Connexin 50 Expression in Ependymal Stem Progenitor Cells after Spinal Cord Injury Activation
Abstract
:1. Introduction
2. Results
2.1. Expression of Cx50 in epSPC and epSPCi under Spontaneous Differentiation Conditions

2.2. Cx50 Expression in Directed-Differentiation Process of epSPCs to Mature Oligodendrocytes
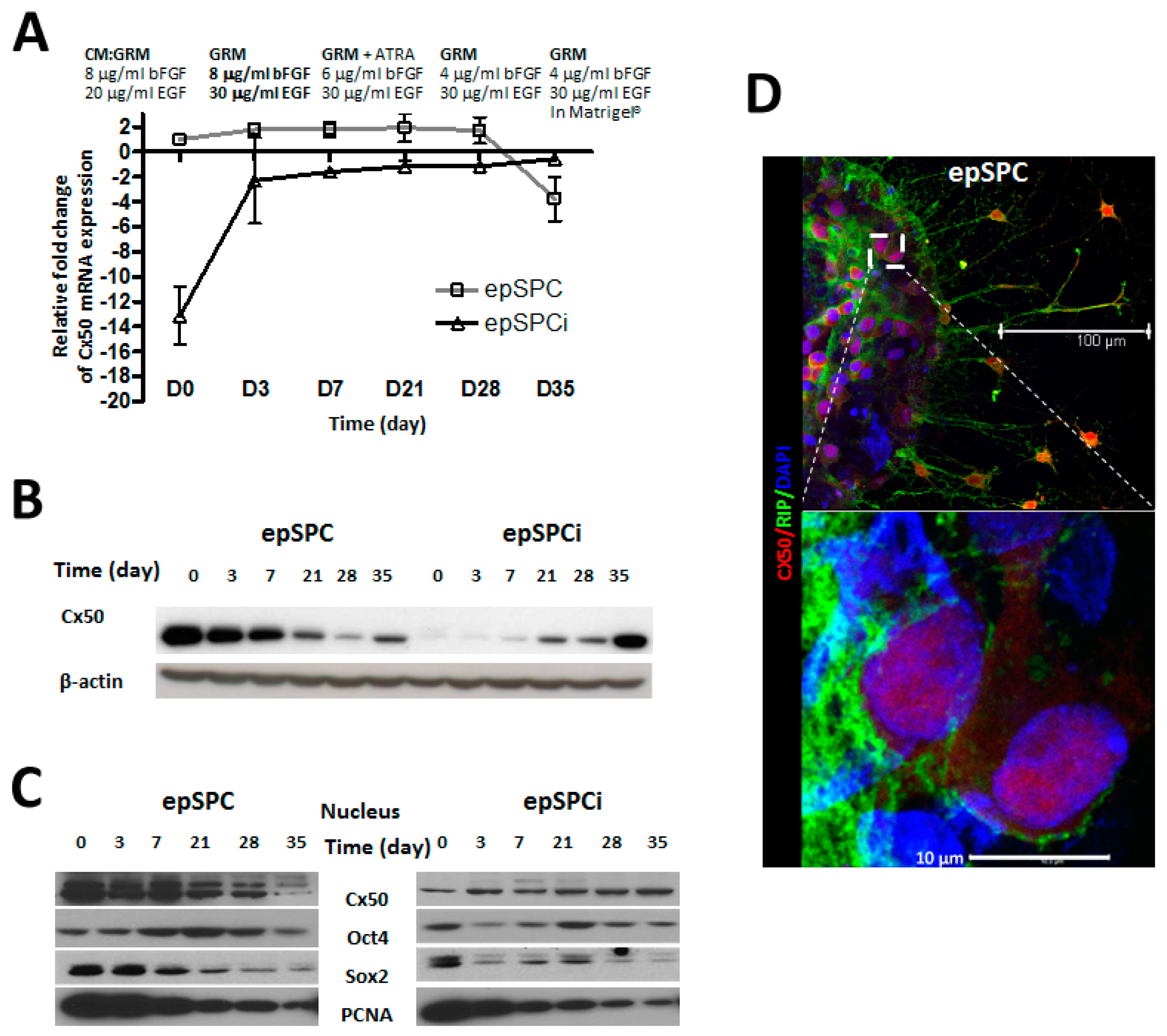
2.3. Cx50 Expression after Spinal Cord Injury and epSPCi Transplantation
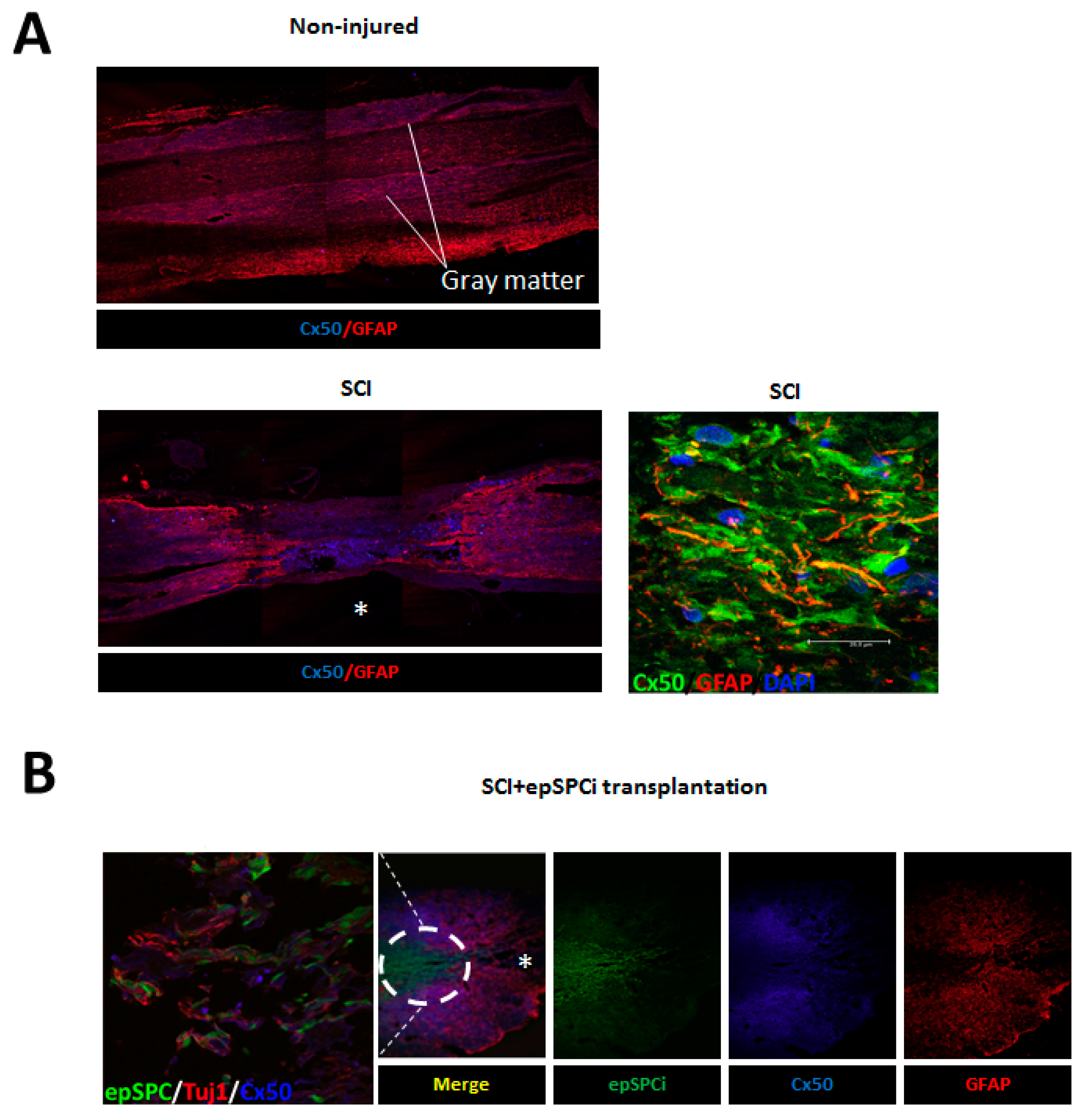
3. Discussion
4. Experimental Section
4.1. Spinal Cord Contusion and GFP-epSPCi Transplantation
4.2. epSPC/epSPCi, GFP-epSPCi Isolation
4.3. Spontaneous Differentiation of epSPC
4.4. Cx50 over-Expression in epSPCi
4.5. Oligodendrocyte-Directed Differentiation
4.6. Sub-Cellular Protein Fractionation
4.7. Isolation and Culture of Rat Spinal Cord Astrocytes
4.8. Real-Time Polymerase Chain Reaction (Taqman)
4.9. Western Blotting Analysis
4.10. Immunocytochemistry
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Orthmann-Murphy, J.L.; Abrams, C.K.; Scherer, S.S. Gap junctions couple astrocytes and oligodendrocytes. J. Mol. Neurosci.: MN 2008, 35, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, W.T.; Cornell-Bell, A.H.; Sontheimer, H. Astrocytes exhibit regional specificity in gap-junction coupling. Glia 1994, 11, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Pastor, A.; Kremer, M.; Moller, T.; Kettenmann, H.; Dermietzel, R. Dye coupling between spinal cord oligodendrocytes: Differences in coupling efficiency between gray and white matter. Glia 1998, 24, 108–120. [Google Scholar] [CrossRef]
- Rash, J.E.; Yasumura, T.; Davidson, K.G.; Furman, C.S.; Dudek, F.E.; Nagy, J.I. Identification of cells expressing Cx43, Cx30, Cx26, Cx32 and Cx36 in gap junctions of rat brain and spinal cord. Cell Commun. Adhes. 2001, 8, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Kunzelmann, P.; Schroder, W.; Traub, O.; Steinhauser, C.; Dermietzel, R.; Willecke, K. Late onset and increasing expression of the gap junction protein connexin30 in adult murine brain and long-term cultured astrocytes. Glia 1999, 25, 111–119. [Google Scholar] [CrossRef]
- Dermietzel, R.; Gao, Y.; Scemes, E.; Vieira, D.; Urban, M.; Kremer, M.; Bennett, M.V.; Spray, D.C. Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res. Brain Res. Rev. 2000, 32, 45–56. [Google Scholar] [CrossRef]
- Li, W.E.; Nagy, J.I. Connexin43 phosphorylation state and intercellular communication in cultured astrocytes following hypoxia and protein phosphatase inhibition. Eur. J. Neurosci. 2000, 12, 2644–2650. [Google Scholar] [CrossRef] [PubMed]
- Dahl, E.; Manthey, D.; Chen, Y.; Schwarz, H.J.; Chang, Y.S.; Lalley, P.A.; Nicholson, B.J.; Willecke, K. Molecular cloning and functional expression of mouse connexin-30,a gap junction gene highly expressed in adult brain and skin. J. Biol. Chem. 1996, 271, 17903–17910. [Google Scholar] [CrossRef] [PubMed]
- Liebmann, M.; Stahr, A.; Guenther, M.; Witte, O.W.; Frahm, C. Astrocytic Cx43 and Cx30 differentially modulate adult neurogenesis in mice. Neurosci. Lett. 2013, 545, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Dermietzel, R.; Traub, O.; Hwang, T.K.; Beyer, E.; Bennett, M.V.; Spray, D.C.; Willecke, K. Differential expression of three gap junction proteins in developing and mature brain tissues. Proc. Natl. Acad. Sci. USA 1989, 86, 10148–10152. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yu, H.X.; Sun, M.L.; Wang, Y.; Xi, W.; Yu, Y.Q. Astrocyte-restricted disruption of connexin-43 impairs neuronal plasticity in mouse barrel cortex. Eur. J. Neurosci. 2014, 39, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Kanczuga-Koda, L.; Sulkowski, S.; Koda, M.; Sulkowska, M. Alterations in connexin26 expression during colorectal carcinogenesis. Oncology 2005, 68, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Mennecier, G.; Derangeon, M.; Coronas, V.; Herve, J.C.; Mesnil, M. Aberrant expression and localization of connexin43 and connexin30 in a rat glioma cell line. Mol. Carcinogen. 2008, 47, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Krutovskikh, V.A.; Troyanovsky, S.M.; Piccoli, C.; Tsuda, H.; Asamoto, M.; Yamasaki, H. Differential effect of subcellular localization of communication impairing gap junction protein connexin43 on tumor cell growth in vivo. Oncogene 2000, 19, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Li, L.; Cai, B.; Liu, C.; Yang, Y.; Gao, Y.; Huang, W.; Yuan, X.; Wang, T.; Zhang, Q.; et al. Connexin 43 is involved in the generation of human-induced pluripotent stem cells. Hum. Mol. Genet. 2013, 22, 2221–2233. [Google Scholar] [CrossRef] [PubMed]
- Duval, N.; Gomes, D.; Calaora, V.; Calabrese, A.; Meda, P.; Bruzzone, R. Cell coupling and Cx43 expression in embryonic mouse neural progenitor cells. J. Cell Sci. 2002, 115, 3241–3251. [Google Scholar] [PubMed]
- Rozental, R.; Morales, M.; Mehler, M.F.; Urban, M.; Kremer, M.; Dermietzel, R.; Kessler, J.A.; Spray, D.C. Changes in the properties of gap junctions during neuronal differentiation of hippocampal progenitor cells. J. Neurosci. 1998, 18, 1753–1762. [Google Scholar] [PubMed]
- Todorova, M.G.; Soria, B.; Quesada, I. Gap junctional intercellular communication is required to maintain embryonic stem cells in a non-differentiated and proliferative state. J. Cell. Physiol. 2008, 214, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.X.; Gu, S. Gap junction- and hemichannel-independent actions of connexins. Biochim. Biophys. Acta 2005, 1711, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Hartfield, E.M.; Rinaldi, F.; Glover, C.P.; Wong, L.F.; Caldwell, M.A.; Uney, J.B. Connexin 36 expression regulates neuronal differentiation from neural progenitor cells. PLoS ONE 2011, 6, e14746. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.E.; Reali, C.; Radmilovich, M.; Fernandez, A.; Trujillo-Cenoz, O. Connexin 43 delimits functional domains of neurogenic precursors in the spinal cord. J. Neurosci. 2008, 28, 3298–3309. [Google Scholar] [CrossRef] [PubMed]
- Roscoe, W.A.; Messersmith, E.; Meyer-Franke, A.; Wipke, B.; Karlik, S.J. Connexin 43 gap junction proteins are up-regulated in remyelinating spinal cord. J. Neurosci. Res. 2007, 85, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Bautista, W.; Nagy, J.I.; Dai, Y.; McCrea, D.A. Requirement of neuronal connexin36 in pathways mediating presynaptic inhibition of primary afferents in functionally mature mouse spinal cord. J. Physiol. 2012, 590, 3821–3839. [Google Scholar] [CrossRef] [PubMed]
- Bautista, W.; McCrea, D.A.; Nagy, J.I. Connexin36 identified at morphologically mixed chemical/electrical synapses on trigeminal motoneurons and at primary afferent terminals on spinal cord neurons in adult mouse and rat. Neuroscience 2014, 263, 159–180. [Google Scholar] [CrossRef] [PubMed]
- Bautista, W.; Nagy, J.I. Connexin36 in gap junctions forming electrical synapses between motoneurons in sexually dimorphic motor nuclei in spinal cord of rat and mouse. Eur. J. Neurosci. 2014, 39, 771–787. [Google Scholar] [CrossRef] [PubMed]
- Bautista, W.; Rash, J.E.; Vanderpool, K.G.; Yasumura, T.; Nagy, J.I. Re-evaluation of connexins associated with motoneurons in rodent spinal cord, sexually dimorphic motor nuclei and trigeminal motor nucleus. Eur. J. Neurosci. 2014, 39, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Goncharenko, K.; Eftekharpour, E.; Velumian, A.A.; Carlen, P.L.; Fehlings, M.G. Changes in gap junction expression and function following ischemic injury of spinal cord white matter. J. Neurophysiol. 2014, 112, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Huettner, J.E.; Lu, A.; Qu, Y.; Wu, Y.; Kim, M.; McDonald, J.W. Gap junctions and connexon hemichannels in human embryonic stem cells. Stem Cells 2006, 24, 1654–1667. [Google Scholar] [CrossRef]
- Moreno-Manzano, V.; Rodriguez-Jimenez, F.J.; Garcia-Rosello, M.; Lainez, S.; Erceg, S.; Calvo, M.T.; Ronaghi, M.; Lloret, M.; Planells-Cases, R.; Sanchez-Puelles, J.M.; et al. Activated spinal cord ependymal stem cells rescue neurological function. Stem Cells 2009, 27, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Mothe, A.J.; Bozkurt, G.; Catapano, J.; Zabojova, J.; Wang, X.; Keating, A.; Tator, C.H. Intrathecal transplantation of stem cells by lumbar puncture for thoracic spinal cord injury in the rat. Spinal Cord 2011, 49, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Villafuertes, R.; Rodriguez-Jimenez, F.J.; Alastrue-Agudo, A.; Stojkovic, M.; Miras-Portugal, M.T.; Moreno-Manzano, V. Purinergic receptors in spinal cord-derived ependymal stem/progenitor cells and its potential role in cell-based therapy for spinal cord injury. Cell Transpl. 2014, 24, 1493–1509. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.B.; Momma, S.; Clarke, D.L.; Risling, M.; Lendahl, U.; Frisen, J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell 1999, 96, 25–34. [Google Scholar] [CrossRef]
- Okano, H.; Okada, S.; Nakamura, M.; Toyama, Y. Neural stem cells and regeneration of injured spinal cord. Kidney Int. 2005, 68, 1927–1931. [Google Scholar] [CrossRef] [PubMed]
- Stenudd, M.; Sabelstrom, H.; Frisen, J. Role of endogenous neural stem cells in spinal cord injury and repair. JAMA Neurol. 2015, 72, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Tonkin, R.S.; Mao, Y.; O’Carroll, S.J.; Nicholson, L.F.; Green, C.R.; Gorrie, C.A.; Moalem-Taylor, G. Gap junction proteins and their role in spinal cord injury. Front. Mol. Neurosci. 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.; Doble, B.W.; Kardami, E. The carboxy-tail of connexin-43 localizes to the nucleus and inhibits cell growth. Mol. Cell. Biochem. 2003, 242, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, C.P.; Holmstrom, N.A.; Lilja, J.A.; Schweinhardt, P.; Hao, J.; Spenger, C.; Wiesenfeld-Hallin, Z.; Kurpad, S.N.; Frisen, J.; Olson, L. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat. Neurosci. 2005, 8, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Theriault, E.; Frankenstein, U.N.; Hertzberg, E.L.; Nagy, J.I. Connexin43 and astrocytic gap junctions in the rat spinal cord after acute compression injury. J. Comp. Neurol. 1997, 382, 199–214. [Google Scholar] [CrossRef]
- Lee, I.H.; Lindqvist, E.; Kiehn, O.; Widenfalk, J.; Olson, L. Glial and neuronal connexin expression patterns in the rat spinal cord during development and following injury. J. Comp. Neurol. 2005, 489, 1–10. [Google Scholar] [CrossRef] [PubMed]
- White, T.W.; Goodenough, D.A.; Paul, D.L. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J. Cell Biol. 1998, 143, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Cronin, M.; Anderson, P.N.; Cook, J.E.; Green, C.R.; Becker, D.L. Blocking connexin43 expression reduces inflammation and improves functional recovery after spinal cord injury. Mol. Cell. Neurosci. 2008, 39, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Lois, C.; Hong, E.J.; Pease, S.; Brown, E.J.; Baltimore, D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 2002, 295, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Kerstetter, A.E.; Miller, R.H. Isolation and culture of spinal cord astrocytes. Methods Mol. Biol. 2012, 814, 93–104. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Jimenez, F.J.; Alastrue-Agudo, A.; Stojkovic, M.; Erceg, S.; Moreno-Manzano, V. Connexin 50 Expression in Ependymal Stem Progenitor Cells after Spinal Cord Injury Activation. Int. J. Mol. Sci. 2015, 16, 26608-26618. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161125981
Rodriguez-Jimenez FJ, Alastrue-Agudo A, Stojkovic M, Erceg S, Moreno-Manzano V. Connexin 50 Expression in Ependymal Stem Progenitor Cells after Spinal Cord Injury Activation. International Journal of Molecular Sciences. 2015; 16(11):26608-26618. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161125981
Chicago/Turabian StyleRodriguez-Jimenez, Francisco Javier, Ana Alastrue-Agudo, Miodrag Stojkovic, Slaven Erceg, and Victoria Moreno-Manzano. 2015. "Connexin 50 Expression in Ependymal Stem Progenitor Cells after Spinal Cord Injury Activation" International Journal of Molecular Sciences 16, no. 11: 26608-26618. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161125981





