zhan, W.B. Relationship between expression of cellular receptor-27.8 kDa and lymphocystis disease virus (LCDV) infection. PLoS ONE 2015, 10, e0127940. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.Q.; Diao, J.; Zhan, W.B.; Sheng, X.Z. Identification of a 37.6 kDa membrane protein from flounder gill cells involved in binding and infection to lymphocystis disease virus. Aquaculture 2012, 358, 132–138. [Google Scholar] [CrossRef]
- Wu, R.H.; Tang, X.Q.; Sheng, X.Z.; Zhan, W.B. Tissue distribution of the 27.8 kDa receptor and its dynamic expression in response to lymphocystis disease virus infection in flounder (Paralichthys olivaceus). J. Comp. Pathol 2015. [Google Scholar] [CrossRef]
- Alonso, M.C.; Cano, I.; Garcia-Rosado, E.; Castro, D.; Lamas, J.; Barja, J.L.; Borrego, J.J. Isolation of lymphocystis disease virus from sole, Solea senegalensis Kaup, and blackspot sea bream, Pagellus bogaraveo (Brünnich). J. Fish Dis. 2005, 28, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Cano, I.; Ferro, P.; Alonso, M.C.; Bergmann, S.M.; Römer-Oberdörfer, A.; Garcia-Rosado, E.; Castro, D.; Borrego, J.J. Development of molecular techniques for detection of lymphocystis disease virus in different marine fish species. J. Appl. Microbiol. 2007, 102, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K. Fish Viruses and Fish Viral Diseases; Comstock Publishing Associates; Cornell University Press: New York, NY, USA, 1988. [Google Scholar]
- Bowser, P.R.; Wooster, G.A.; Getchell, R.G. Transmission of walleye dermal sarcoma and lymphocystis via waterborne exposure. Aquat. Anim. Health 1999, 11, 158–161. [Google Scholar] [CrossRef]
- Cano, I.; Ferro, P.; Alonso, M.C.; Sarasquete, C.; Garcia-Rosado, E.; Borrego, J.J.; Castro, D. Application of in situ detection techniques to determine the systemic condition of lymphocystis disease virus infection in cultured gilt-head seabream, Sparus aurata L. J. Fish Dis. 2009, 32, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal research: reporting in vivo experiments: the ARRIVE guidelines. J. Gene Med. 2010, 12, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.F.; Zhan, W.B.; Xing, J.; Sheng, X.Z. Development and characterization of monoclonal antibody to the lymphocystis disease virus of Japanese flounder Paralichthys olivaceus isolated from China. J. Virol. Methods 2006, 135, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.B.; Wang, Y.H.; Fryer, J.L.; Okubo, K.; Fukuda, H.; Yu, K.K.; Meng, Q.X. Production of monoclonal antibodies (Mabs) against white spot syndrome virus (WSSV). J. Aquat. Anim. Health 1999, 11, 17–22. [Google Scholar] [CrossRef]
- Lin, Y.B.; Zhan, W.B.; Li, Q.; Zhang, Z.D.; Wei, X.M.; Sheng, X.Z. Ontogenesis of haemocytes in shrimp (Fenneropenaeus chinensis) studied with probes of monoclonal antibody. Dev. Comp. Immunol. 2007, 31, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Faulk, D.M.; Carruthers, C.A.; Warner, H.J.; Kramer, C.R.; Reing, J.E.; Zhang, L.; D’Amore, A.; Badylak, S.F. The effect of detergents on the basement membrane complex of a biologic scaffold material. Acta Biomater. 2014, 10, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Fan, H.; Qi, X.; Qi, Y.H.; Shi, Z.Y.; Wang, H.; Cui, L.B.; Zhou, M.H. Development and evaluation of a SYBR Green-based real time RT-PCR assay for detection of the emerging avian influenza A (H7N9) virus. PLoS ONE 2013, 8, e80028. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, X.; Wu, R.; Tang, X.; Xing, J.; Zhan, W. Tissue Localization of Lymphocystis Disease Virus (LCDV) Receptor-27.8 kDa and Its Expression Kinetics Induced by the Viral Infection in Turbot (Scophthalmus maximus). Int. J. Mol. Sci. 2015, 16, 26506-26519. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161125974
Sheng X, Wu R, Tang X, Xing J, Zhan W. Tissue Localization of Lymphocystis Disease Virus (LCDV) Receptor-27.8 kDa and Its Expression Kinetics Induced by the Viral Infection in Turbot (Scophthalmus maximus). International Journal of Molecular Sciences. 2015; 16(11):26506-26519. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161125974
Chicago/Turabian StyleSheng, Xiuzhen, Ronghua Wu, Xiaoqian Tang, Jing Xing, and Wenbin Zhan. 2015. "Tissue Localization of Lymphocystis Disease Virus (LCDV) Receptor-27.8 kDa and Its Expression Kinetics Induced by the Viral Infection in Turbot (Scophthalmus maximus)" International Journal of Molecular Sciences 16, no. 11: 26506-26519. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161125974