A Comparative Study on Two Cationic Porphycenes: Photophysical and Antimicrobial Photoinactivation Evaluation
Abstract
:1. Introduction

2. Results and Discussion
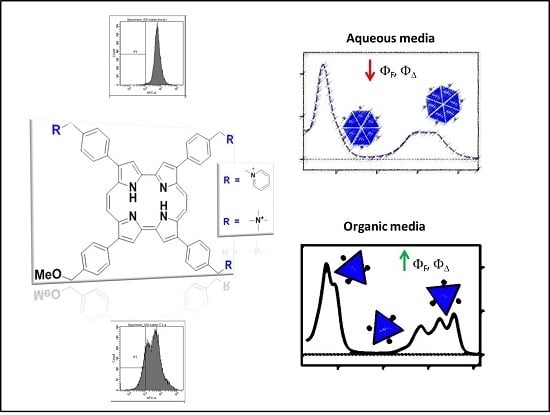
2.1. Photophysical Characterization of Porphycene Derivatives
2.1.1. Absorption and Fluorescence
| Compound | Solvent | λAbs/nm a | λFluo/nm b | ΦF c | τS/ns | ΦΔ d | τΔ/μs |
|---|---|---|---|---|---|---|---|
| TPPo e | Toluene | 659 (5.0 × 104) | 667 | 0.150 | 4.8 | 0.230 | - |
| Py3MeO-TBPo (1) | MeOH | 655 (5.1 × 104) | 664 | 0.075 | 2.6 | 0.193 | 10.0 |
| Water | 644 (2.6 × 104) | 656 | 0.005 | 1.8 | 0.004 | 3.7 | |
| NMe3MeO-TBPo (2) | MeOH | 657 (4.7 × 104) | 669 | 0.054 | 2.6 | 0.180 | 9.6 |
| Water | 641 (2.0 × 104) | 660 | 0.002 | 2.0 | 0.003 | 4.1 |

2.1.2. Near-Infrared Phosphorescence

2.2. Photodynamic Inactivation Studies



2.3. Flow Cytometry Analysis

2.4. Discussion
| Microorganism | Concentration (μM) | Fluence (J·cm−2) | Log10 Reduction | Other Studies |
|---|---|---|---|---|
| S. aureus | 2 | 20 | 6 | [27,43,45] |
| MRSA | 0.5 | 22.5 | 6 | [27,44,45,46,48] |
| E. coli | 10 | 60 | 6 | [27,43,45,48] |
| P. aeruginosa | 20 | 100 | 6 | [27,43,45,47,48] |
| C. albicans | 35 | 30 | >4 | [27,49,50] |
| C. krusei | 10 | 30 | >4 | [27,49,50] |
3. Experimental Section
3.1. Chemicals
3.2. Synthesis
3.3. Spectroscopic Techniques and Measurements
3.4. Flow Cytometry
3.5. Microbial Techniques
3.5.1. Microbial Strains and Growth Conditions
3.5.2. Photodynamic Inactivation Procedure
3.6. Statistical Analyses
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anonymous. Microbiology by numbers. Nat. Rev. Microbiol. 2011, 9. [Google Scholar] [CrossRef]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- St Denis, T.G.; Dai, T.; Izikson, L.; Astrakas, C.; Anderson, R.R.; Hamblin, M.R.; Tegos, G.P. All you need is light: Antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence 2011, 2, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Kutter, E.; de Vos, D.; Gvasalia, G.; Alavidze, Z.; Gogokhia, L.; Kuhl, S.; Abedon, S.T. Phage therapy in clinical practice: Treatment of human infections. Curr. Pharm. Biotechnol. 2010, 11, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.M.; Jabra-Rizk, M.A.; O’May, G.A.; Costerton, J.W.; Shirtliff, M.E. Polymicrobial interactions: Impact on pathogenesis and human disease. Clin. Microbiol. Rev. 2012, 25, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Prebiotics and probiotics; modifying and mining the microbiota. Pharmacol. Res. 2010, 61, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Tegos, G.P.; Hamblin, M.R. Disruptive innovations: New anti-infectives in the age of resistance. Curr. Opin. Pharmacol. 2013, 13, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.; Mroz, P. Advances in Photodynamic Therapy: Basic, Translational, and Clinical; Artech House: Norwood, MA, USA, 2008. [Google Scholar]
- Planas, O.; Boix-Garriga, E.; Rodríguez-Amigo, B.; Torra, J.; Bresolí-Obach, R.; Flors, C.; Viappiani, C.; Agut, M.; Ruiz-González, R.; Nonell, S. Chapter 9: Newest approaches to singlet oxygen photosensitisation in biological media. In Photochemistry; Fasani, E., Albini, A., Eds.; Royal Society of Chemistry: Cambridge, UK, 2015; Volume 42, pp. 233–278. [Google Scholar]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D. More adventures in photodynamic therapy. Int. J. Mol. Sci. 2015, 16, 15188–15193. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Huang, Y.Y.; Hamblin, M.R. Photodynamic therapy for localized infections—State of the art. Photodiagnosis Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, F.F.; Huang, Y.-Y.; Hamblin, M.R. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat. Antiinfect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.Y.; Dai, T.; Hamblin, M.R. Photodynamic therapy for infections: Clinical applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Babilas, P.; Schreml, S.; Landthaler, M.; Szeimies, R.M. Photodynamic therapy in dermatology: State-of-the-art. Photodermatol. Photoimmunol. Photomed. 2010, 26, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, H.; Ozcakir-Tomruk, C.; Tanalp, J.; Yilmaz, S. Photodynamic therapy in dentistry: A literature review. Clin. Oral Investig. 2013, 17, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M. Photolysis of oral bacteria and its potential use in the treatment of caries and periodontal disease. J. Appl. Bacteriol. 1993, 75, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Merchat, M.; Bertolini, G.; Giacomini, P.; Villanueva, A.; Jori, G. Meso-substituted cationic porphyrins as efficient photosensitizers of gram-positive and gram-negative bacteria. J. Photochem. Photobiol. B 1996, 32, 153–157. [Google Scholar] [CrossRef]
- Malik, Z.; Ladan, H. Photodynamic inactivation of Gram-negative bacteria: Problems and possible solutions. J. Photochem. Photobiol. B Biol. 1992, 14, 262–266. [Google Scholar] [CrossRef]
- Minnock, A.; Vernon, D.I.; Schofield, J.; Griffiths, J.; Parish, J.H.; Brown, S.T. Photoinactivation of bacteria. Use of a cationic water-soluble zinc phthalocyanine to photoinactivate both gram-negative and gram-positive bacteria. J. Photochem. Photobiol. B 1996, 32, 159–164. [Google Scholar] [CrossRef]
- Jori, G.; Fabris, C.; Soncin, M.; Ferro, S.; Coppellotti, O.; Dei, D.; Fantetti, L.; Chiti, G.; Roncucci, G. Photodynamic therapy in the treatment of microbial infections: Basic principles and perspective applications. Lasers Surg. Med. 2006, 38, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Jori, G.; Coppellotti, O. Inactivation of pathogenic microorganisms by photodynamic techniques: Mechanistic aspects and perspective applications. Antiinfect. Agents Med. Chem. 2007, 6, 119–131. [Google Scholar] [CrossRef]
- Alves, E.; Costa, L.; Carvalho, C.M.B.; Tomé, J.P.C.; Faustino, M.A.; Neves, M.G.; Tomé, A.C.; Cavaleiro, J.; Cunha, A.; Almeida, A. Charge effect on the photoinactivation of Gram-negative and Gram-positive bacteria by cationic meso-substituted porphyrins. BMC Microbiol. 2009, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stockert, J.C.; Cañete, M.; Juarranz, A.; Villanueva, A.; Horobin, R.W.; Borrell, J.I.; Teixido, J.; Nonell, S. Porphycenes: Facts and prospects in photodynamic therapy of cancer. Curr. Med. Chem. 2007, 14, 997–1026. [Google Scholar] [CrossRef] [PubMed]
- Ragàs, X.; Sánchez-García, D.; Ruiz-González, R.; Dai, T.; Agut, M.; Hamblin, M.R.; Nonell, S. Cationic porphycenes as potential photosensitizers for antimicrobial photodynamic therapy. J. Med. Chem. 2010, 53, 7796–7803. [Google Scholar] [CrossRef] [PubMed]
- Hager, B.; Strauss, W.S.L.; Falk, H. Cationic hypericin derivatives as novel agents with photobactericidal activity: Synthesis and photodynamic inactivation of Propionibacterium acnes. Photochem. Photobiol. 2009, 85, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Späth, A.; Leibl, C.; Cieplik, F.; Lehner, K.; Regensburger, J.; Hiller, K.-A.; Bäumler, W.; Schmalz, G.; Maisch, T. Improving photodynamic inactivation of nacteria in dentistry: Highly effective and fast killing of oral key pathogens with novel tooth-colored type-II photosensitizers. J. Med. Chem. 2014, 57, 5157–5168. [Google Scholar] [CrossRef] [PubMed]
- Rubio, N.; Prat, F.; Bou, N.; Borrell, J.I.; Teixido, J.; Villanueva, A.; Juarranz, A.; Canete, M.; Stockert, J.C.; Nonell, S. A comparison between the photophysical and photosensitising properties of tetraphenyl porphycenes and porphyrins. New J. Chem. 2005, 29, 378–384. [Google Scholar] [CrossRef]
- Jiménez-Banzo, A.; Ragàs, X.; Kapusta, P.; Nonell, S. Time-resolved methods in biophysics. 7. Photon counting vs. analog time-resolved singlet oxygen phosphorescence detection. Photochem. Photobiol. Sci. 2008, 7, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, C.; Schmidt, R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem. Rev. 2003, 103, 1685–1758. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, F.; Helman, W.P.; Ross, A.B. Quantum yields for the photosensitized formation of the lowest electronically excited state of molecular oxygen in solution. J. Phys. Chem. Ref. Data 1993, 22, 113–262. [Google Scholar] [CrossRef]
- Redmond, R.W.; Gamlin, J.N. A compilation of singlet oxygen yields from biologically relevant molecules. Photochem. Photobiol. 1999, 70, 391–475. [Google Scholar] [CrossRef]
- García-Díaz, M.; Nonell, S.; Villanueva, A.; Stockert, J.C.; Cañete, M.; Casadó, A.; Mora, M.; Sagristá, M.L. Do folate-receptor targeted liposomal photosensitizers enhance photodynamic therapy selectivity? Biochim. Biophys. Acta 2011, 1808, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-González, R.; Acedo, P.; Sánchez-García, D.; Nonell, S.; Cañete, M.; Stockert, J.C.; Villanueva, A. Efficient induction of apoptosis in HeLa cells by a novel cationic porphycene photosensitizer. Eur. J. Med. Chem. 2013, 63, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.; García-Díaz, M.; Mora, M.; Sagrista, M.L.; Nonell, S.; Villanueva, A.; Stockert, J.C.; Canete, M. Liposomal temocene (m-THPPo) photodynamic treatment induces cell death by mitochondria-independent apoptosis. Biochim. Biophys. Acta 2013, 1830, 4611–4620. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, Y.; Gutterman, M.; Malik, Z.; Ehrenberg, B. Inactivation of gram-negative bacteria by photosensitized porphyrins. Photochem. Photobiol. 1992, 55, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; O’Donnell, D.A.; Murthy, N.; Rajagopalan, K.; Michaud, N.; Sherwood, M.E.; Hasan, T. Polycationic photosensitizer conjugates: Effects of chain length and Gram classification on the photodynamic inactivation of bacteria. J. Antimicrob. Chemother. 2002, 49, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Tegos, G.P.; Anbe, M.; Yang, C.; Demidova, T.N.; Satti, M.; Mroz, P.; Janjua, S.; Gad, F.; Hamblin, M.R. Protease-stable polycationic photosensitizer conjugates between polyethyleneimine and chlorin(e6) for broad-spectrum antimicrobial photoinactivation. Antimicrob. Agents Chemother. 2006, 50, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Caminos, D.A.; Spesia, M.B.; Durantini, E.N. Photodynamic inactivation of Escherichia coli by novel meso-substituted porphyrins by 4-(3-N,N,N-trimethylammoniumpropoxy)phenyl and 4-(trifluoromethyl)phenyl groups. Photochem. Photobiol. Sci. 2006, 5, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Alves, E.; Carvalho, C.M.B.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, A.; Almeida, A. Sewage bacteriophage photoinactivation by cationic porphyrins: A study of charge effect. Photochem. Photobiol. Sci. 2008, 7, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Dimaano, M.; Rozario, C.; Nerandzic, M.; Donskey, C.; Lam, M.; Baron, E. The photodynamic antibacterial effects of silicon phthalocyanine (Pc) 4. Int. J. Mol. Sci. 2015, 16, 7851–7860. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.-R.; Eastel, J.M.; Ngai, K.L.K.; Cheung, Y.-Y.; Chan, P.K.S.; Hui, M.; Ng, D.K.P.; Lo, P.-C. Photodynamic inactivation of bacteria and viruses using two monosubstituted zinc(II) phthalocyanines. Eur. J. Med. Chem. 2014, 84, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Feterl, M.; Mulyana, Y.; Warner, J.M.; Collins, J.G.; Keene, F.R. In vitro susceptibility and cellular uptake for a new class of antimicrobial agents: Dinuclear ruthenium(II) complexes. J. Antimicrob. Chemother. 2012, 67, 2686–2695. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, B.N.; Tuchina, E.S.; Tuchin, V.V.; Khlebtsov, N. Multifunctional Au nanoclusters for targeted bioimaging and enhanced photodynamic inactivation of Staphylococcus aureus. RSC Adv. 2015, 5, 61639–61649. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Caruso, E.; Banfi, S.; Barbieri, P. Effect of Organic Matter on the in Vitro Photoeradication of Pseudomonas aeruginosa by Means of a Cationic Tetraaryl-porphyrin(dagger). Photochem. Photobiol. 2012, 88, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Schastak, S.; Ziganshyna, S.; Gitter, B.; Wiedemann, P.; Claudepierre, T. Efficient photodynamic therapy against gram-positive and gram-negative bacteria using THPTS, a cationic photosensitizer excited by infrared wavelength. PLoS ONE 2010, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Oriel, S.; Nitzan, Y. Mechanistic aspects of photoinactivation of Candida albicans by exogenous porphyrins. Photochem. Photobiol. 2012, 88, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Rezusta, A.; López-Chicón, P.; Paz-Cristobal, M.P.; Alemany-Ribes, M.; Royo-Díez, D.; Agut, M.; Semino, C.; Nonell, S.; Revillo, M.J.; Aspiroz, C.; et al. In vitro fungicidal photodynamic effect of hypericin on Candida species. Photochem. Photobiol. 2012, 88, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Gruden, C.; Skerlos, S.; Adriaens, P. Flow cytometry for microbial sensing in environmental sustainability applications: Current status and future prospects. FEMS Microbiol. Ecol. 2004, 49, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Nebe-von-Caron, G.; Stephens, P.; Hewitt, C.; Powell, J.; Badley, R. Analysis of bacterial function by multi-colour fluorescence flow cytometry and single cell sorting. J. Microbiol. Methods 2000, 42, 97–114. [Google Scholar] [CrossRef]
- Ananta, E.; Heinz, V.; Knorr, D. Assessment of high pressure induced damage on Lactobacillus rhamnosus GG by flow cytometry. Food Microbiol. 2004, 21, 567–577. [Google Scholar] [CrossRef]
- Amor, K.B.; Breeuwer, P.; Verbaarschot, P.; Rombouts, F.M.; Akkermans, A.D.L.; de Vos, W.M.; Abee, T. Multiparametric flow cytometry and cell sorting for the assessment of viable, injured, and dead bifidobacterium cells during bile salt stress. Appl. Environ. Microbiol. 2002, 68, 5209–5216. [Google Scholar] [CrossRef] [PubMed]
- Nguefack, J.; Budde, B.B.; Jakobsen, M. Five essential oils from aromatic plants of Cameroon: Their antibacterial activity and ability to permeabilize the cytoplasmic membrane of Listeria innocua examined by flow cytometry. Lett. Appl. Microbiol. 2004, 39, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Paparella, A.; Taccogna, L.; Aguzzi, I.; Chaves-López, C.; Serio, A.; Marsilio, F.; Suzzi, G. Flow cytometric assessment of the antimicrobial activity of essential oils against Listeria monocytogenes. Food Control 2008, 19, 1174–1182. [Google Scholar] [CrossRef]
- Magde, D.; Wong, R.; Seybold, P.G. Fluorescence quantum yields and their relation to lifetimes of rhodamine 6G and fluorescein in nine solvents: Improved absolute standards for quantum yields. Photochem. Photobiol. 2002, 75, 327–334. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-González, R.; Agut, M.; Reddi, E.; Nonell, S. A Comparative Study on Two Cationic Porphycenes: Photophysical and Antimicrobial Photoinactivation Evaluation. Int. J. Mol. Sci. 2015, 16, 27072-27086. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161125999
Ruiz-González R, Agut M, Reddi E, Nonell S. A Comparative Study on Two Cationic Porphycenes: Photophysical and Antimicrobial Photoinactivation Evaluation. International Journal of Molecular Sciences. 2015; 16(11):27072-27086. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161125999
Chicago/Turabian StyleRuiz-González, Rubén, Montserrat Agut, Elena Reddi, and Santi Nonell. 2015. "A Comparative Study on Two Cationic Porphycenes: Photophysical and Antimicrobial Photoinactivation Evaluation" International Journal of Molecular Sciences 16, no. 11: 27072-27086. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161125999