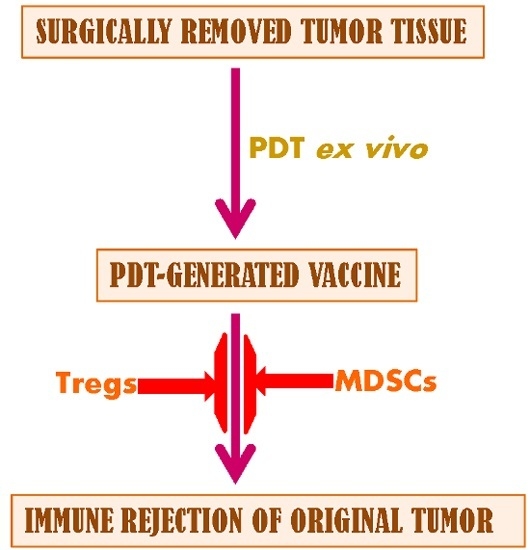
Immunoregulatory Cell Depletion Improves the Efficacy of Photodynamic Therapy-Generated Cancer Vaccines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Low-Dose Cyclophosphamide Combined with Vaccine Treatment


2.1.2. Single and Double Cyclophosphamide Treatment

2.1.3. PDT Vaccine and Tregs


2.1.4. PDT Vaccine and MDSCs

2.2. Discussion
3. Experimental Section
3.1. Tumor Model

3.2. PDT Vaccine Preparation and Treatment


3.3. Flow Cytometry
3.4. Foxp3 Gene Expression Analysis
3.5. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gollnick, S.O.; Vaughan, L.; Henderson, B.W. Generation of effective antitumor vaccines using photodynamic therapy. Cancer Res. 2002, 62, 1604–1608. [Google Scholar] [PubMed]
- Korbelik, M. Cancer vaccines generated by photodynamic therapy. Photochem. Photobiol. Sci. 2011, 10, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R. Photodynamic therapy: Oncologic horizons. Future Oncol. 2014, 10, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M. PDT-associated host response and its role in the therapy outcome. Lasers Surg. Med. 2006, 38, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M.; Banáth, J.; Canals, D.; Hannun, Y.A.; Separovic, D. Ceramide and sphingosine-1-phosphate act as photodynamic therapy-elicited damage-associated molecular patterns: Cell surface exposure. Int. Immunopharmacol. 2014, 20, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Kepp, O.; Semeraro, M.; Bravo-San Pedro, J.M.; Bloy, N.; Buque, A.; Huang, X.; Zhou, H.; Senovilla, L.; Kroemer, G.; Galluzzi, L. eIF2α phosphorylation as a biomarker of immunogenic cell death. Semin. Cancer Biol. 2015, 33, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M.; Stott, B.; Sun, J. Photodynamic therapy-generated vaccines: Relevance of tumour cell death expression. Br. J. Cancer 2007, 97, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Kepp, O.; Senovilla, L.; Vitale, I.; Vacchelli, E.; Adjemian, S.; Agostinis, P.; Apetoh, L.; Aranda, F.; Barnaba, V.; Boly, N.; et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology 2014, 3, 955691. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Mroz, P.; Wu, M.X.; Hamblin, M.R. Photodynamic therapy plus low-dose cyclophosphamide generates antitumor immunity in a mouse model. Proc. Natl. Acad. Sci. USA 2008, 105, 5495–5500. [Google Scholar] [CrossRef] [PubMed]
- Reginato, E.; Mroz, P.; Chung, H.; Kawakubo, M.; Wolf, P.; Hamblin, M.R. Photodynamic therapy plus regulatory T-cell depletion produces immunity against a mouse tumor that expresses a self-antigen. Br. J. Cancer 2013, 109, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- Buckner, J.H.; Ziegler, S.F. Functional analysis of FOXP3. Ann. N. Y. Acad. Sci. 2008, 1143, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Stewart, T.J.; Smyth, M.J. Improving cancer immunotherapy by targeting tumor-induced immune suppression. Cancer Metastasis Rev. 2011, 30, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.M.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Ha, G.-H.; Kim, S.-H.; Kang, C.-D. Combination of cancer immunotherapy with clinically available drugs that can block immunosuppressive cells. Immunol. Investig. 2014, 43, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Mougiakakos, D.; Choudhury, A.; Lladser, A.; Kiessling, R.; Johansson, S.C. Regulatory T cells in cancer. Adv. Cancer Res. 2010, 107, 57–117. [Google Scholar] [PubMed]
- Nishikawa, H.; Sakaguchi, S. Regulatory T cells in tumor immunity. Int. J. Cancer 2010, 127, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Haile, L.A.; Greten, T.F.; Korangy, F. Immune suppression: The hallmark of myeloid derived suppressor cells. Immunol. Investig. 2012, 41, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Sistigu, A.; Viaud, S.; Chaput, N.; Bracci, L.; Proietti, E.; Zitvogel, L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin. Immunopathol. 2011, 33, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Sakaguchi, S. Foxp3: A critical regulator of the development and function of regulatory T cells. Microbes Infect. 2004, 6, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Radis, C.D.; Kahl, L.E.; Baker, G.L.; Wasko, M.C.; Cash, J.M.; Gallatin, A.; Stolzer, B.L.; Agrawal, A.K.; Medsger, T.A., Jr.; Kwoh, C.K. Effects of cyclophosphamide on the development of malignancy and on long-term survival of patients with rheumatoid arthritis a 20-year followup study. Arthritis Rheum. 1995, 38, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Iclozan, C.; Antonia, S.; Chiappori, A.; Chen, D.-T.; Gabrilovich, D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol. Immunother. 2013, 62, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Guo, W.; Cui, J.; Qian, X.; Yi, L.; Chang, M.; Cai, Q.; Zhao, Q. A tritherapy combination of a fusion protein vaccine with immune-modulating doses of sequential chemotherapies in an optimized regimen completely eradicates large tumors in mice. Int. J. Cancer 2011, 128, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Khurana, D.; Martin, E.A.; Kasperbauer, J.L.; O’Malley, B.W., Jr.; Salomao, D.R.; Chen, L.; Strome, S.E. Characterization of a spontaneously arising murine squamous cell carcinoma (SCC VII) as a prerequisite for head and neck cancer immunotherapy. Head Neck 2001, 23, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M. Photodynamic therapy-generated vaccines. Methods Mol. Biol. 2010, 635, 147–153. [Google Scholar] [PubMed]
- Korbelik, M. Distribution of disulfonated and tetrasulfonated aluminum phthalocyanine between malignant and host cell populations of a murine fibrosarcoma. J. Photochem. Photobiol. 1993, 20, 173–181. [Google Scholar] [CrossRef]
- Korbelik, M.; Cecic, I.; Merchant, S.; Sun, J. Acute phase response induction by cancer treatment with photodynamic therapy. Int. J. Cancer 2008, 122, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M.; Merchant, S. Photodynamic therapy-generated cancer vaccine elicits acute phase and hormonal response in treated mice. Cancer Immunol. Immunother. 2012, 61, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korbelik, M.; Banáth, J.; Saw, K.M. Immunoregulatory Cell Depletion Improves the Efficacy of Photodynamic Therapy-Generated Cancer Vaccines. Int. J. Mol. Sci. 2015, 16, 27005-27014. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161126008
Korbelik M, Banáth J, Saw KM. Immunoregulatory Cell Depletion Improves the Efficacy of Photodynamic Therapy-Generated Cancer Vaccines. International Journal of Molecular Sciences. 2015; 16(11):27005-27014. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161126008
Chicago/Turabian StyleKorbelik, Mladen, Judit Banáth, and Kyi Min Saw. 2015. "Immunoregulatory Cell Depletion Improves the Efficacy of Photodynamic Therapy-Generated Cancer Vaccines" International Journal of Molecular Sciences 16, no. 11: 27005-27014. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161126008